Mixed Babesia canis and Anaplasma platys phagocytophilum Infection in Dog
Himalini*, Rajiv Singh, Rajinder Kumar Bhardwaj and Ajay Kumar Gupta
Veterinary Clinical Medicine, India
Submission: August 01, 2018; Published: August 14, 2018
*Corresponding author: Himalini, Veterinary Clinical Medicine, SKUAST-Jammu (J&K), India; Email: himalini.12@gmail.com
How to cite this article: Himalini, Rajiv S, Rajinder K B,Ajay K G. Mixed Babesia canis and Anaplasma platys phagocytophilum Infection in Dog. Dairy and Vet Sci J. 2018; 7(2): 555707. DOI: 10.19080/JDVS.2018.07.555707
Abstract
A 3 yr old male Non-descript dog presented to Referral Veterinary Hospital, Sher-e-Kashmir University of Agricultural Sciences and Technology Jammu with history of fever, anorexia, tick infestation, vomition, dark yellow urine and epistaxis. On clinical examination temperature 103.6ºF and pale mucous membrane were observed. Blood smear was found negative. Blood sample collected for CBC, LFT, KFT, PCR, and oxidative indices. PCR was positive for Babesia canis and SNAP4Dx test positive for Anaplasma platys phagocytophilum. Animal was treated with fluids, Analgin (Injection Vetalgin @ 0.5 – 1 mg /kg b.wt I/M) and Oxytetracycline (Injection terramycin @ 20mg/kg b.wt I/V in NSS). No further therapeutic intervention could be done as the animal collapsed within 24 hrs.
Keywords: Tick infestation; Epistaxis; Dog; CBC; LFT; KFT; Oxidative indices; PCR; Babesia canis; SNAP4Dx test; Anaplasma platys phagocytophilum; Vetalgin; Terramycin; Piroplasms; Anorexia; Tick infestation; Vomition
Introduction
Babesia species are tick-transmitted apicomplexan parasites infecting a wide range of wild and domestic vertebrate hosts [1]. Traditionally, identification of species has been based on host specificity and morphology of the intraerythrocytic piroplasms. Based on these, canine piroplasms have been originally recognised to belong to two distinct species, the large (4–5mm) Babesia canis and the small (–2.5mm) Babesia gibsoni. The association of Babesia species. with other haemoprotozoa can be attributed to the presence of the vector tick R. sanguineus Babesiosis can range from a very mild to a hyper acutely fatal disease. Parasites mainly affect erythrocytes, and typically cause haemolytic anaemia, but can also result in multiple organ dysfunctions [2]. It is characterized by erythrocyte destruction, anaemia, hypoxic tissue injury leading to disseminated pigmenturia, thrombocytopenia, γ- hyperglobulinemia, and waxing and waning of fever, pallor, jaundice, extravascular haemolysis, hypoxic injury, spleenomegaly and systemic inflammation [3]. Anaplasma phagocytophilum is a gram-negative, obligate intracellular bacteria that parasitize neutrophils in dogs, people, cats, cattle, sheep, and goats and horses worldwide. Anaplasma phagocytophilum is an obligate, intracytoplasmic coccus that belongs to the family Anaplasmataceae. The agent affects mostly neutrophils and rarely eosinophils; it is present within intracytoplasmatic vacuoles (morulae) [4]. Canine cyclic thrombocytopenia is caused by Anaplasma platys, a gram-negative, obligate intracellular bacterium that infects platelets [5].
Case History and Observation
A 3-yr. old male Non-descript dog presented to Referral Veterinary Hospital, Sher-e-Kashmir University of Agricultural Sciences and Technology Jammu with history of fever, anorexia, tick infestation, vomition, dark yellow urine and epistaxis. The owner also reported that vaccination and deworming were regular. On clinical examination animal had pyrexia (103.6 °F) and pale oral and conjuctival mucous membrane. Ticks were present on body with pityriasis (Figures 1-4).
Blood smear examination was done but found negative. Blood sample was collected for CBC, LFT, KFT and Oxidative indices. PCR was performed using standard protocol for B. canis having a 450 bp product. SNAP4Dx test is multivalent (enzyme linked immunosorbent assay) based test uses synthetic peptide reagents for in vitro diagnosis of Dirofilaria immitis antigen, A. platys/ phagocytophilum antibodies, B. burgdorferi antibodies, E. canis/ ewingii antibodies.

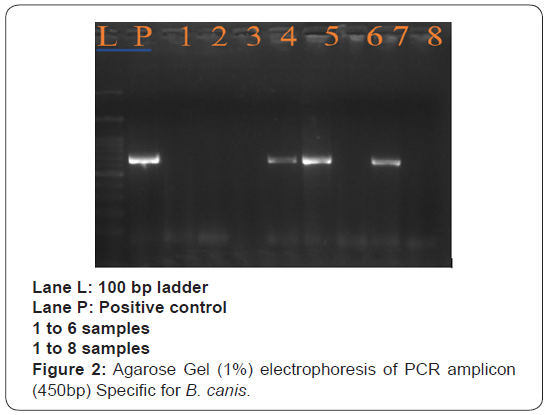
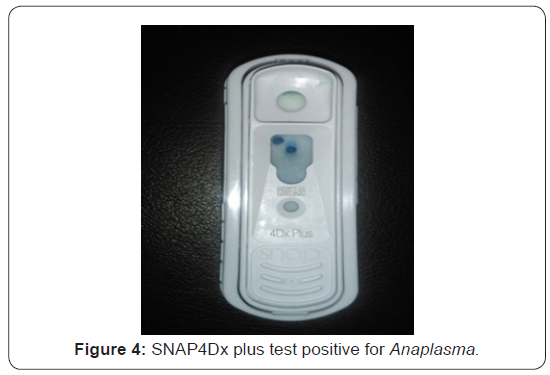
Treatment and Discussion
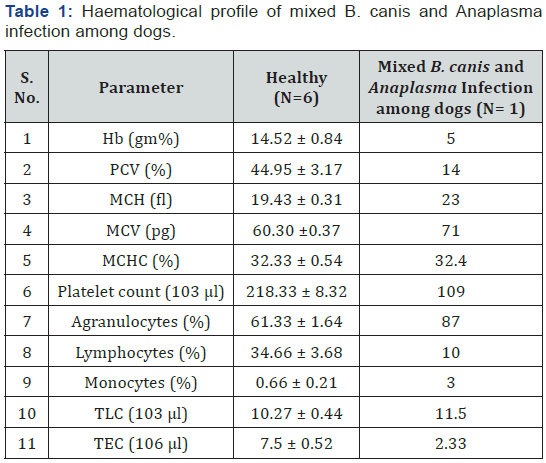
Diagnosis of B. canis and Anaplasma platys/phagocytophilum was made based on the clinical signs and the demonstration of organism in agarose gel (1%) electrophoresis of PCR amplicon (450bp) specific for B. canis. SNAP4Dx plus test kit shows positive result as blue dot specific for Anaplasma platys/ phagocytophilum. Hb, PCV and TEC were low in mixed B. canis and Anaplasma infection among dogs. Erythrocytic indices showed an increase in MCV, MCH and MCHC value among affected dogs. Platelets showed decreased level among affected dogs. Monocytosis and Lymphopenia along with an increase in total leukocyte count among affected dogs (Table1).
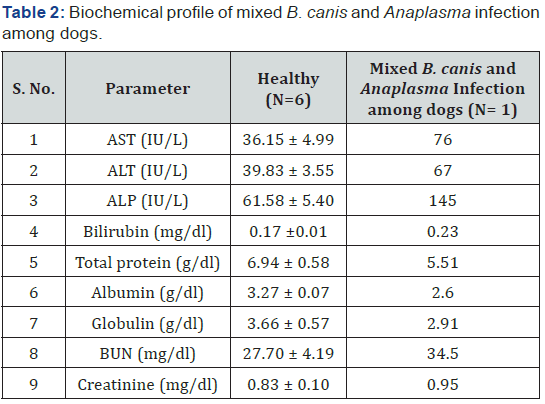
There was decrease in the value of protein, albumin and globulin in mixed B. canis and Anaplasma infection among dogs. BUN and Creatinine showed a non-significant difference. AST, ALT, ALP and Bilirubin showed an increase among affected dogs (Table2). Values of CAT, SOD and GSH showed a decrease in mixed B. canis and Anaplasma infection among dogs. An increase in value of LPO was observed among affected dogs (Table 3). Animal was treated with fluids, Analgin (Injection Vetalgin @ 0.5 – 1mg /kg b.wt I/M) and Oxytetracycline (Injection terramycin @ 20mg/kg b.wt I/V in NSS). No further therapeutic intervention could be done as the animal collapsed within 24 hrs.
Canine babesiosis is a tick-borne disease caused by intraerythrocytic piroplasms of the genus Babesia, named after Dr. Victor Babes, who in 1887 established the etiology of the cattle disease in Romania. Canine babesiosis has been attributed to infection with either Babesia canis, large babesia species [6] or Babesia gibsoni., Babesia conradae, the small Babesia species [7]. The large babesia of dogs have a wide distribution which includes South Africa [6] while the small babesiosis of dogs occur in South-East Asia, North East Africa, Spain, Australia and the USA [7]. Babesia gibsoni have been reported in India, Korea, Malaysia, Cyylon and the USA [8]. The situation regarding canine babesiosis in India is far from clear. Babesiosis can range from a very mild to a hyper acutely fatal disease. Parasites mainly affect erythrocytes, and typically cause haemolytic anaemia, but can also result in multiple organ dysfunctions [2]. It is characterized by erythrocyte destruction, anaemia, hypoxic tissue injury leading to disseminated pigmenturia, thrombocytopenia, γ- hyperglobulinemia, and waxing and waning of fever, pallor, jaundice, extravascular haemolysis, hypoxic injury, spleenomegaly and systemic inflammation.

Anaplasma phagocytophilum is a gram-negative, obligate intracellular bacteria that parasitize neutrophils in dogs, people, cats, cattle, sheep, and goats and horses worldwide. Anaplasma phagocytophilum is an obligate, intracytoplasmic coccus that belongs to the family Anaplasmataceae. The agent affects mostly neutrophils and rarely eosinophils; it is present within intracytoplasmatic vacuoles (morulae) [4]. Canine cyclic thrombocytopenia is caused by Anaplasma platys, a gramnegative, obligate intracellular bacterium that infects platelets [5]. Clinical signs of anaplasmosis vary but the disease most commonly presents as an acute febrile syndrome with elevated body temperature, lethargy, anorexia and reluctance to move [9]. More localized signs may be present, especially in the chronic phase of the disease, affecting the musculoskeletal system (lameness), the gastrointestinal system (diarrhoea), or the central nervous system [9,10]. Hematological changes in dogs infected with Anaplasma species are anaemia, thrombocytopenia, and leucopenia. The most consistent laboratory abnormality is thrombocytopenia which occurs in approximately 90% of dogs [11]. The platelet count in thrombocytopenic dogs has been reported to range from 5,000 to 164,000 platelets/ml [11,12]. Both the parasites are sensitive to oxytetracyclines but further therapeutic intervention could be done as the animal collapsed within 24 hrs.
References
- Kuttler KL (1988) World-wide impact of babesiosis. In: Ristic M(Eds.), Babesiosis of Domestic Animals and Man. CRC Press, Boca Raton, Finland, p. 1-22.
- Welzl C, Leisewitz AL, Jacobson LS, Vaughan-Scott T, Myburgh E (2001) Systemic inflammatory response syndrome and multiple-organ damage/dysfunction in complicated canine babesiosis. Journal of the South African Veterinary Association 72(3): 158-162.
- Irwin PJ, Jefferies R (2009) Arthropod-transmitted diseases of companion animals in Southeast Asia. Trends in Parasitology 20: 27- 34.
- Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, et al. (2001) Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. International Journal of Systematic and Evolutionary Microbiology 51: 2145-2165.
- Greene CE (2006) Infectious Diseases of the Dog and Cat. (3rd edn), Elsevier Health Sciences, New York, USA.
- Uilenberg G, Franssen FF, Perie NM, Spanjer AA (1989) Three groups of Babesia canis distinguished and a proposal for nomenclature. Veterinary Record 11(1): 33-40.
- Kjemtrup AM, Conrad PA (2006) A review of the small canine piroplasms from California: Babesia conradae in the literature. Veterinary Parasitology 138(1-2): 112-117.
- Conrad P, Thomford J, Yamane I, Whiting J, Bosma L, et al. (1991) Hemolytic anemia caused by Babesia gibsoni infection in dogs. Journal of the American Veterinary Medical Association 199: 601-605.
- Harrus S, Waner T, Bjoersdorff A, Shaw SE (2005) Ehrlichiosis and anaplasmosis. In: Shaw, S.E., Day, M.J. (Eds.), Arthropod-borne Infectious Diseases of the Dog and Cat. Manson Publishing, London, UK, pp.120-133.
- Greig B, Asanovich KM, Armstrong PJ (1996) Geographic, clinical, serologic, and molecular evidence of granulocytic ehrlichiosis, alikely zoonotic disease, in Minnesota and Wisconsin dogs. Journal of Clinical Microbiology 34: 44-48.
- Poitout FM, Shinozaki JK, Stockwell PJ, Holland CJ, Shukla S K (2005) Genetic variants of Anaplasma phagocytophilum infecting dogs in western Washington State. Journal of Clinical Microbiology 43(2): 796- 801.
- Kohn B, Galke D, Beelitz P, Pfister K (2008) Clinical features of canine granulocytic ehrlichiosis in 18 naturally infected dogs. Journal of Veterinary Internal Medicine 22(6): 1289-1295.






























