- Mini Review
- Abstract
- Introduction
- Materials and Methods
- Results
- Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Temporal Pattern of Antimicrobial Susceptibility of ESKAPE Pathogens
- Herbal Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Correlation between Antimicrobial Activity of Herbal and Conventional Antimicrobial Drugs
- Comparison between Antimicrobial Activities of Different Antimicrobials on ESKAPE Group of Bacteria Isolated from Different Sources
- Discussion
- Acknowledgement
- References
ESKAPE Pathogens in Animals and their Antimicrobial Drug Resistance Pattern
Bhoj R Singh*
Division of Epidemiology, ICAR-Indian Veterinary Research Institute, India
Submission: July 27, 2018; Published: August 24, 2018
*Corresponding author: Bhoj R Singh, Head, Division of Epidemiology, ICAR-Indian Veterinary Research Institute, Izatnagar-243122, Bareilly, UP, India. Phone: +91-8449033222, Email: brs1762@ivri.res.in
How to cite this article: Bhoj R Singh. ESKAPE Pathogens in Animals and their Antimicrobial Drug Resistance Pattern. Dairy and Vet Sci J. 2018; 7(3): 555715. DOI: 10.19080/JDVS.2018.07.555715
- Mini Review
- Abstract
- Introduction
- Materials and Methods
- Results
- Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Temporal Pattern of Antimicrobial Susceptibility of ESKAPE Pathogens
- Herbal Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Correlation between Antimicrobial Activity of Herbal and Conventional Antimicrobial Drugs
- Comparison between Antimicrobial Activities of Different Antimicrobials on ESKAPE Group of Bacteria Isolated from Different Sources
- Discussion
- Acknowledgement
- References
Abstract
The analytical study, of 3240 bacterial isolates from veterinary clinical cases and related sources since 2011 to 2017, aimed to understand the extent of infections in animals associated with ESKAPE group (439) of pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) and to map their antimicrobial sensitivity pattern. All the six ESKAPE pathogens caused infection in animals, the most common being Enterobacter species followed by K. pneumoniae, P. aeruginosa S. aureus, E. faecium and A. baumannii. None of the antibiotics was effective on all the ESKAPE bacterial isolates however, carbapenems (80.4%), tigecycline (76.8%), chloramphenicol (75.8%) and cefepimes (74.6%) inhibited majority of the isolates. More than 76% ESKAPE bacteria had multiple drug resistance (MDR), significantly (p, <=0.05) more common in E. faceium (87.5%) and P. aeruginosa (94.5%) isolates. Metallo-β-lactamase (MBL) and extended spectrum-β-lactamase (ESBL) production was detected in 5% and 54% ESKAPE isolates, respectively. Only colistin, cefepime and gentamicin were effective on >=75% P. aeruginosa isolates but cinnamaldehyde could inhibit almost 98% of P. aeruginosa and >90% isolates of other ESKAPE bacteria (except E. faecium). Cinnamaledehyde, cinnamon oil, carvacrol and ajowan oil were on the most effective herb killing almost 90% of ESKAPE pathogens. Ciprofloxacin (r, -0.12) and colistin (r, -0.14) antibacterial activity had significant (p, <=0.05) negative correlation with that of ajowan oil. All the herbal drugs tested except sandalwood oil (SWO) and Zanthoxylum rhetsa seed carp essential oil (ZREO) were slightly more often active against carbapenem resistant (CR) strains. However, lemongrass oil (LGO) and ajowan oil were significantly (p, 0.01) more often active against CR strains than on non-CR strains. Most of the herbs were less active on ESBL and MBL positive ESKAPE bacteria except ZREO, showing more activity towards MBL and ESBL positive bacteria, indicating a promising area of research to find the active ingredient of ZREO to counter infections by ESBL and MBL strains.
Keywords:ESKAPE; MDR; MBL; ESBL; Herbal antimicrobial drug resistance (HADR); Aztreonam; Nalidixic Acid; Vancomycin; Tetracycline; Piperacillin; Cotrimoxazole; Erythromycin; Citral; Colistin; Pathogens; Epidemiology; Antibiotics; Bacteria
Abbreviations:CO: Cinnamon Oil; BO: Betel leaf Oil; GO: Guggul Oil; HBO: Holy Basil Oil; Ajowan Oil; Sandal Wood Oil; PO: Patchouli essential Oil; LGO: Lemon Grass Oil; HADR: Herbal Antimicrobial Drug Resistance; MBL: Metallo-β-Lactamse; ADRT: Antimicrobial Drug Resistance Traits; VRE: Vancomycin Resistant Enterococci
- Mini Review
- Abstract
- Introduction
- Materials and Methods
- Results
- Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Temporal Pattern of Antimicrobial Susceptibility of ESKAPE Pathogens
- Herbal Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Correlation between Antimicrobial Activity of Herbal and Conventional Antimicrobial Drugs
- Comparison between Antimicrobial Activities of Different Antimicrobials on ESKAPE Group of Bacteria Isolated from Different Sources
- Discussion
- Acknowledgement
- References
Introduction
The ESKAPE group of pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) is the leading cause of nosocomial infections in humans throughout the world [1,2]. The ESKAPE bacteria are among the most common causes of life-threatening nosocomial infections amongst critically ill and immunocompromised individuals with characteristic potential drug resistance [3]. The ESKAPE group of bacteria have similar kind of drug resistance mechanism as possessed by many of the non-ESKAPE bacteria [2,4]. There is hardly any antimicrobial drug which may be effective universally on all ESKAPEs because of wide spectrum of antimicrobial drug resistance traits (ADRT). The ADRT is the outcome of evolution spanning billions of years and over the course, bacteria have developed mechanisms to avert, expel, negate, destroy or withstand compounds structurally like the antibiotics in clinical use [5]. Lot of research is going on ESKAPE bacteria in medical sciences, but little is understood about their occurrence in animals and its relevance to health of livestock owners and veterinarians [6]. In the present study the data available in clinical epidemiology laboratory of Division of Epidemiology, Indian Veterinary Research Institute for antimicrobial sensitivity assays of bacteria associated with infections in animals has been analysed to understand the drug resistance pattern of ESKAPE pathogens occurring in animals.
- Mini Review
- Abstract
- Introduction
- Materials and Methods
- Results
- Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Temporal Pattern of Antimicrobial Susceptibility of ESKAPE Pathogens
- Herbal Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Correlation between Antimicrobial Activity of Herbal and Conventional Antimicrobial Drugs
- Comparison between Antimicrobial Activities of Different Antimicrobials on ESKAPE Group of Bacteria Isolated from Different Sources
- Discussion
- Acknowledgement
- References
Materials and Methods
From the antibiogram database of bacteria (3240) isolated from referred veterinary clinical cases (2011-2017) available in clinical epidemiology laboratory of Division of Epidemiology, Indian Veterinary Research Institute, Izatnagar data for ESKAPE group of bacteria was extracted and analysed in Microsoft Excel-2010. The zone of growth inhibition (ZI) data was available against discs containing one μL of agarwood oil (AWO), ajowan oil (AO), betel leaf oil (BO), carvacrol (Cr), cinnamon oil (CO), cinnamaldehyde (CIN), citral (CTR), guggul oil (GO), holy basil oil (HBO), lemongrass oil (LGO), patchouli essential oil (PO), sandalwood oil (SWO), thyme essential oil (TO) and Zanthoxylum rhetsa seed carp essential oil (ZREO). Any zone of growth inhibition around a disc containing the abovementioned herbal compound was counted as susceptible [7]. The ZI data for antibiotics discs purchased from Difco-BBL including ampicillin (10μg), aztreonam (30μg), carbapenems (imipenem/ meropenem/ ertapenem 10μg), cefepime (30μg), ceftriaxone (10μg), chloramphenicol (25μg), ciprofloxacin (10μg), colistin (10μg), cotrimoxazole (25μg), erythromycin (15μg), gentamicin (30μg), nalidixic acid (30μg), nitrofurantoin (300μg), piperacillin (100μg), tetracycline (30μg), tigecycline (15μg), vancomycin (30μg), and E-test (BioMerieux, India) results for ESBL and MBL was also retrieved from the data base for analysis. To determine sensitivity CLSI [8] criteria was followed where so ever applicable. For the selected 439 bacterial isolates, sensitivity pattern for herbal antimicrobials and conventional antimicrobial drugs was included for the analytical purposes to determine carbapenem resistance, ESBL and MBL production. The bacteria resistant to one or more carbapenems (meropenem 10μg, imipenem 10μg, ertapenem 10μg) were classified as carbapenem resistant (CR).
The data for 439 ESKAPE strains was analysed for finding association between different parameters of antimicrobial drug sensitivity in different types of bacteria, isolated from different disease conditions and in different years using chi-square/ Fisher’s exact tests and odds ratio.
- Mini Review
- Abstract
- Introduction
- Materials and Methods
- Results
- Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Temporal Pattern of Antimicrobial Susceptibility of ESKAPE Pathogens
- Herbal Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Correlation between Antimicrobial Activity of Herbal and Conventional Antimicrobial Drugs
- Comparison between Antimicrobial Activities of Different Antimicrobials on ESKAPE Group of Bacteria Isolated from Different Sources
- Discussion
- Acknowledgement
- References
Results
From the antimicrobial sensitivity database at clinical epidemiology laboratory of Division of Epidemiology, Indian Veterinary Research Institute for 3240 bacteria isolated from veterinary clinical cases and related sources since 2011- 2017, a total of 439 bacteria (13.5%) were classified into ESKAPE group (Table 1). All 6 pathogens of ESKAPE category were isolated from animals in association with one or other disease conditions. Of those, most of the isolates belonged to Enterobacter species (44.4%) followed by Klebsiella pneumoniae (22.1%), Pseudomonas aeruginosa (16.6%), Staphylococcus aureus (12.98%), Enterococcus faecium (3.6%) and Acinetobacter boumannii (0.23%). The most common source of ESKAPE bacteria was samples of abscess and wound infections in animals (18.7%), followed by cases of abortion and infertility (17.5%), healthy animals (17.1%), animals died of infection (15%), otopharyngeal infections (5.2%), nasal swabs (3.9%), mastitis (2.5%), conjunctivitis (2.5%), genital tract infections (2.3%), and urinary tract infections (1.4%). ESKAPE pathogens (S. aureus) could also be detected in two injectables for animal use.

*Enterobacter aerogenes 11, E. agglomerans 171, E. amnigenus 6, E. gregoviae 6, E. nimipressuralis 1; # from two FMD Vaccine vials.
- Mini Review
- Abstract
- Introduction
- Materials and Methods
- Results
- Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Temporal Pattern of Antimicrobial Susceptibility of ESKAPE Pathogens
- Herbal Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Correlation between Antimicrobial Activity of Herbal and Conventional Antimicrobial Drugs
- Comparison between Antimicrobial Activities of Different Antimicrobials on ESKAPE Group of Bacteria Isolated from Different Sources
- Discussion
- Acknowledgement
- References
Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
Resistance to conventional antimicrobial drugs varied for different antibiotics, with respect to source of bacteria (Table 2), year of isolation (Figure 1) and type of infection caused. Resistance in isolates of ESKAPE bacteria was the commonest (>80%) for ampicillin and was the least for carbapenems (~20% isolates). The ESKAPE group of bacteria (Table 3) often detected with potential to produce ESBL (53.99%), MBL (5.01%) and had carbapenem resistance (19.59%). Pseudomonas aeruginosa and E. faecium isolates were commonly resistant to carbapenems but metallo-β-lactamase type carbapenem resistance was detected in P. aeruginosa only and not in E. faecium isolates (Figure 2). Besides, K. pneumoniae and Enterobacter species isolates also had MBL activity.

ESBL, extended spectrum β-lactmase; MBL, metallo-β-lactmase

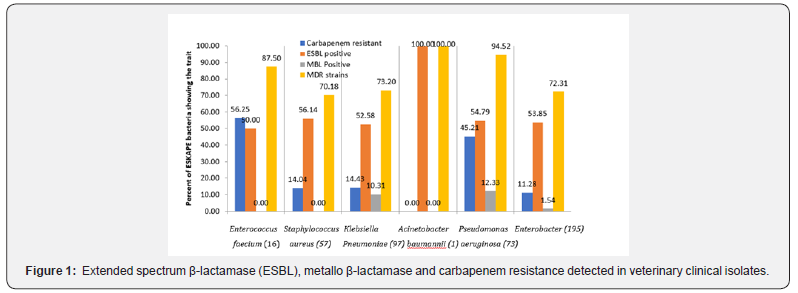
Multiple drug resistance (MDR) was common (76.5%) among ESKAPE bacteria under study. However, isolates of P. aueruginosa (94.5%) and E. faecium (87.5%) were significantly (p < 0.01) more often MDR type than other bacteria. Antimicrobial activity of different antimicrobials on ESKAPE pathogens under study (Table 3) revealed that aztreonam was almost ineffective on Gram positive bacteria (GPBs). Among GPBs, E. faecium were more often (p, < 0.05) resistant to most of the antimicrobials (except ampicillin and tigecycline) than S. aureus strains. Resistance to most of the antibiotics was common among P. aeruginosa, followed by K. pneumoniae, and Enterobacter isolates were more often sensitive. However, colistin was more often (p, < 0.01) effective on P. aeruginosa (90.5%) than either on Klebsiella (67.2%) or on Enterobacter (71.5%) isolates.
- Mini Review
- Abstract
- Introduction
- Materials and Methods
- Results
- Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Temporal Pattern of Antimicrobial Susceptibility of ESKAPE Pathogens
- Herbal Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Correlation between Antimicrobial Activity of Herbal and Conventional Antimicrobial Drugs
- Comparison between Antimicrobial Activities of Different Antimicrobials on ESKAPE Group of Bacteria Isolated from Different Sources
- Discussion
- Acknowledgement
- References
Temporal Pattern of Antimicrobial Susceptibility of ESKAPE Pathogens
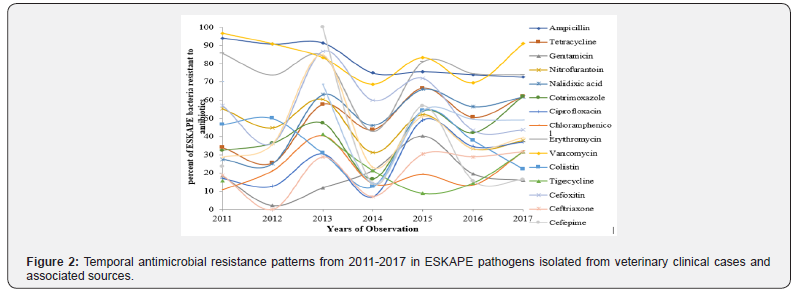
Temporal pattern of antimicrobial resistance among ESKAPE pathogens from animals (Figure 2) revealed significant (p, <=0.02) increase in tetracycline resistance after 2012 (25.5%) with several ups and downs. Similar undulating patterns were also evident for other antimicrobials. Carbapenem resistance (CR) and metallo-β-lactamse (MBL) mediated resistance trends were in inverted U shape, minimum number of CR isolates in 2013 with shallow plateaus at either of the sides of time line with significant heights (p, <=0.02) in 2011-2012, and 2016- 2017. While extended spectrum β-lactamase (ESBL) positivity was highest in ESKAPEs in the year 2014 (75%) after a steady increase and then shown downward trend for two years and again a significant (p, 0.03) increase in share of resistant ESKAPEs in 2017.
- Mini Review
- Abstract
- Introduction
- Materials and Methods
- Results
- Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Temporal Pattern of Antimicrobial Susceptibility of ESKAPE Pathogens
- Herbal Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Correlation between Antimicrobial Activity of Herbal and Conventional Antimicrobial Drugs
- Comparison between Antimicrobial Activities of Different Antimicrobials on ESKAPE Group of Bacteria Isolated from Different Sources
- Discussion
- Acknowledgement
- References
Herbal Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
Like conventional antimicrobial drug resistance, resistance to herbal antimicrobials also varied among isolates of ESKAPE bacteria from different sources (Table 4). Ajowan oil, thyme oil and carvacrol were equally active on all types of ESKAPEs but significantly less active against P. aeruginosa (p, <=0.01). The resistance pattern of ESKAPE pathogens to different herbal antimicrobials tested (Table 5) revealed that Klebsiella pneumoniae and Enterobacter spp. isolates were equally susceptible to betel leaf oil but the oil was significantly (p, <=0.01) less active against S. aureus and P. aeruginosa isolates. About 90% of the ESKAPEs isolated from different sources were resistant to guggul oil and agarwood oil but S. aureus was significantly (p, <=0.01) more often sensitive than isolates of other ESKAPEs. About 85% of the ESKAPE isolates were resistant to patchouli oil but S. aureus isolates (32.7%) were more often sensitive (p, <=0.01) to patchouli oil than other ESKAPEs.
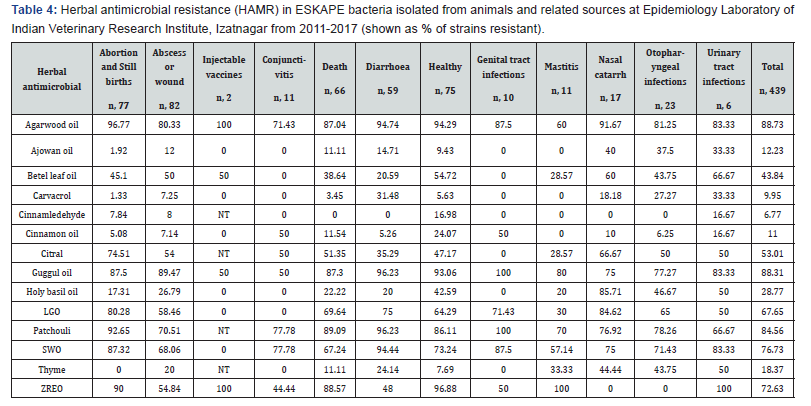
NT, not tested; LGO, lemongrass oil; SWO, sandalwood oil; ZREO, Zanthoxylum rhetsa fruit carp essential oil.
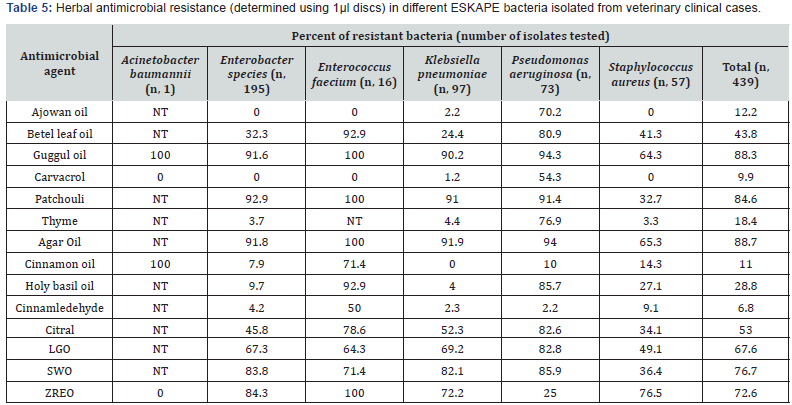
Though cinnamon oil was inactive against almost 11% of ESKAPEs, K. pneumoniae were invariably sensitive to the oil. Similarly, cinnamaldehyde inhibited >93% ESKAPEs and P. aeuginosa and K. pneumoniae were significantly (p <=0.01) more often sensitive to it than other pathogens. Holy basil oil (HBO) was very active antimicrobial against K. pneumoniae (96%) and Enterobacter species (>90%) but significantly (p, <=0.01) a smaller number of E. faecium (< 7%) and P. aeruginosa (< 15%) isolates were sensitive to HBO.
Citral and lemongrass oil (LGO) had similar pattern of antimicrobial activity but activity was more potent in citral than in LGO inhibiting 67.7% and 53% ESKAPEs, respectively. Pseudomonas aeruginosa and K. pneumoniae strains were more often resistant to LGO and citral than isolates of other pathogens (p, <=0.01). Zanthoxyllum rhetsa seed carp essential oil (ZREO) failed to inhibit >72 ESKAPE strains but was significantly (p, <=0.02) better antimicrobial (75%) on P. aeruginosa strains.
Resistance in ESKAPE bacteria isolated in year 2017 to ajowan oil was higher than those isolated in year 2015 (p, 0.005) and 2016 (p, 0.0004). Similarly, resistance in ESKAPE bacteria isolated in year 2017 to betel-leaf oil was higher than in year 2015 (p, 0.008) and slightly more than those isolated in 2016 (p, 0.09). After 2012, resistance to guggul oil and agarwood oil decreased significantly (p, <=0.05), while resistance to cinnamon oil was more commonly observed in 2013 thereafter it ran on down path (p, <=0.01). Similarly, resistance to carvacrol among ESKAPEs was recorded maximum in year 2013 followed by 2017, and was significantly (p, <=0.05) higher than among isolated in other years. Resistance to patchouli oil, LGO, SWO and citral shown a significant (p, <=0.05) reduction after 2012 but resistance increased significantly (p, 0.006) again in year 2017. Resistance to HBO was significantly more common in ESKAPEs in year 2015 than in those isolated in 2011 (p, 0.02) and 2016 (p, 0.04). In 2017, cinnamaldehyde resistance was significantly lower (p, <=0.01) than in year 2015 and 2016. Resistance to ZERO was maximum in 2013 and significantly higher than ESKAPES isolated in other years (p, <=0.05). Resistance to ajowan oil in ESKAPE bacteria isolated in year 2017 was higher than in year 2015 (p, 0.005) and 2016 (p, 0.0004).
- Mini Review
- Abstract
- Introduction
- Materials and Methods
- Results
- Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Temporal Pattern of Antimicrobial Susceptibility of ESKAPE Pathogens
- Herbal Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Correlation between Antimicrobial Activity of Herbal and Conventional Antimicrobial Drugs
- Comparison between Antimicrobial Activities of Different Antimicrobials on ESKAPE Group of Bacteria Isolated from Different Sources
- Discussion
- Acknowledgement
- References
Correlation between Antimicrobial Activity of Herbal and Conventional Antimicrobial Drugs
Correlation among antibacterial activity indicated by diameter of ZI by different herbal (Table 6) and conventional antimicrobials (Table 7) often revealed good correlation in antibacterial activity of of herbal and conventional antimicrobials. All of the herbal drugs tested except SWO and ZREO were often more active against CR strains but activity of ajovan oil and LGO was significantly (p, 0.01) better on CR strains than on non-CR strains of ESKAPE pathogens. All the herbs except ZREO were effective against ESBL and MBL producing strains than non ESBL and non-MBL producing isolates of ESKAPES like most of the conventional antibiotics. However, activity of ZERO on ESBL (p, 0.01) and MBL (p, 0.05) isolates of ESKAPE pathogens was significantly higher than on non-ESBL and on MBL isolates of ESKAPES. Most of the antibiotics tested except colistin were less active (p, <=0.05) on ESBL and MBL producing isolates than on non-ESBL and on MBL isolates of ESKAPE bacteria.

The positive correlations were common, and the drugs not mentioned either in negative or in no correlation columns had statistically significant (p, ≤0.05) positive correlation.

The positive correlations were common, and the drugs not mentioned either in negative or in no correlation columns had statistically significant (p, ≤0.05) positive correlation.
All ZIs by herbal drugs were positively (p, <=0.01) correlated (r, 0.12- 0.69) with each other (Table 4) except of ZREO. The ZIs induced by ZREO had negative correlation (p, 0.05) with ZI by carvacrol (r, -0.18), thyme (r, -0.12), cinnamon oil (r, -0.13) and LGO (r, -0.19) indicating a probability of different mechanism of action or some novel kind of active ingredient possibly having some similarity with one present in ajowan oil (r, 0.15), betel leaf oil (r, 0.16), guggul oil (r, 0.16), patchouli oil (r, 0.27) and agarwood oil (r, 0.32) with significant positive (p, <=0.01) correlation.
The ZI by ciprofloxacin and cinnamon had significant (p, 0.01) positive correlation (r, 0.19) but significantly (p, <=0.05) negative correlation with ZIs by ajowan oil (r, -0.12), patchouli oil (r, -0.15) and agarwood oil (r, -0.15). On the other hand, ZI by ciprofloxacin had positive (p, <=0.05) correlation with ZI induced by most of the antibiotics except a negative correlation (r, -0.23) with ZIs by vancomycin (p, 0.01). Besides ciprofloxacin, ZI by colistin also had negative (p, 0.05) correlation with ZI induced by ajowan oil (r, -0.14). The correlation was also negative (r, -0.12) among ZI induced by vancomycin and cinnamaldehyde (p, 0.05). Aztreonam (active on G-ve bacteria) was the antibiotic having significant (p, <=0.01) negative correlation with ZIs by guggul oil (r, -0.15), patchouli oil (r, -0.28), agarwood oil (r, -0.28), SWO (r, -0.19) and ZREO (r, -0.23) similar to that with ZI by erythromycin (r, -0.28) and vancomycin (r, -0.46), the drugs more active on active on Gram positive bacteria.
- Mini Review
- Abstract
- Introduction
- Materials and Methods
- Results
- Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Temporal Pattern of Antimicrobial Susceptibility of ESKAPE Pathogens
- Herbal Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Correlation between Antimicrobial Activity of Herbal and Conventional Antimicrobial Drugs
- Comparison between Antimicrobial Activities of Different Antimicrobials on ESKAPE Group of Bacteria Isolated from Different Sources
- Discussion
- Acknowledgement
- References
Comparison between Antimicrobial Activities of Different Antimicrobials on ESKAPE Group of Bacteria Isolated from Different Sources
Results of comparison among ESKAPE group of bacteria isolated from 11 different sources revealed that bacteria associated with abortions were more often (p, <= 0.05) sensitive to tetracycline, nalidixic acid, cotrimoxazole, ciprofloxacin, chloramphenicol and ceftriaxone than bacteria isolated from many other sources. On the other hand, bacteria associated with gastrointestinal tract infections causing diarrhoea were more often (p, <= 0.05) resistant to ampicillin, cotrimoxazole, chloramphenicol, erythromycin, vancomycin, and tigecycline than those isolated from other disease conditions. Bacteria isolated from urinary tract infection cases were more (p, <= 0.05) often resistant to cotrimoxazole and ciprofloxacin than bacteria isolated in association with many other ailments. However, bacteria isolated from apparently healthy animals were more often (p, <= 0.05) sensitive to tetracycline, nalidixic acid, chloramphenicol, and tigecycline than those associated with otopharyngeal infections. Like antibiotics, ESKAPE bacteria associated with abortions were also more often (p, <= 0.05) sensitive to ajowan oil, carvacrol, and thyme oil than those associated with other ailments. The ESKAPE bacteria causing diarrhoea were more often resistant to guggul oil, patchouli oil, carvacrol and SWO than those isolated from cases of many of other illness.
Bacteria isolated from cases of diarrhoea and otopharyngeal infections in animals were more often (p≤ 0.05) resistant to carbapenem drugs than those associated with abortions, deaths and abscess or wound infections. However, bacteria associated with genital tract infections were more commonly (p≤ 0.05) sensitive to β-lactam antibiotics, rarely producing ESBL than bacteria from other sources. Bacteria associated with death, diarrhoea and otopharyngeal infections more commonly had (p≤ 0.02) multiple drug resistance (MDR) than bacteria associated with other ailments specifically than those caused abortions, abscess/ wounds, conjunctivitis, genital tract infections and those isolated from healthy animals.
- Mini Review
- Abstract
- Introduction
- Materials and Methods
- Results
- Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Temporal Pattern of Antimicrobial Susceptibility of ESKAPE Pathogens
- Herbal Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Correlation between Antimicrobial Activity of Herbal and Conventional Antimicrobial Drugs
- Comparison between Antimicrobial Activities of Different Antimicrobials on ESKAPE Group of Bacteria Isolated from Different Sources
- Discussion
- Acknowledgement
- References
Discussion
In the present analysis ESKAPE pathogens in animals were commonly detected either as a cause of infections or associated with animals almost in 13.5% cases in Bareilly area. Though ESKAPE bacteria are more often reported as cause of nosocomial infections, in the present study all the ESKAPE bacteria were associated with non-nosocomial infections indicating their common occurrence in animals. Further, nosocomial infections are not given a due importance in veterinary clinical cases but post-surgery infections with highly drug resistant bacteria were not uncommon [9-12]. In medical practice 5-10% of human patients may acquire infection in hospital. In US, CDC estimated that ESKAPE pathogens cause over 2 million illnesses and approximately 23,000 deaths per year [13]. In the study majority of ESKAPE bacteria had MDR (76.5%), produced ESBL (53.9%) and almost every fifth isolate was also resistant to one or more carbapenems. The ESKAPE pathogens are known for their drug resistance; and transfer of antimicrobial drug resistance from companion and domestic animals to humans is a well-documented fact [14]. Therefore, detection of ESKAPE pathogens in sizable number of cases in animals indicated the need for systematic studies on ESKAPE pathogens in veterinary practice.
Detection of multiple drug resistant enterococci from animal sources is not uncommon, even the vancomycin resistant enterococci (VRE) are of common occurrence [15]. However, isolation of E. faecium has rarely been reported from animals. None of 16 E. faecium isolates was resistant to vancomycin but 8 isolates from pigs had intermediated resistance to vancomycin. Isolation of E. faecium from apparently healthy animals in India is of more significance due to sharing of house by human and their domestic animals. Staphylococcus aureus, is an important pathogen of animals associated with mastitis, wound infections and abscesses [16]. In the study S. aureus was one of the most common ESKAPE bacteria isolated from animals suffering from mastitis, wounds, abscesses and otorrhoea cases. Its importance further increased due to communicability of the infection to humans [17]. Though vancomycin resistance was not observed, isolates from 29 cases were intermediate resistant to the drug and from 27 cases has methicillin and ampicillin resistance.
The undulating but upward trends of antimicrobial drug resistance in most of the ESKAPEs may not be easily explained, it might be outcome of variation in pattern of clinical cases referred to the Laboratory depending on varying trends of different diseases in animals in Bareilly (Indian Veterinary Research Institute, Annual reports 2011-2017) or the changing pattern of antimicrobial drug use [18]. Specificity of some drugs against specific group of pathogens observed in the analysis as aztreonam resistance in GPBs, and erythromycin and vancomycin resistance in GNBs is a well-documented phenomenon due to specific targets of the drugs present in specified group of bacteria [19]. Colistin was the most effective antibiotic to inhibit P. aeruginosa active against >90% isolates, besides it acted best on P. aeruginosa than any of the other ESKAPE bacteria. Though Colistin is known to be effective on other ESKAPE bacteria [19], its clinical utility is limited due to limited systematic use owing to its toxicity.
Herbal antimicrobials are known for their wide spectrum activity on drug resistant bacteria [20] and some of the screened antimicrobials as cinnamaledehyde, cinnamon oil, ajowan oil and carvacrol shown promising activity against most of the ESKAPE bacteria. However, clinical utility of herbal antimicrobials to combat infections with ESKAPE bacteria is still a farfetched goal either as solo-therapy or in combination with antibiotics [21]. Besides, emergence of herbal drug resistance may be another threat for their use [22-24]. However, the study revealed that like colistin, an effective antibiotic on P. aeruginosa, some of the herbal antimicrobials (cinnamldehyde from cinnamon oil and carvacrol from thyme and ajowan oil) may be used topically for infections with ESKAPE bacteria and needs evaluation. Carvacrol, a wide spectrum herbal compound, was very effective in control of most of the ESKAPE bacteria in the study; it was quite ineffective on P. aeruginosa isolates. It might be due to ability of P. aeruginosa efflux pump to throw out the antimicrobial. In a recent study [25] two efflux pump genes detected in P. aeruginosa have been reported modulating carvacrol resistance. In future, with more research on antimicrobial herbal drug resistance (AHDR) mechanism more may be understood about the spread of AHDR in ESKAPE and other bacteria.
The analysis of antimicrobial drug resistance pattern of ESKAPE bacteria isolated from animals revealed that infections with ESKAPE bacteria are common in animals and majority of the isolates had multiple drug resistance and sizeable number were positive for ESBL and MBL production. Therefore, continuous and elaborately planned studies are required to understand the role of domestic and pet animals in circulation of ESKAPE bacteria. Besides, the study indicated that there are potentially good antibiotics and herbal antimicrobials which may be used to treat ESKAPE bacterial infections using the proper clinical microbiology techniques to select the best drug to be used.
- Mini Review
- Abstract
- Introduction
- Materials and Methods
- Results
- Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Temporal Pattern of Antimicrobial Susceptibility of ESKAPE Pathogens
- Herbal Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Correlation between Antimicrobial Activity of Herbal and Conventional Antimicrobial Drugs
- Comparison between Antimicrobial Activities of Different Antimicrobials on ESKAPE Group of Bacteria Isolated from Different Sources
- Discussion
- Acknowledgement
- References
Acknowledgement
Author thankfully acknowledge the permission and financial assistance granted by the Director and Joint Director (R) of Indian Veterinary Research Institute, Izatnagar
- Mini Review
- Abstract
- Introduction
- Materials and Methods
- Results
- Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Temporal Pattern of Antimicrobial Susceptibility of ESKAPE Pathogens
- Herbal Antimicrobial Drug Resistance in ESKAPE Bacteria from Animals
- Correlation between Antimicrobial Activity of Herbal and Conventional Antimicrobial Drugs
- Comparison between Antimicrobial Activities of Different Antimicrobials on ESKAPE Group of Bacteria Isolated from Different Sources
- Discussion
- Acknowledgement
- References
References
- Navidinia M (2016) The Clinical importance of emerging ESKAPE pathogens in nosocomial infections. J Paramed Sci 7: 43-47.
- Santajit S, Indrawattana N (2016) Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res Int. 2016: 2475067
- Rice LB (2010) Progress and challenges in implementing the research on ESKAPE pathogens. Infect Ctrl Hosp Epidemiol 31(1): S7–S10.
- Kumar S, Singh BR (2013) an overview of mechanisms and emergence of antimicrobials drug resistance. Adv Anim Vet Sci 1(2S): 7-14.
- Pendleton JN, Gorman SP, Gilmore BF (2013) Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther 11(3): 297-308.
- Singh BR (2017) Do ESKAPE group of pathogens isolated from sick and apparently healthy animals are as important as those from humans? Research Gate.
- Singh BR (2013) Antimicrobial sensitivity assay and antimicrobial chemotherapy in animals: A practical approach. In: Singh BR, Somvanshi R (Eds.), Diseases of Animals: Diagnosis and Management, Izatnagar: Indian Veterinary Research Institute, Izatnagar, Bareilly, India p. 7-31.
- Clinical and Laboratory Standards Institute (2014) Performance standards for antimicrobial disk susceptibility tests. 24th Informational Supplement M100-S24 and M11-A8, Clinical and Laboratory Standards Institute, Wayne, Pennsylvania, PA, USA.
- Morley PS (2004) Surveillance for Nosocomial Infections in Veterinary Hospitals. Vet Clin North Am Equine Pract 20: 561-576.
- Campbell MT (2010) Nosocomial infections. CVC in San Diego proceedings, USA.
- Weese JS (2010) Nosocomial Infections are Not Just for Referral Hospitals. World Small Animal Veterinary Association World Congress Proceedings, Guelph, ON, Canada.
- Binns S, Coyne K, Weese JS (2017) Hospital-Associated Infections.
- Johnson JA (2002) Nosocomial infections. Veterinary Clinics: Small Animal Practice 32: 1101-1126.
- Pomba C, Rantala M, Greko C, Baptiste KE, Catry B, et al. (2017) Public health risk of antimicrobial resistance transfer from companion animals. J Antimicrob Chemother 72(4): 957-968.
- Singh BR (2009) Prevalence of vancomycin resistance and multiple drug resistance in enterococci in equids in North India. J Infect Developing Ctri 3(7): 498-503.
- Peton V, Le Loir Y (2014)Staphylococcus aureus in veterinary medicine. Infect Genet Evol 21: 602-615.
- Vincze S (2015) Staphylococcus aureus in Companion Animals: An Infection Source for the Community? Ph.D. Dissertation, Freie Universität Berlin, Germany
- Singh BR (2010) Antimicrobial drug uses by veterinarians in equine clinical cases in India. Res J Vet Sci 3: 165-178.
- Davey P, Wilcox MH, Irving W, Thwaites G (2015) Antimicrobial chemotherapy. (7th Edn), Oxford University Press, Oxford, UK.
- Buhner SH (2012) Herbal antibiotics: Natural alternatives for treating drug-resistant bacteria. (2nd Edn), Storey Publishing, LLC, USA.
- Bhardwaj M, Singh BR, Sinha DK, Vadhana P, Vinodhkumar OR, et al. (2016) Potential of herbal drug and antibiotic combination therapy: A new approach to treat multidrug resistant bacteria. Pharmaceutica Analytica Acta 7(11): 1-4.
- Singh BR (2016) Antimicrobial and herbal drug resistance pattern of important pathogens of animal health importance at Bareilly. 16th Indian Veterinary Congress Bhubaneswar, India, p. 71-79.
- Vadhana P, Singh BR, Bhardwaj M, Singh SV (2015) Emergence of herbal antimicrobial drug resistance in clinical bacterial isolates. Pharm Anal Acta 6: 434.
- Singh BR, Singh V, Ebibeni N, Singh RK (2013) Antimicrobial and herbal drug resistance in enteric bacteria isolated from faecal droppings of common house lizard/gecko (Hemidactylus frenatus). Int J Microbiol p. 8.
- Vadhana P (2017) Molecular Studies on Resistance against Carvacrol in Escherichia coli / Pseudomonas aeruginosa. Ph.D. Thesis, Indian Veterinary Research Institute, Izatnagar, India.






























