Bovine Tuberculosis Slaughter Surveillance in Albania, Importance of Its Traceback Investigation Based on Singel Cervical Comparative Skin Test
Xhelil Koleci* and Anita Koni
Public Health Department, Faculty of Veterinary, Agricultural University of Tirana, Europe
Submission: June 14, 2018; Published: August 15, 2018
*Corresponding author: Xhelil Koleci, Public Health Department, Faculty of Veterinary, Agricultural University of Tirana, Europe; Email: xhelil.koleci@ubt.edu.al
How to cite this article: Xhelil Koleci, Anita Koni. Bovine Tuberculosis Slaughter Surveillance in Albania, Importance of Its Traceback Investigation Based on Singel Cervical Comparative Skin Test. Dairy and Vet Sci J. 2018; 7(2): 555710. DOI: 10.19080/JDVS.2018.07.555710
Abstract
Bovine tuberculosis is a bacterial contagious disease due by Mycobacterium bovis that primary affects cattle and widest range of mammals. Mycobacterium bovis and Mycobacterium caprae are responsible for zoonotic tuberculosis. In Albania, bovine tuberculosis is endemic, however information on the spread of disease is not available and sustainable control measures are not fully enforced. In this study, we present data from a pilot survey for bovine TB after receiving an unofficial information of generalized bovine TB in a slaughtered calf in Dibra region. In total, there were tested 277 cattle by comparative tuberculin skin test and prevalence of bovine tuberculosis was 1.1% at individual level and 1.4% at farm level. There were identify a variety of risk factors that facilitate spreading bovine tuberculosis in animals and interfere with its control programs, such as lack of correct animal identification, animal movement control, age structure etc. Active surveillance in the abattoirs and traced back to their farm of origin and tested all in-contact animals with simultaneous comparative cervical skin test is a rational approach as a first step for control of bovine tuberculosis disease. An active surveillance in large commercial farms must be initiate and when the necessary infrastructure will be available, a national active surveillance must be implemented. In addition to field tests, gross examination during meat inspection, histopathological, microbiological and molecular methods must be employed systematically for bTB diagnosis and epidemiological study. More efforts must be pay in collaboration with human health in the framework of “One Health” philosophy, which will provide the full background of zoonotic tuberculosis in human and animals.
Keywords:Mycobacterium Bovis; Bovine tuberculosis; Bovine PPD; Avian PPD; Mammals; One Health; Mineralisation; Necrosis; Giant cells; Macrophages; Lymphocytes; Hematoxilin-Eosin; Zoonotic potential
Abbrevations: M. bovis: Mycobacterium Bovis; bTB: Bovine tuberculosis; HE: Hematoxilin-Eosin; SICCT: Single Intradermal Comparative Cervical Tuberculin Test
Introduction
Bovine tuberculosis (bTB) due by Mycobacterium bovis (M. bovis) is a zoonotic disease, spread worldwide, affects the widest range of mammals, including humans [1-3]. Cattle are target animals for M. bovis. Infected cattle serve as main sources of infection, and in different parts of the world there are identify a certain reservoir of infection [1,4]. M. bovis is slow growing bacteria, able to survive in the environment conditions, which interfere with pathogen isolation and in other hand contaminated environment, including manure may serve as source of infection [5].
M. bovis transmission occur in direct and indirect routes, and it is present in aerosol of infected animals, sputum, excretions, secretions and tissues. The inhalation is most common and efficient method of infection, where the infected dose ranges from 1-10 bacteria [5,6]. Ingestion is another important method for transmission from infected animal to susceptible animal, but the infected doses is very high compare to the inhalation route. The tubercular lesions could be localised in any tissues or organs, but in general there are known two main forms: pulmonary and extrapulmonary forms. In pulmonary form, which is typically for relatively old animals infected by inhalation, while the extrapulmonary form is related with digestive route of infection and mesenterial lymph nodes often are involve. Despite the above mention forms, in advanced stages of active bTB, generalised form is likely to occur. Number of mycobacteria have a positive correlation with developing of granulomatous lesions and they are in a large number in lymph nodes, lungs, serous membranes, and M. bovis is present in aerosol, faeces, milk, urine etc. Transmission of disease between herds is most likely to occur by introducing infected animals to the free herds, while contaminated vehicles, visitors etc play role in speeding of bTB to the new herds. Applying strict animal movement control and biosecurity measures play important role in reducing the risk of transmission of bTB. Appling the quarantine measures at national and regional level and isolation procedures at farm levels are extremely important for limiting spreading of bTB.
The importance of bTB for animal health and zoonotic potential enforced program control application, which date back since early 1900. Two main factors play significant positive impact on bTB control: milk pasteurisation and identifying of infected animal by using tuberculin skin test. There is estimated that M. bovis is responsible for up to 10% of all human cases [6]. Any control program aims to eliminate bTB, either at regional and/or local level is based on systematic programs of tuberculin skin test, stamping out of positive cattle; active surveillance at abattoirs; strict animal movement control; frequently testing of infected herds; depopulation of wild reservoir animals, heat treatment and milk pasteurisation, farmer training, providing education program etc [7]. In Albania, the first bTB cases were recorded during 1935-1940, but the official reports and control program of bTB started in 1960 [8]. In 1959, the prevalence of bTB was 2.6% at animal level. In 1964, the serious outbreaks occurred in several districts. Interestingly, in 1977 and subsequent years, bTB cases have been reported after ten years in areas that were officially bTB free. The control program was evaluating as effective and the incidence of bovine tuberculosis from 1973 to 1987, decreased 16 to 24 times. The tuberculin test involved almost 50% of the cattle population. In 1988, bTB prevalence at national level was 0.116% [2]. In the 1990, the socio-economic changes have had an impact on farm organisation and veterinary services. Animals were tested on incidental basis and no information was available on disease prevalence. Bovine and avian tuberculin produced at ISUV was used for the field diagnosis. No quality assurance testing was performed during production process. The meat inspection was not done in all cases as many animals are not slaughter under control. In last years, number of tested animals is reduced and laboratory data on bTB confirmation almost do not exists.
In this study, we present results from a pilot study on usefulness of surveillance of bTB based on SICCT by tracing back the farm origin of a calf infected by bTB. In addition, gross lesions and histological changes of affected tissue are described.
Material and Methods
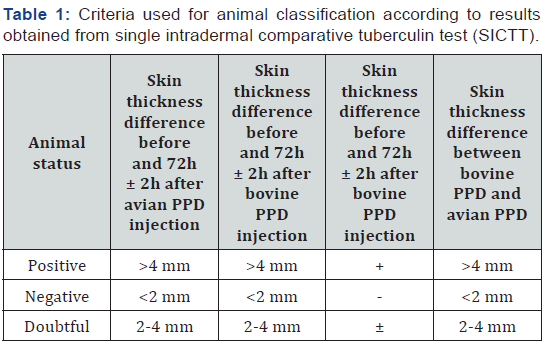
In this study, we tested all eligible cattle present in 141 holdings in entirely epidemiological unit and tissue samples were collected from slaughtered animals, which were judged as positive in comparative tuberculin skin test. The criteria for including animals were age over 1-month, healthy status and animals that belong to the village that was consider as epidemiological unit. In addition, there was used certified bovine and avian PPD tuberculin, appropriate syringes, clipper and calliper. The details data for each animal were recorded according designated template for the skin test purpose. In total, there were tested 277 animals by single intradermal comparative cervical tuberculin test (SICCT). Briefly, the tested animal ear tag was recorded, animal was restrained, the injection sites were prepared, the skin was measured, recorded and both tuberculin were correctly intradermal injected. The test was applicated according to European Union guideline 64/432/EEC. The injection sites were prepared at the 1/3d of middle of the neck. The doses of the tuberculin were 0.1 ml, which contain 3000UI and 2800 UI for bovine and avian PPD, respectively. The results were read 72 ± 2h and the data were recorded in the same template used at day zero. The criteria for classification of animal health status are described in Table 1. The criteria for classification of animal health status are described in Table 1.
The Tissues
The positive animals were slaughtered, and detailed meat inspection was carried out. The gross lesions were identified, recorded and selected pair samples were collected; fresh samples were used for isolation of suspected mycobacteria (data not show), the tissues fixed in 10% buffer solution of formalin were used for histopathological examination. The samples were collected from the initial case with suspected bTB lesions (liver, spleen and mesenteric lymph nodes) detected during routine meat inspection and from three positive animals (lung lesions, retropharyngeal, mediastinal and mammary lymph nodes) in SICT.
Gross Lesion Examination
Examination was done based on knife and eye method and presence of tubercular lesions was judged by naked-eye. From organs with visible tubercular lesions, both fresh and fixed samples were collected and submitted to the laboratory of animal infectious disease, Faculty of Veterinary Medicine, Tirana.
Histopathological Examination
The fixed samples were stained by using the standard haematoxylin-eosin (H&E) method, and prepared microscopic slides were exanimate under light microscope.
Results and Discussion
The single comparative skin test results are present in Table 2. In this study, 277 cattle from 140 holdings were tested with single intradermal cervical comparative skin test (SICCT). The final judgment for classification of individual animals for their health status was based on difference of skin thickness and presence of inflammatory oedema, fluctuation and presence of necrosis etc.
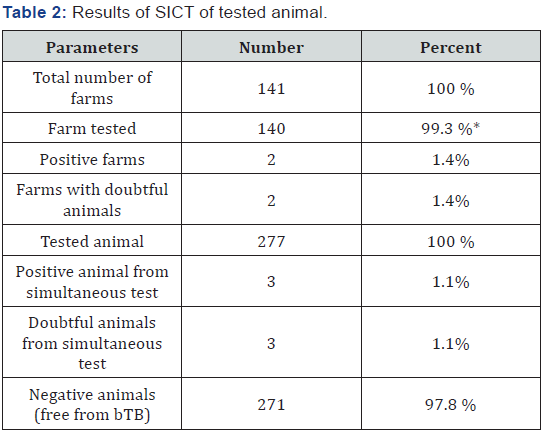
Based on the study results, disease prevalence of bovine tuberculosis of cattle population at village Blliçe was 1.1% at individual level and 1.4% at farm level. Referring to OIE criteria where disease prevalence should be less than 0.2% in order to classify the unit free from bovine tuberculosis at farm level, the study area could be classify as affected and bTB as enzootic status. Based on available data three main risk factor that affect the control program and/or can have impact on bTB spreading were identified.
Herd Size
The average size of farms is two animals/holding, 43% of them own only one cattle, while 1.5% of farmers have over five cattle per farm. Only a very few (0.7%) have 10 animal/ holding. This trend of cattle distribution per farm follow the national pattern, where 73% of the farms have a size ranged 1-4 animals and only 5% have a size bigger than 50 animals per farm. Apparently, this size farm does not support bTB transmission, if the close herd management is in place. In the reality, animals managed in one village have close contact between them, they share the routes, water sources, pastures and often the bulls. Those conditions are risk factors that facilitate bTB spreading within and between heads. We strongly suggest the extensions program must consider practical aspects of biosecurity and correct implementation at farm level.
Age Herd Structure
bTB is a typical chronic disease, so it is assuming that clinical disease, if appear, could be detect generally in older animals. In our study, the overall age of tested animals was 5.8 years old, ranged from 1 month to 20 years old. Age structure is accepted as a risk factor for developing typical tubercular lesions. At advanced stage of the disease, the number of mycobacteria and severity of lesions obviously increase. It is important to highlight that older animal and animal where the bTB is in advanced case, the cell mediated immunity response is affected negatively and either official skin test and/or INF-± test do not detect all affected animals. To increase sensitivity, we recommend using ELISA test as a ̏cleaver̋ choice aims to detect the infected animals, which may be negative in SICCT and ± - INF tests.
Correct Identification of Animals
The first step for disease surveillance is animal identification and strict animal movement control. Animal identification was identified as a poor and risk factor for control of infectious disease in general and bTB in particular. Furthermore, present farm buildings conditions are not adequate for implementing of cleaning and disinfection procedures. As it is shown in Table 3, 121 cattle (43.7%) were unidentified, missing one ear tag was recorded in 301(0, 8%) animals and only 102 of them or 36.8% were correctly identified. Furthermore, 24 animals (8.7%) were not ear tagged, because the farmers refused. Based on measures made 72±2h, three animals were positive on SICCT. Detailed data for reactors is given in Table 4 and Figure 1.

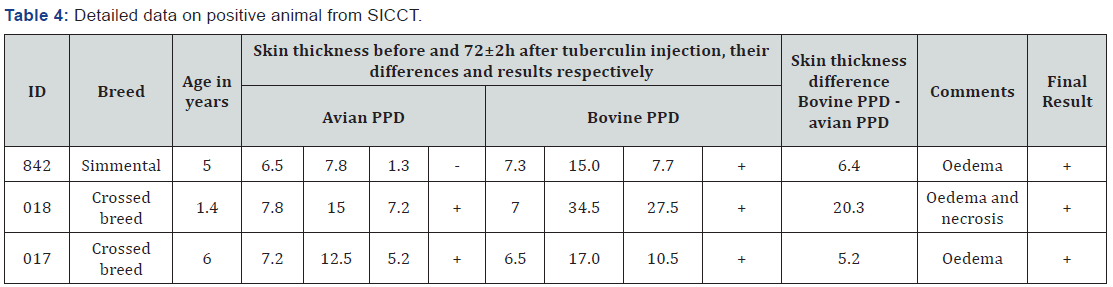
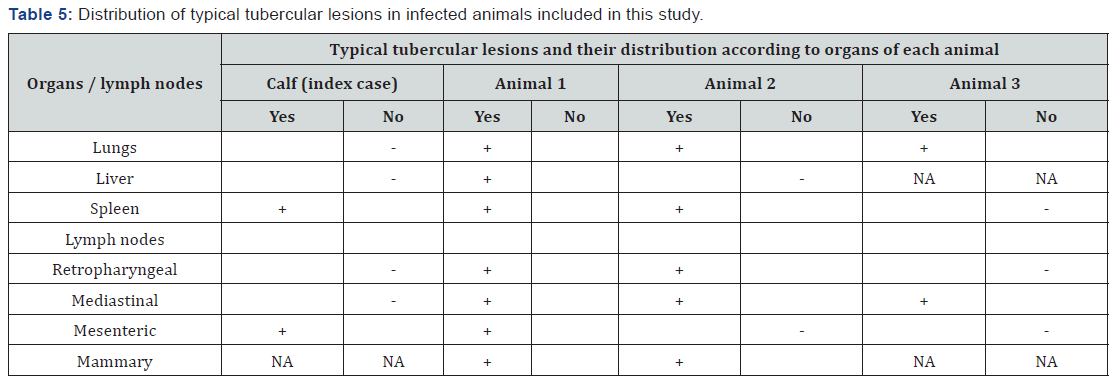

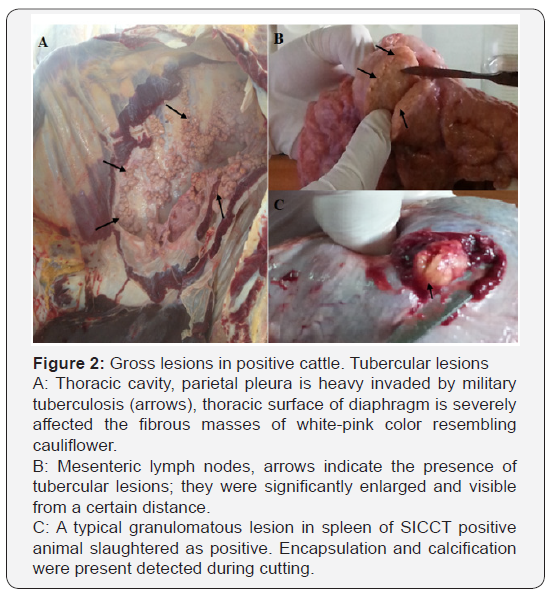
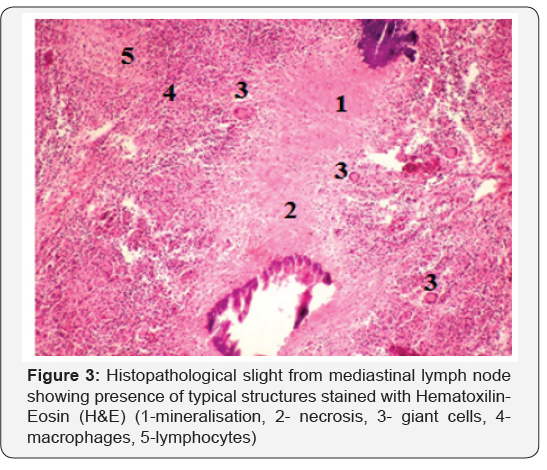
Positive animals, according to the veterinary low, were slaughtered at regional sanitary abattoir and detailed meat inspection was performed. All affected organs were recorded, filmed and proper samples were collected. The results of close examination of animal carcasses are presented in Table 5 and Figure 2. Histopathological examination of samples collected from carcases, showed presence of cell alterations and presence of typical structures in response to M. bovis infection. Microscopical examination indicate presence of typical changes in all samples, however the intensity of caseous necrosis, lymphocytic infiltration, presence of giant cells etc varies according to the stage of lesions (Figure 3).
Conclusion
According to our results, incidence of bTB is increased and control of M. bovis infection is an important issue of veterinary services, it is becoming a serious challenge. Drafting of a rational and realistic bTB eradication program is necessary; however, it is necessary to consider the risk factors that may have impact on failing the control program. We identify that lack of correct animal identification and strict animal movement control, unavailability of logistic infrastructure for performing skin test and lack of funds for farmer compensation; cleaning and disinfection interfere with the control program [2,5].
Drafting and implementing a rational strategy based on screening of commercial dairy farms, strict slaughterhouse surveillance and following up the origin of suspected case will be a realistic approach for controlling of bTB. In addition, transferring the new diagnostic tools, smartly using the available diagnostic test and involving the scientific institutions and human health capacities will support positively the bTB control program [7].
References
- Menzies FD, Neill SD (2002) Cattle-to-cattle transmission of bovine tuberculosis. Veterinary Journal 2000, 160(2):92-106. Microbes Infect 4: 471–80.
- Morris RS, Pfeiffer DU, Jackson R (1994) The epidemiology of Mycobacterium bovis infections. Veterinary Microbiology 40(1-2): 153-177.
- World Organisation for Animal Health (OIE) (2009) Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Chapter 2: 4-7.
- de la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, et al. (2006) Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, gamma-interferon assay and other ancillary diagnostic techniques. Res Vet Sci 81(2): 190–210.
- Quinn PJ, Markey BK, Leonard FC, Hartigan P, Fanning S, et al. (2011) Mycobacterium species in Veterinary Microbiology and Microbial Disease Textbook (2nd edn), pp. 161-176.
- Kaneene JB, Pfeiffer D (2006) Epidemiology of Mycobacterium bovis. In: Thoen CO, Steele JH, Gilsdorf MJ (Eds.), Mycobacterium bovis Infection in Animals and Humans. Ames, IA: Blackwell Publishing p. 34-48.
- Wedlock DN, Skinner MA, de Liste GW, Buddle BM (2002) Control of Mycobacterium bovis infections and the risk to human populations. Microbes and Infection 4(4): 471-480.
- Heba E, Duka S (2000) ELISA test for the detection of M. bovis antibodies and its value in the diagnosis of infection in cattle. Bulletin of Agricultural Sciences 4: 94-100.






























