Abnormalities in Unhatched Chicks of Japanese Quails Fed with Zinc Oxide Nanoparticles
Zeinab Khoobbakht1*, Mohammad Roostaei-Ali Mehr1, Mehrdad Mohammadi1, Mohammad Mehdi Sohani2 and Fahimeh Mohammadghasemi3
1Department of Animal Science, College of Agriculture, Guilan University, Rasht, Iran
2Department of Biotechnology, College of Agriculture, Guilan University, Rasht, Iran
3Cellular and molecular research center, Guilan University of medical science, Iran
Submission: December 12, 2017; Published: March 29, 2018
*Corresponding author: Zeinab Khoobbakht, Department of Animal Science, College of Agriculture, Guilan University, Rasht, Iran, Email: zkhoobbakht@gmail.com
How to cite this article: Zeinab Khoobbakht, Mohammad Roostaei-Ali Mehr, Mehrdad Mohammadi, Mohammad Mehdi Sohani, Fahimeh Mohammadghasemi. Abnormalities in Unhatched Chicks of Japanese Quails Fed with Zinc Oxide Nanoparticles. Dairy and Vet Sci J. 2018; 5(3): 555662. DOI: 10.19080/JDVS.2018.05.555662
Abstract
According to some reports, zinc oxide nanoparticles (ZnONs) are toxic. Therefore, their toxicity was assessed in Japanese quail offspring, which breeders were fed with ZnONs. Two abnormal chicks were observed in this report. It is concluded that ZnONs have the teratogenic potential in Japanese quail embryos and these results confirm that ZnONs could pose the potential risk for animal and human health.
Keywords: Japanese quail; Zinc oxide nanoparticles; Embryonic abnormalities
Introduction
In the past few years, with the rapid development of nanotechnology and owing to their wide industrial and commercial applications, manufactured nanomaterials are now used in pharmaceutics, commodities, biomedical products, human and animal foods [1,2]. Nanoscale materials possess more novel, unique and specific physicochemical properties such as durability and chemical reactivity compared to bulk materials [3]. Thus, because of their small size, nanoparticles (NPs) can penetrate into the cell membrane and interfere in important cell functions. Studies have demonstrated that the size of NPs also affects their efficiency [4,5]. Recently, Zinc nanoparticles have been considered as an important factor animal science [6] that one of its applications is as dietary zinc source. Some of researchers reported that bioavailability of Zn nanoparticles is high and demonstrated that use of ZnONs in broiler diets led to improve growth performance [7]. Also there has shown that dietary supplementation with ZnONs in rat causes of fertility increasement [8]. However, in some of the studies adverse effects of nanoparticles on the reproduction and embryos of laboratory animals were reported [9]. There are few reports in related to supplementation of diet with ZnONs on the Japanese quail embryo. Therefore the aim of this report is to describe Japanese quail embryo abnormality due to use of ZnONs in diet.
Case Presentation
In our investigation, two malformed chicks were observed in offspring of breeder Japanese quails fed with zinc oxide nanoparticles (Figure 1). The embryos have been died between the 14th to 17th days of incubation. In the first case, the chicken brain was protruded (nude brain) and was without the eyes and upper beak was incomplete, but there was no abnormality in the internal organs after an autopsy. In second case (Figure 2), the chicken was smaller than first case and its appearance characteristics were: no upper break, protruded brain, two backbone attached to one neck, that two naturally developed wing and leg connected to each spine, and both backbone had formed a cavity. After the autopsy, we observed an abnormal heart, and enlarged liver in meddle of abdominal cavity. The gizzard size was relatively large with cylindrical shape that located in the centre of cavity and two series of intestine with normal appearance was attached to gizzard that each of them individually was terminated to a vent.
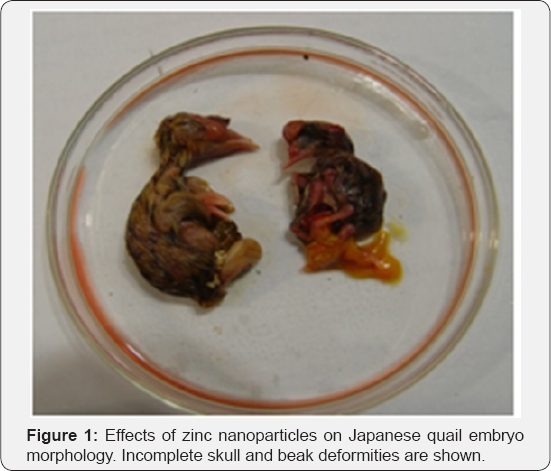

Discussion
Several parameters influence what happens at the nanobio interface and thus determine the toxic potential of any given NP [3]. Besides the chemical composition of the NP, size, surface modification, shape and polarity have to be considered to be biologically active. The reasons suggested for cellular damage caused by NP exposure include production of toxic radicals [10] and interaction with DNA [11]. Therefore, the results of nanoparticle toxicity studies can be of considerable heterogeneity. In somatic cells such insults cause inflammation or even malignant transformation. Multitude studies reported, most have shown the ability of NPs to effectively cross biological barriers including the ones protecting the reproductive and brain tissue. In germ line cells, either defect might lead to impaired fertility and congenital defects in the offspring [12]. It was noted that NPs distributed to the placenta and fetuses of exposed pregnant dams and to the milk and pups of exposed lactating dams [13].
In our investigation, two malformed chicks were observed in offspring of breeder Japanese quails fed with zinc oxide nanoparticles. It shows that nanoparticle supplementation in breeder Japanese quail diet may have detrimental effects on offspring development. This was supported with the previous studies that have indicated prenatal NPs exposure NPs found in many products causes alteration to the cerebral cortex, poor fetal growth, development retardation, low birth weight in unborn and newborn infants and other dysfunctions in offspring [1416]. Also researchers detected a cytotoxic effect of NPs on rodent testes and spermatogenesis caused the consequent fluctuation of sperm and testosterone levels [17]. It is concluded that ZnONs have the teratogenic potential in Japanese quail embryos and these results confirm that ZnONs could pose potential risk for animal and human health.
References
- Baek M, Chung HE, Yu J, Lee JA, Kim TH, et al. (2012) Pharmacokinetics, tissue distribution, and excretion of zinc oxide nanoparticles. Int J Nanomedicine 7: 3081-3097.
- Jo E, Seo G, Kwon JT, Lee M, Lee CB, et al. (2013) Exposure to zinc oxide nanoparticles affects reproductive development and biodistribution in offspring rats. J Toxicol Sci 38(4): 525-530.
- Nel A, Xia T, Madler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311(5761): 622-627.
- Canton I, Battaglia G (2012) Endocytosis at the nanoscale. Chem Soc Rev 41(7): 2718-2739.
- Zhao F, Zhao Y, Liu Y, Chang X, Chen C, et al. (2011) Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 7(10): 1322-1337.
- Dawei A, Zhisheng W, Anguo Z (2010) Protective effects of Nano-ZnO on the primary culture mice intestinal epithelial cells in in vitro against oxidative injury. WRJAS 6(2): 149-153.
- Ahmadi F, Ebrahimnezhad Y, Sis NM, Ghiasi J (2013) The effects of zinc oxide nanoparticles on performance, digestive organs and serum lipid concentrations in broiler chickens during starter period. IJB 3(7): 2329.
- Afifi M, Almaghrabi OA, Kadasa NM (2015) Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes BioMed Research International 2015: 2015.
- Talebi AR, Khorsandi L, Moridian M (2013) The effect of zinc oxide nanoparticles on mouse spermatogenesis. J Assist Reprod Genet 30(9): 1203-1209.
- Oberdörster G, Oberdorster E, Oberdorster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 823-839.
- Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, et al. (2009) NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials 30(23-24): 3891-3914.
- Taylor U, Barchanski A, Kues W, Barcikowski S, Rath D (2012) Impact of metal nanoparticles on germ cell viability and functionality, Reprod Domest Anim 47 (Suppl 4): 359-368.
- Sumner SC, Fennell TR, Snyder RW, Taylor GF, Lewin AH (2010) Distribution of carbon-14 labeled C60 ([14C] C60) in the pregnant and in the lactating dam and the effect of C60 exposure on the biochemical profile of urine. J Appl Toxicol 30(4): 354-360.
- Jo E, Seo G, Kwon JT, Lee M, Lee Bc, et al. (2013) Exposure to zinc oxide nanoparticles affects reproductive development and biodistribution in offspring rats. J Toxicol Sci 38(4): 525-530.
- Yon JM, Jung AY, Lin C, Lee JG, Jung KY, et al. (2011) Teratogenic effects of nano-and micro-sized particles of zinc oxide during mouse organogenesis. JBR 12(2): 102-110.
- Yamashita K, Yoshioka Y, Higashisaka K, Mimura K, Morishita Y, et al. (2011) Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat Nano technol 6(5): 321-328.
- Yoshida S, Hiyoshi K, Ichinose T, Takano H, Oshio S, et al. (2009) Effect of nanoparticles on the male reproductive system of mice. Int J Androl 32(4): 337-342.






























