Amelioration of Acetaminophen-Induced Hepatotoxicity in Rat by Co-Administration of Quercetin and Resveratrol in Rats
Roohollah Mohseni1,2, Ebrahim Abbasi-Oshaghi1,3*, Hamid Reza Ghasemi Basir4, Amin Molaie5, Hamid Reza Zareie5, Fatemeh Mirzaei3, Mehrdad Ahmadi5, Mehrnoush Mousavi 5 and Ghazal Alagha5
1Department of Biochemistry, Hamadan University of Medical Sciences, Iran
2Student research committee, Hamadan University of Medical Sciences, Iran
3Neurophysiology Research Center, Hamadan University of Medical Sciences, Iran
4Department of Pathology, Hamadan University of Medical Sciences, Iran
5Department of Medical Laboratory Sciences, Hamadan University of Medical Sciences, Iran
Submission: April 11, 2019; Published: May 02, 2019
*Corresponding author: Ebrahim Abbasi Oshaghi, Department of Clinical Biochemistry, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
How to cite this article: Roohollah M, Ebrahim AO, Hamid RGB, Amin M, Hamid RZ, et al. Amelioration of Acetaminophen-Induced Hepatotoxicity in Rat by Co-Administration of Quercetin and Resveratrol in Rats. Dairy and Vet Sci J. 2019; 11(4): 555817. DOI: 10.19080/JDVS.2019.11.555817
Abstract
Monotherapy of resveratrol (RES) and quercetin (QE) showed potential useful effects. While, the combination properties are remain unknown. Therefore, in the current study we aimed to investigate the hepatoprotective effects of oral co-administration of RES and QE against acetaminophen (AA) induced hepatotoxicity in rats. Acute hepatotoxicity was induced by a single dose of 640 mg/kg AA. Then RES and QE were administrated orally for one week. The male Wistar rats were randomly divided into 6 groups including; normal, hepatotoxic group (AA received rats), N-acetylcysteine (NAC) group: AA + 150mg/kg/day NAC, QE group: AA + 20mg/kg/day QE, RES group: AA + 30 mg/kg/day RES and combination group: AA + RES (30mg/kg/day) + QE (20mg/kg/day). At the end of experiment, serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT) were measured. Also, serum level of triglyceride, total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) and very low-density lipoprotein were measured (VLDL-C). Then the liver structure was assessed by histopathological examination. Serum and liver total antioxidant capacity (TAC) and malondialdehyde (MDA) were measured by photometry and fluorescence spectroscopy method, respectively in serum and tissue homogenate.
The combination treatment significantly alleviated activity of specific liver enzymes and modulated lipid profiles parameters more than RES and QE treatment alone. Histopathological analysis indicated combination treatment remarkably led to improve liver injury more than RES and QE treatment separately. Also, co-administration of RES and QE normalized TAC and MDA levels in serum and tissue more significantly than single treatment. Oral co-administration of RES and QE normalized AA-induced hepatotoxicity by reducing lipid levels, MDA, liver enzymes and histopathological changes.
Keywords: Acetaminophen; N-acetylcysteine; Quercetin; Resveratrol
Abbrevations: TAC: Total Antioxidant Capacity; MDA: Malondialdehyde; AST: Aspartate Aminotransferase; GGT: Gamma-Glutamyl Transferase; NAC: N-Acetylcysteine; AA: Against Acetaminophen; ALT: Alanine Aminotransferase; RES: Monotherapy of Resveratrol; QE: Quercetin
Introduction
Acetaminophen (AA) widely utilized in clinical interventions for the management of fever and mild-to-moderate pain [1]. Cheap price and well availability of AA is mainly responsible for its abuse that can be led to liver injury [2]. In the United States, approximately 50% of patients with liver injury were due to drug induced liver toxicity [3]. Malnutrition, fasting, abdominal pain, nausea symptom is the most common clinical sign of the drug induced hepatotoxicity [4]. N-acetylcysteine (NAC) was used commonly in AA overdose patients to reduce acute liver failure, and in some cases, accompanied by nausea, vomiting and skin allergy. In this context, herbal medicine could be considered as an alternative therapeutic approach for improving AA-induced liver damage.
From ancient times until now natural polyphenols have become widely used for prevention and treatment of many diseases [5]. Their consumption accommodated with fewer side effects than [6]. Natural polyphenols have known as a free radical scavenger where relived oxidative stress and inflammation [7]. Resveratrol (RES) is one of the most popular natural polyphenols with a huge number of beneficial effects in clinical interventions [8]. Many reports introduced RES as an effective protective supplement against drug hepatotoxicity by relieving tissue oxidative stress [9-11]. Recent study demonstrated the hepatoprotective effect of RES against alcohol consumption and AA toxicity [12]. On the other hand, quercetin (QE) is one of the most prominent dietary antioxidants that widely found in herbal sources. Previous studies showed the hepatoprotective effect of QE via decreasing oxidative stress [13,14]. QE ameliorated diethylnitrosamine induced hepatotoxicity in rats’ model through reducing the serum level of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and improving hepatic lipid peroxidation and glutathione content (GSH) [15]. However low bioavailability of RES and QE that is mainly due to poor stability and intestinal absorption limit their clinical applications when administrated orally.
Previous experimental researches identified intraduodenal and intraperitoneally co-administration of natural RES and QE enhance their bioavailability [16,17]. Over the last years, various experiment has been done to elevate the bioavailability of natural polyphenols. Here combination therapy may be considered as an attractive choice. Recent in vitro study showed co-administration of QE, RES and curcumin enhances intestinal absorption of RES and curcumin across epithelial cells [16]. On the other hand, recent studies reported that co-administration of RES and QE suppressed cell proliferation, cell cycle progression and primary mammary tumor growth more effective than single administration [18,19]. Mikstacka showed that co-administration of RES and QE effectively decreased lipid peroxidation in human erythrocytes [20]. Moreover, the previous attempt demonstrated RES plus QE prevented liver steatosis through increasing fatty acid oxidation and inhibiting de novo lipogenesis [21]. Considering previous reports that mention above, it could be concluded that the co-administration of RES plus QE may lead to enhance its hepatoprotective effect against AA induced hepatotoxicity. Therefore, in the current study, we investigated the hepatoprotective effect of RES and QE treatment against AA hepatotoxicity when administrated together and separately.
Materials and Methods
Animal study design
The 6-week-old male Wistar rats weighing 220-230g were purchased from Hamadan University of Medical Sciences. Animals were housed in a temperature-controlled room (23±2 °C) and humidity environment (60±5%) with 12-hour light/dark cycles and standard chow diet. After acclimatization for one week in the animal house, the rats were randomly divided into 6 groups (7 for each group) as following:
a. group 1: normal healthy controls which received normal saline.
b. group 2: received 640 mg/kg AA (hepatotoxic group).
c. Group 3: received 640 mg/kg AA + 150 mg/kg/day NAC (positive control).
d. Group 4: received 640 mg/kg AA + 20mg/kg/day QE.
e. group 5: received 640 mg/kg AA + 30mg/kg/day RES and
f. group 6: received 640 mg/kg AA + 20mg/kg/day QE + 30mg/kg/day RES (combination group).
AA hepatotoxicity was induced by single dose of AA 640 mg/kg orally. Then the single and combined administration of QE and RES were performed orally for one week. In the present study low dose of RES and QE was selected according to previous studies [22-24]. All procedures of this study were carried out under supervision of Ethics Committee of Hamadan University of Medical Sciences (Ethics code: IR.UMSHA.REC.1395.383).
At the end of study, after overnight fasting, all animals were anesthetized with ketamine 100mg/kg and sacrificed. Blood samples were collected from the heart and centrifuged at 3000×g for 10 min at 4 °C then serum was separated and stored at -20 °C for further biochemical analysis. Liver tissues also were excised and small portion was fixed in 10% formalin for histopathological examination. Another section immediately was frozen at the liquid nitrogen and stored at -70 °C for the determination of oxidative stress parameters.
Tissue Homogenate Preparation
Small pieces of liver tissues were weighed and homogenized using lysis buffer and then centrifuged for 15 minutes at 10000g at 4 °C. The supernatant was separated and maintained at -20 °C for further analysis.
Protein Quantification Assay
The total protein concentration was measured by Bradford method [25]. Bovine serum albumin was used as standard.
Serum Biochemical Measurement
Specific liver enzymes including; AST, ALT, gamma-glutamyl transferase (GGT) and serum lipid profile includes; triglyceride (TG), total cholesterol (Cho) and high-density lipoprotein cholesterol (HDL-C) were measured by colorimetric assay kit (Pars Azmun, Tehran, Iran). The levels of low-density lipoprotein cholesterol (LDL-C) and very low-density lipoprotein (VLDL) was calculated according to the Friedewald formula.
Haematoxylin-eosin Staining
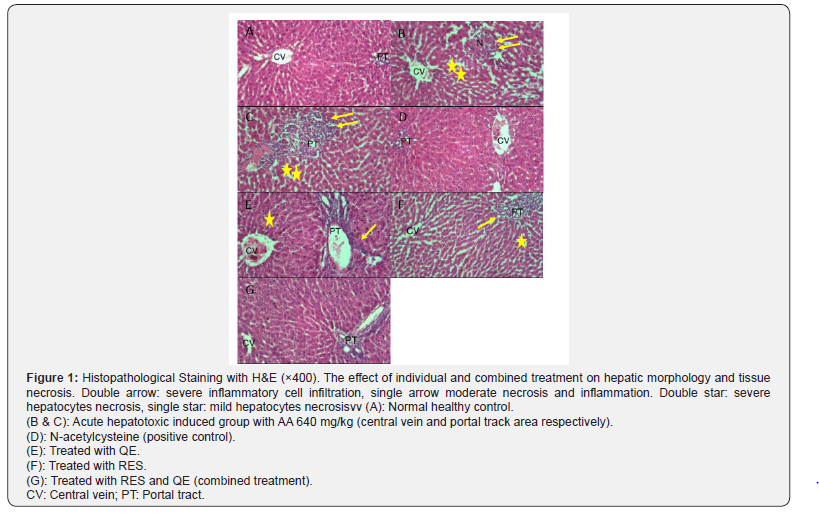
The formalin treated samples were processed using standard method. Briefly, paraffin-embedded tissue samples were cut into 5-mm thickness, after rehydration, slides stained with haematoxylin and eosin (H & E). Liver structure characteristics including; tissue inflammation, hepatocytes necrosis and foam cells was semi-quantitatively evaluated in 10 different fields and compared with control and treated-groups by an experienced pathologist
Total Antioxidant Capacity
The reducing potential of biological fluid was determined by ferric reducing antioxidant potential assay (FRAP) [26]. Briefly, Fe III was reduced to Fe II by reducing potential of the sample. Reaction between Fe II and tripyridyltriazine (Fe II-TPTZ) produce a colored complex with maximum absorbance at 593nm. In this study, total antioxidant capacity (TAC) in serum and liver homogenates were measured.
Lipid Peroxidation Assay
Quantitative measurement of malondialdehyde (MDA), as the end product of lipid peroxidation, represents lipid damage cause by free radicals in the biological samples. The reaction between MDA and thiobarbituric acid produce a colored complex. The concentrations of peroxidized lipids were expressed as nmol/mg protein. 1,1,3,3- tetraethoxypropane was used as standard [27].
Statistical Analysis
All data were analyzed with SPSS 16.0 software (IBM, Armonk, NY, USA). Statistical significances were determined using one-way analysis of variance (ANOVA) following by Tukey post-hoc test. All data were expressed as mean ± standard deviation (SD). P<0.05 was considered significant.
Results
Liver Biomarkers
As shown in Table 1, acute hepatotoxicity significantly led to increase specific liver enzymes compared to the control group. Our results revealed that administration of RES and QE alone, inhibited elevation of serum biochemical parameters as compared with hepatotoxic control group. NAC treatment decreased these markers more than RES and QE. Interestingly, combination treatment decreased specific liver enzymes dramatically in a similar manner to NAC treatment and restored these parameters to the normal level. As shown in Table 2 AA treatments led to serum lipid profile disturbance. RES and QE treatment reduced TG level slightly but didn’t impact on the other parameters. NAC treatment significantly led to decrease in TG and VLDL-C levels but other parameters didn’t change. Interestingly combination treatment decreased TG, VLDL-C, LDL-C more than NAC. Also, combination treatment increased serum content of HDL-C.
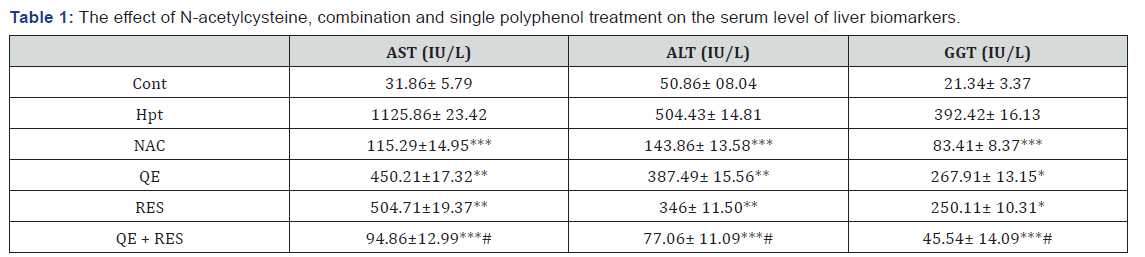
ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; GGT: Gamma-Glutamyltransferase. Cont: control; Hpt: Hepatotoxic; NAC: N-Acetylcysteine, QE: Quercetin; RES: Resveratrol.
Data are expressed as means ± SD. *p<0.05,**p<0.01,***p<0.001 compared to hepatotoxic group, # there was a significant difference between combination and single treatment.
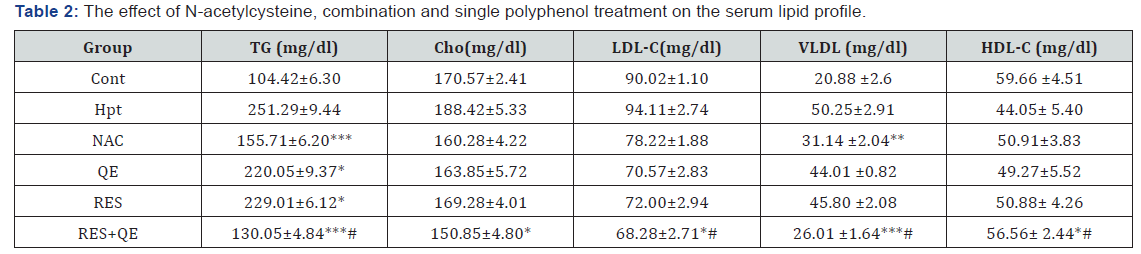
TG: Triglyceride; Chlo: Cholesterol; LDL-C: Low-Density Lipoprotein-Cholesterol; VLDL: Very Low-Density Lipoprotein; HDL-C: High-Density Lipoprotein-Cholesterol; Cont: Control; Hpt: Hepatotoxic; NAC: N-Acetylcysteine; QE: Quercetin: RES: Resveratrol
Data are expressed as means ± SD. *p<0.05, **p<0.01, ***p<0.001 compared to hepatotoxic group, # there was a significant difference between combination and single treatment.
Histopathological Examination
H&E examination was depicted in the Figure 1. As expected the architecture of hepatic lobule and portal tract in the healthy control group were normal, while in the hepatotoxic group the lobular necrosis and portal chronic inflammation are noted. Complete protection from necrosis and inflammation were observed in NAC treatment compared to the hepatotoxic group. Moreover, QE and RES treatment revealed mild portal inflammation without necrosis, that showed partial protection and then in the combination treatment group was demonstrated trivial portal inflammation only, that showed a supportive effect from partial protection more than QE and RES individually.
Total Antioxidant Capacity in Serum and Liver Tissue
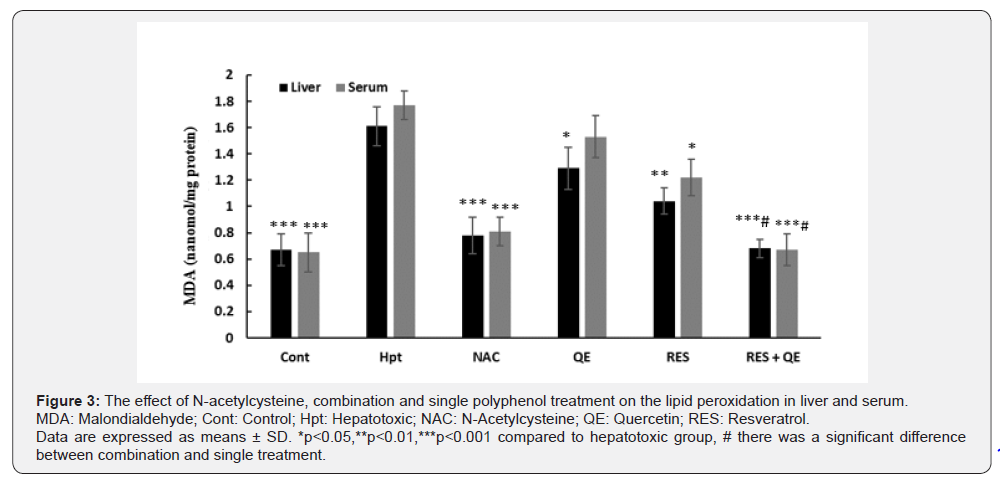
As illustrated in the Figure 2, TAC levels were significantly reduced in serum and tissue following AA treatment in the hepatotoxic group compared to the control group. Administration of RES and QE avoided reduction of TAC in serum and liver samples. The NAC treatment decreased this factor in serum and tissue more than RES and QE treatment. Interestingly co-administration of RES and QE normalized TAC level in serum and liver.

Lipid Peroxidation in Serum and Liver Tissue
Animal treatment by RES inhibited elevation of MDA level cause by AA toxicity partially compared to hepatotoxic group. QE intake led to decrease MDA level just in liver. NAC treatment reduced hepatic and serum level of MDA more significantly than RES and QE. Surprisingly, MDA level in combination treatment was significantly lower than RES and QE groups and showed similar pattern like the NAC (Figure 3).
Discussion
Over the last years, poor pharmacokinetic properties of natural polyphenols limit their usage in clinical interventions. A huge amount of data has been proved the beneficial effect of natural polyphenols when used together [16,20]. Therefore, in the current study, we investigated the hepatoprotective effect of RES and QE against AA-induced hepatotoxicity when administrated together in male Wistar rats.
The overdose of AA in induce experimental hepatotoxicity model and elevate liver enzymes, which in turn represented successful induction of acute hepatic injury. Single treatment partially inhibited elevation of serum level of ALT, AST and GGT. But oral co-administration of RES and QE restored serum levels of liver specific markers near to the normal level similar to NAC. Consistent with earlier reports by Gupta et al, intraperitoneally administration of 100mg/kg QE normalized ALT and AST level in diethylnitrosamine (DEN) induced hepatotoxicity model [15]. Also, Wang et al., showed the high dose of RES 100mg/kg treatment inhibited elevation of ALT and AST level significantly in AA induced liver injury in mice [12].
These results indicated orally co-administration of low dose of RES and QE have showed protective effect similar to intraperitoneally administration of the high dose of RES and QE. In can be concluded co-administration of RES and QE lead to improve their bioavailability through increase gastric absorption cooperatively when administrated orally. The serum level of TG, Cho, VLDL and LDL-C were remarkably elevated during AA hepatotoxicity and HDL-C content was depleted. Compatible with the previous studies RES and QE treatment separately didn’t significant effect on the lipid profile parameters. Heebøll showed that administration of high dose of RES (408 mg/kg) prevent hepatic lipid accumulation partially in non-alcoholic fatty liver disease (NAFLD) mice model [17]. Also, previous attempt showed administration of high dose of QE (100mg/ kg) had the hypolipidemic and hepatoprotective effect against high cholesterol diet induced hepatotoxicity in Swiss albino mice. Likely, low intestinal absorption and rapid metabolism of polyphenol was due to weak its hypolipidemic effect. Increase amount of oral intake and combination therapy could be consider as an alternative approach for this problem. In agreement to our propose lipid profile was significantly improved by combination therapy even though better than NAC treatment.
Histopathological examination validated earlier serum biochemical results. Similar to NAC treatment, combination treatment compensated tissue necrosis and inflammatory cell infiltration near to the normal liver. Also, hepatocyte lipid accumulation was markedly reduced by polyphenol treatment. According to the Arias et al. finding the antilipidemic effect of RES and QE supplement may be due to reducing fatty acid oxidation and suppression of hepatic lipogenesis [21]. Histological observation was in line with serum lipid profile changes, whereas combination treatment had a more constructive effect on the vacuolated hepatocytes compared to the single treatment. Consistent with our results, Arias et al. reported that RES and QE have a synergistic effect against lipid accumulation in adipocytes when they are consumed together [28]. Biochemical and histopathological alteration declared that combination treatment revealed the protective effect of REQ and QE cooperatively when administrated together. The co-administration of RES and QE decreased TAC level better than single treatment. Regarding the previous attempt by Atmaca et al. it could be concluded oral co-administration of RES and QE represented anti-oxidant properties similar to RES treatment intraperitoneal [6]. They showed intraperitoneally injection of RES was accompanied with a profound modulatory effect on the serum and hepatic oxidative stress status in sodium fluoride-induced hepatotoxicity in rats [6].
It seems to increase intestinal absorption of polyphenols when combined to etch other may lead to increase circulation time of RES and QE in blood and improve their protective effect. Compatible to our propose Lund et al. investigated the effect of co-administration of RES, QE and curcumin on their intestinal absorption [16]. They showed that permeability of RES was elevated across epithelial cells about 3.1 folds when combined with QE.
Similar to the TAC results, single treatment approach reduced serum and tissue MDA level slightly. Although Sebai reported interaprituneal RES treatment, reduced hepatic oxidative stress cause by lipopolysaccharide in rat [29]. Also, post-treatment of rats with DEN induced hepatotoxicity during interaprituneal injection of QE at dose of 10 and 30 mg/kg retuned hepatic MDA level. Interestingly combination treatment orally declared similar effect to interaprituneal administration of RES and QE. NAC and combination treatment returned serum and tissue level of MDA content near to the normal level. Mikstacka found similar results. They reported combination treatment suppressed oxidative injury of membrane lipids in human erythrocyte synergistically more than RES and QE treatment alone [20]. This useful finding presents additional evidence to a cooperative effect of RES and QE treatment in attenuation of acute hepatotoxicity. Indeed, combination treatment can be considered as an appropriate alternative treatment for NAC treatment.
Conclusion
Co-administration of RES plus QE showed potential hepatoprotective effects against AA induced toxicity by normalizing lipid profile, liver enzymes and oxidative stress. It can be concluded that combination treatment could serve as an interesting strategy to clinical use of natural polyphenols against liver damage cause by oxidative stress. Further in-depth investigations will be needed to understand the molecular mechanisms of our findings.
Acknowledgement
This work has been supported by a grant from Hamadan University of Medical Sciences (grant number: 9509025023).
Declaration of Interest
The authors declare no conflict of interests






























