Relationships between Plasma L-Carnitine Levels and Genetic Characteristics of Japanese Black Steers
Seizi Sukemori1*, Satoshi Odo2 and Mitsuo Sato3
1FacuJty of Agriculture, Tokyo University of Agriculture, Japan
2Graduate School of Engineering, Tokyo University of Agriculture and Technology, Japan
3Fuji Farm, Tokyo University of Agriculture, Japan
Submission: October 16, 2017; Published: October 26, 2017
*Corresponding author: Seizi Sukemori, Faculty of Agriculture, Tokyo University of Agriculture, Atsugi, Kanagawa, Japan, Tel: +81-46-294-6598; Email: sukemori@nodai.ac.jp
How to cite this article: Seizi S, Satoshi O, Mitsuo S. Relationships between Plasma L-Carnitine Levels and Genetic Characteristics of Japanese Black Steers. Dairy and Vet Sci J. 2017; 4(3): 555639. DOI: 10.19080/JDVS.2017.04.555639
Abstract
The present study aimed to observe changes in plasma L-carnitine levels in accordance with the growth of two representative strains of steers. Steers were classified into two groups: one group (11 steers) represented beef with fine marbling texture, and the other group (7 steers) was a high-gain group. Calves were separated from their mothers 10 days after birth and then fed with artificial milk. Blood was collected at the beginning of artificial nursing and again when the calves were 30 days old, which was when early weaning was carried out. The levels of L-carnitine in both the blood plasma and artificial milk samples were determined. The L-carnitine contents in artificial milk from two different farms were 47.9mg/L and 87.4mg/L. The difference in L-carnitine contents in the current results might be caused by seasonal variation of L-carnitine in milk as a result of skimmed milk resources. Although the linear regression equations showed variations in plasma L-carnitine levels in each strain, the levels were not significantly different, however the fine marbling strain showed a decreasing trend, and a slight increasing trend was shown in the strain of high-gain steers. The present results suggest that endogenous synthesis of L-carnitine may be controlled by the genetic characteristics of Japanese Black steer
Keywords: L-carnitine; Steers; Strain character; Synthetase
Introduction
The calving transition period, which lasts several days before and after calving, is a time of high stress for cows, and this stress affects not only cows but also the possibility of calves receiving milk with damaged composition. Some treatments, such as glucose precursors, minerals used as an oral drench solution, L-carnitine or administration of rumen-protected amino acids, have been examined as methods to minimize the negative effects caused by calving [1-6]. In studies of the nutritional role of L-carnitine supplementation on growth performance and meat characteristics, early postnatal skeletal myo-fiber formation in lightweight piglets from large litters was reported by Losel D et al. [6,7] and Madsen JG et al [8] with L-carnitine supplementation occurring during suckling. These authors reported increases in myo-fiber number, but growth performance was not influenced. Meat production performance of Japanese Black cattle requires large carcass size, i.e., beef cattle strains that show high daily gains preferred by farmers. In contrast, beef with fine marbling texture generally is preferred by consumers [8,9] but preference for beef with coarse marbling has recently increased, as this type of beef is considered a healthier option by older consumers.
Based on the genetic modification of meat characteristics, strains with fine marbling and beef strains with coarse marbling have been developed. Production of beef with fine marbling is an original technique developed in Japan, and beef steers that provide fine marbling show lower weight gain tendencies than do steers that provide beef with coarse marbling [10]. This difference in daily gain between the steers may be induced by other factors, such as nutrient metabolism. Additionally, there are few studies on the endogenous synthesis of nutrients that do not consider ingestion supply. Rumen microbes undergo reproductive functions that produce amino acids using protein ingested from feed, therefore the requirements of amino acids have never been of concern in cattle farming. Recently, it was revealed that a spot supply of specific amino acids for the regulated lactation period, is produced as a result of a rumen- bypass characteristic found in high-performance dairy cattle [5,11]. Colostrums has high levels of lactose and milk fat, both of which can be used as an energy source, and L-carnitine, which accelerates the utilization of long-chain fatty acids in p-oxidation, is also present at high levels in colostrums. In a study of humans, it was estimated that 25% of L-carnitine was produced by endogenous synthesis from lysine and methionine in the liver and/or kidneys of mature individuals, and the remaining 75% of total body carnitine levels came from the diet [12].
Therefore, a young animal such as a calf must take in L-carnitine in the form of milk or a starter; additionally domestic steers recognized to have bovine spongiform encephalopathy and physiologically inactive organs require a regulated intake of animal products rich in L-carnitine resources. Artificial milking practices caused by the early separation of mother and child such as in dairy cattle, have increased in the feeding of beef cattle. In this system, artificial milk is produced from skimmed milk and acts as a resource that supplies L-carnitine. The L-carnitine content in milk changes in the lactation phase and varies in colostrums and regular milk, and the content decreases with heat stress [2]. In previous studies [13,5], although the supplementation of L-carnitine into artificial milk tended to improve daily gains, significant differences could not be recognized, and it was deduced that the statistical results were induced by the endogenous synthesis of L-carnitine, which was dominant in the strain
In this study, changes of plasma L-carnitine levels in accordance with growth of steers from two strains, one that was representative of fine marbling and one that was representative of high gains, were observed to provide further clarification of our previous hypotheses.
Materials and Methods
In this study, the steers used in the experiments were fed under custom conditions which included feed-stuffs from two collaborating farms, and were cared for in accordance with the guidelines of the Animal Welfare Act of the Tokyo University of Agriculture. Blood collection was carried out by veterinarian in each farm.
Steers and artificial milk
The two collaborating farms were located in Kumamoto Prefecture (K farm) and Shimane Prefecture (S farm). Steers were classified into two groups, one of which was a fine marbling group composed of 11 steers (4 in the K farm and 7 in the S farm) and the other was a high-gain group composed of 7 steers (2 in the K farm and 5 in the S farm). They were given their mother's milk until 9 days old, and steer separation occurred at 10 days, after which the calves were fed artificial milk. The makers of commercial artificial milk were different.
Blood collection and treatment
Blood was collected when the calves were 10 days old, which was the starting point of artificial nursing, and 30 days old at which time early weaning was carried out. Blood plasma was separated from whole blood by moderate centrifugation (1500xg) and stored at -20 °C for determination of L-carnitine levels.
Analysis
The same volume of 2% sulfo-salicylic acid solution was added to the stored blood plasma and artificial milk that was prepared at typical concentrations for the removal of protein sediments. The L-carnitine levels in the supernatant of the blood plasma and in the artificial milk samples were determined by UV 21 nm by liquid chromatography (LC-20AD, Shimadzu, Co. Ltd., Kyoto, Japan) and under the following conditions: a Unison UK-C18 column, 20nM phosphate buffer including a 5nM octane sulfonic acid sodium carrier, and a flow rate of 0.8mL/min.
Statistics
The results as shown with real values were statistically analyzed with one-way analysis of variance with a significance level of P<0.05. The differences between the values of 10 days and 30days in each calf, as shown Δ20 values, were treated using the Student t-test with significance level of P<0.05, between the farms in the strain with fine marbling and the strains in the S farm. Furthermore, a linear regression equation for chronological variation was calculated for each strain.
Results and Discussion
The obtained real values and A20 values of plasma L-carnitine level and the difference between day 30 value and day 10 values in each calf are shown in Table 1.

Values of plasma L-carnitine level are shown as means ± SE
Δ20: Changed plasma L-carnitine level in the calf during 20 days supplied with artificial milk from day 10 to day 30.
Different small shoulder letter shows a statistic trend: a,b; P=0.095 and cd; P=0.10.
L-carnitine contents in artificial milk
The L-carnitine content in the artificial milk used at the K farm was 47.9mg/L and at the S farm 87.4mg/L. The L-carnitine content from the S farm was approximately 1.8 times higher than that of the K farm. In our previous studies, we reported values ranging from 16-64mg/L in colostrums and a value of 47.5mg/L in artificial milk [3-6]. The high values in these studies were closer to the K farm value, and the lowest value of 16mg/L was calculated from the colostrums of Japanese Black cows (unpublished), as shown in Table 2. In the groups representing fine marbling of both two farms, there was no significant difference in the Δ20 values (Figure 1). Therefore the difference of supplied L-carnitine amounts through artificial nursing did not affect the plasma L-carnitine level. It is recognized that the contents in milk change with environmental conditions, especially atmospheric temperature [4], and this change is reflected in the L-carnitine content of the skimmed milk that was used to produce artificial milk. The existence of plasma proteins that provide immunological substances can have an effect on the L-carnitine content in artificial milk. The difference in L-carnitine contents in the current result might be caused by one or a combination of the above mentioned reasons.


Δ20: Changed plasma L-carnitine level in the calf with the fine marbling strain in both farms during supply with artificial milk for 20 days. There were no significant differences.
Chronological variations in plasma L-carnitine levels in steers

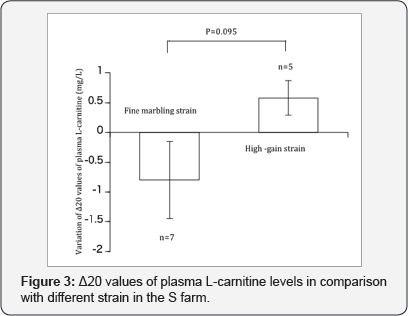
In the procedure of L-carnitine synthesis, ascorbic acid, riboflavin and iron are required as co-factors. Vaz et al. proposed that e-N trimethyllysine hydroxylase mediates the first step in the endogenous synthesis of L-carnitine [14]. The activity of y-butyrobetaine dioxygenase is also necessary to form L-carnitine, and if this enzyme has very low activity, animals become highly dependent on active uptake from blood [15]. Activities of both enzymes are essential factors for L-carnitine biosynthesis, but little research has been conducted on the relationship between the endogenous synthesis of L-carnitine and the growth performance of nursing calves; however many studies have been conducted with infants [13]. In the present study, there was no significant difference between the different strains at the same time after birth. After the separation of mother and child at the age of 10 days, the amount of L-carnitine ingested by steers was different at each farm. It changed in direction, meaning an increase or decrease in plasma L-carnitine level, reflected the endogenous synthesis activities, i.e., an increase reflecting high synthesis and low consumption of L-carnitine, and a decrease showed the opposite relationship. While the obtained linear regression equations were not statistically significant, there was a decreasing trend in the beef strain with fine marbling and a slight increasing trend in the plasma L-carnitine levels in the high-gain beef strain. This present trend was emphasized in the fine marbling strain with significant level of P=0.1 (Figure 2), and the variation of A20 values during different strains in the S farm showed opposite direction with decreasing trend (P=0.095) (Figure 3). It was recognized that the present difference in the supplied L-carnitine did not affect the plasma level, as in the abovementioned. In a previous study [16], a positive relationship between L-carnitine levels of the blood and daily gain of bulls was recognized but was insignificant. The effect of L-carnitine supplementation of feed on the daily gain of steers was also unable to reach full potential, i.e., it was obviously at a low plasma level, as in the previous result [4], and we deduced that the influence of supplemental L-carnitine on the growth of steers may be related to different levels of endogenous synthesis and supplement levels. The current results support the results of our previous studies, although further verification with more heads of steer is necessary.
Δ20: Changed plasma L-carnitine level in the calf with the fine marbling strain in both farms during supply with artificial milk for 20 days. There were no significant differences between the different strains.
In studies on the nutritional roles of administered L-carnitine, it has been reported that dietary L-carnitine prevents fat accumulation in dairy cows [17] and young bulls [18], and there is no improvement in the growth performance of young bulls. This result might be, because the steers are too large; we recorded body weights of 310±24 kg, for steers used for L-carnitine administration tests in our analysis.
Conclusion
Plasma L-carnitine levels in immature steers varied with growth under different conditions of energy production including de novo synthesis and consumption for the energy production as β-oxidation, and this balance may be controlled by the genetic characteristics of beef strain. Current results relate to a particular type of beef production used in Japan, but the supplementation of L-carnitine in the starter feed proves to be useful and therefore is suggested.
Acknowledgement
The authors sincerely thank Mr. N. Fukuyama and Miss Y Urano for their valuable assistance in conducting this work.
References
- Guagnini FS, Pineda A, Gonsalves RS, Dalpizzola L, Driemeier D, et al. (2017) The effect of early postpartum oral drench solution on blood p-hydroxybutyrate concentration, milk yield, and milk composition in Holstein cows. Dairy and Vet Sci 1(1): 1-6.
- Erfle JD, Sauer FD, Fisher LJ (1973) Interrelationships between milk carnitine and blood and milk components and tissue carnitine in normal and ketotic cows. J Dairy Sci 57(6): 671-676.
- Sato M, Noguchi T, Watanabe N, Odo S, Ikeda S, et al. (2011) Effects of L-carnitine supplementation on the milk yields and exodus amounts of it by dairy cattle. Tokai J Anim Sci 22: 37-41.
- Sato M, Watanabe N, Odo S, Ikeda S, Sukemori S (2011) Effect of supplementation of L-carnitine on the production of dairy and beef cattle. Proc Jpn Soc Anim Nutr Metab 55: 35-54.
- Sato M, Noguchi T, Watanabe N, Odo S, Ikeda S, et al. (2012) Milk yield, plasma and milk concentration of L-carnitine of beef cows fed L-carnitine and growth of their calves. Tokai J Anim Sci 23: 27-31.
- Sato M, Ikeda S, Odo S, Sukemori S (2013) The effects of rumen protected lysine supplementation on milk yield and L-carnitine in plasma and milk of dairy cattle. Tokai J Anim Sci 24: 25-30.
- Losel D, Kalbe C, Rehfeldt C (2009) L-carnitine supplementation during suckling intensifies the early postnatal skeletal myofiber formation in piglets of low birth weight. J Anim Sci 87(7): 2216-2226.
- Losel D, Rehfeldt C (2013) Effects of L-carnitine supplementation to suckling piglets on carcass and meat quality at market age. Animal 7(7): 1191-1198.
- Madsen JG, Seoni E, Kreuzer M, Silacci P, Bee G (2017) Influence of L-carnitine and L-arginine on protein synthesis and maturation of the semitendinosus muscle of lightweight piglets. J Anim Physiol Anim Nutr.
- Kitagawa T, Yamaji T, Aoki Y, Murakami K, lida F (2016) Relationships between sensory traits influencing overall judgement in sensory evaluation and fat content of beef from Japanese Black cattle. Nihon Chikusan Gakkaiho 87(3): 235-241.
- O'Quinn TG, Brooks JC, Polkinghorne RJ, Garmyn AJ, Johnson BJ, et al. (2012) Consumer assessment of beef strip loin steaks of varying fat levels. J Anim Sci 90(2): 626-634.
- Pond WG, Church DC, Pond KR (Eds.) (1995) Basic Animal Nutrition and Feeding, 4th (edn.), Chapter 20 Dairy cattle, 401-414.
- Rebouche CJ (1997) Carnitine function and requirement during the life cycle. FASEB J 6(15): 3379-3386.
- Vaz FM, Ofman R, Westinga K, Willem JW, Wanders RJA (2001) Molecular and biochemical characterization of rat £-N trimethyllysine hydroxylase, the first enzyme of carnitine biosynthesis. J Biol Chem 276: 33512-33517.
- Ringseis R, Wen G, Eder K (2012) Regulation of genes involved in carnitine homeostasis by PPAR a across different species (rat, mouse, pig, cattle, chicken, and human). PPAR Res 2012 (2012): 1-11.
- Sukemori S, Ikeda S, Noguchi T, Watanabe N, Odo S, et al. (2011) Relationship between plasma L-carnitine concentration and production performance in hybrid beef cattle and Holstein cows sired by different strain bulls. Vitamin 85(10): 552-555.
- Carlson DB, McFadden JW, Litherland NB, Drackley JK (2006) Dietary L-carnitine prevents fat accumulation in the liver of transition dairy cows.
- Hajilou M, Dehghan-Banadaky M, Zali A, Rezayazdi K (2014) The effects of dietary L-carnitine and rumen-protected choline on growth performance, carcass characteristics and blood and rumen metabolites of Holstein young bulls. J Applied Anim Res 42: 89-96.






























