Effect of Prostaglandin F2α on Growth of Streptococcus uberis associated with Bovine Mastitis
Autran CA1, Price W2, Dalton JC3 and Ahmadzadeh A1*
1Department of Animal and Veterinary Science, University of Idaho, USA
2College of Agricultural and Life Sciences, USA
3Caldwell R & E Center, Caldwell, ID, USA
Submission: July 11, 2017; Published: September 18, 2017
*Corresponding author: A. Ahmadzadeh, Department of Animal and Veterinary Science, University of Idaho, 875 Perimeter Dr. MS 2330, Moscow, ID 83844, Tel: 208 885 7409; Fax: 208 885 6420; Email: amin@uidaho.edu
How to cite this article: Autran CA, Price W, Dalton JC, Ahmadzadeh A. Effect of Prostaglandin F2α on Growth of Streptococcus uberis associated with Bovine Mastitis. Dairy and Vet Sci J. 2017; 3(4): 555619. DOI: 10.19080/JDVS.2017.03.555619
Abstract
Certain fatty acids have been shown to inhibit the growth of mastitis pathogens. Moreover, prostaglandin in F2α (PGF2α) inhibits the growth of Staphylococcusareus in vitro. The objective was to determine the antibacterial effects of PGF2α (in the form of dinoprosttromethamine) on growth of Streptococcus uberis (Strep. uberis) in vitro. Flasks containing tryptic soy broth were inoculated with Strep. uberis, and subsequently treated with PGF2α at concentrations of 0 (control), 0.6, 1.2, 2.4, and 4.8 mg/ml . Cultures were sampled every 4h over 24h to determine bacterial growth. The experiment was repeated 3 times in duplicate. Bacterial growth was assessed by counting colony forming units (CFU) in triplicate. Data were analyzed by repeated measures analysis of variance and reduced and full dummy variable regression models to determine the effect of PGF2α concentrations on growth patterns of Strep. uberis over time. There was an effect of treatment and treatment by time interaction on mean log CFU for Strep. uberis (P < 0.05). At the time of inoculation (0h), mean log CFU values were not different among treatments. At 12 and 24h of growth, mean log CFU for all PGF2α concentrations were smaller and differed (P < 0.05) from the control, in a dose dependent manner. The predicted growth curve pattern over 24h for each treatment was different (P < 0.05) when compared with the control, and the growth rate over time for treatments 2.4 and 4.8 mg/ml was slower and different from the control (P < 0.05). These results provide evidence that PGF2α has inhibitory effects on growth of Strep. uberis in vitro.
Keywords: Fatty acids, mastitis, prostaglandin F2α, Streptococcus uberis
Abbreviations: PGF2α: Prostaglandin F2α; Strep. uberis: Streptococcus uberis; CFU: Colony Forming Units
Introduction
The spread of mastitis pathogens causes large economic losses to the dairy industry. These losses include increased involuntary culling rate, reduced milk production, increase domatic cell count, and discarded milk [1,2]. Costs associated with mastitis infections in the U.S. dairy industry have been estimated at nearly US $2 billion per year [3]. Streptococcus uberis is one of the most common gram-positive causes of clinical mastitis and contributes to a large proportion of subclinical mastitis cases [4,5]. Streptococcus uberis infections are often difficult to cure with traditional intra-mammary antibiotic preparations, especially in older animals [6,7]. Similar to treatment trials focused on Staphylococcus aureus (S. aureus) intra-mammary infection, increased somatic cell counts before treatment was associated with a decreased probability of cure [6]. This may explain why the response of Strep. uberis mastitis to treatment can be poor, even after extended therapy [7]. If cure rates are low, it is generally not considered cost-effective to treat cows with chronic cases infections [8].
The bactericidal activities of various fatty acids as an alternative to antibiotics have been studied and reviewed [9,10]. Kelsey et al. [11] demonstrated that lauric acid, capric acid, myristic acid and linoleic acid inhibited growth of two different mastitis strains of S. aureus. Arachidonic acid, a fatty acid derived from linoleic acid [12] inhibited gram-positive bacteria such as S. aureus and S. pyogenes [13]. Considering that both linoleic and arachidonic acid have been shown to affect bacterial growth, we speculated that PGF2α, synthesized from these fatty acids, may have similar antibacterial properties. In fact, the results from a recent study in our laboratory [14, 15]; indicate that PGF2α, inhibits the growth of S. aureus and Mycoplasma bovis. Given that Strep. uberis is one of the most common gram-positive causes of clinical mastitis, our hypothesis was that PGF2α would inhibit the growth of Strep. uberis. The objective of this study was to determine the effect of PGF2α on Strep. uberis in vitro by characterizing the growth response of Strep. uberis to PGF2α in the form of dinoprosttromethamine.
Materials and Methods
Experimental design and treatment
Bacterial cultures were prepared by inoculating a single colony into 3ml of tryptic soy broth (TSB) (EMD Chemicals Inc., Darmstadt, NJ) followed by overnight incubation at 37 °C with shaking at 250rpm. In order to obtain a sufficient culture for the experiment, culture tubes containing 10ml of fresh TSB were inoculated at 1:100 with 3 ml of overnight Strep. uberis culture, and once more incubated overnight at 37 °C with shaking at 250rpm. Prostaglandin F2α in the form of dinoprosttromethamine (Zoetis, Florham Park, NJ) was added to flasks for a final concentration of 0, 0.6, 1.2, 2.4 and 4.8 mg/ml (2 flasks/treatment). Flasks, which included both treatment and controls, were inoculated with the 10 ml overnight culture of Strep. uberis at a concentration of 1:100. Flasks were incubated at 37 °C and shaken at 250rpm for 24h; at 0h, and every 4h thereafter, 1ml samples were taken from each flask to determine bacterial growth. The entire experiment was repeated three times, in duplicate, in different days to account for variation associated with a day effect, categorized as run.
Determination of bacterial growth
To determine colony forming units (CFU), samples of 0.5ml were taken from flasks for plating. Serial dilutions were performed before samples were placed on agar plates (EMD Chemicals Inc., Darmstadt, NJ). The CFU counts were done in duplicate per sample from each flask. Plates were incubated at least 12h, or until colonies were apparent, at a constant temperature of 37 °C. The CFU counts from each of the two agar plates were averaged for each of the corresponding flasks.
Statistical analysis
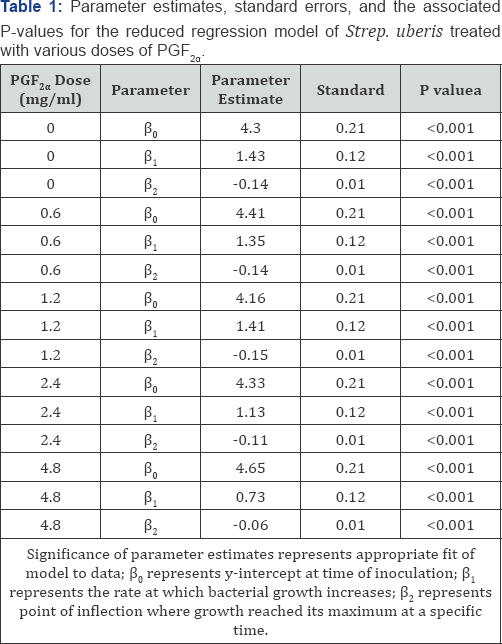
The number of live cells, as measured by log CFU, was determined by averaging the number of cells for the duplicate concentrations of both plates at each 4h time point. An analysis of variance (repeated measures) was carried out using the mixed procedure of SAS (SAS Institute, Cary, NC) where the model included treatment, time (repeated factor) and their interaction. To further analyze the effect of treatments over time on the growth pattern and growth rate of Strep. uberis, a full model dummy variable regression procedure was also performed. The coincidence or equality of the estimated regression lines, the rate of bacterial growth over time, and the point at which the inflection of the growth curve occurred (an indication of maximum bacterial growth) were determined. The estimation of the reduced models for each treatment was carried out using PROC REG procedures of SAS, and that of the full model was carried out using PROC GLM procedures of SAS. The fitted reduced model for each treatment took the form of
Y = β0 + β 1x + β 2x2 + ε1
Where Y was the logarithmic value of the number of live cells (log CFU/ml), x represented time, β0 was the intercept (estimated log CFU/ml at time 0), β1 was the rate of increase for bacterial growth, β2 was the point of inflection, and ε represented the random error under the classical regression assumptions. The adequacy of the fit was determined by the significance of the parameter estimates (declared at P <0.05), their corresponding magnitudes and signs, and the examination of the estimated residuals.
Results
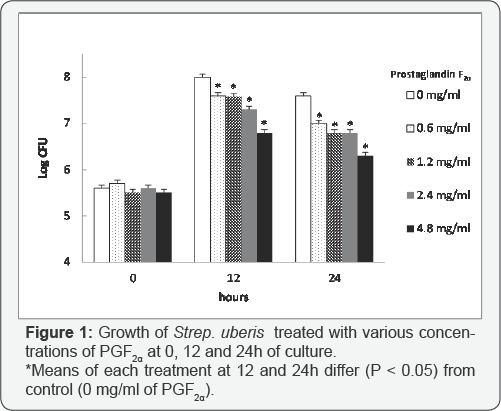
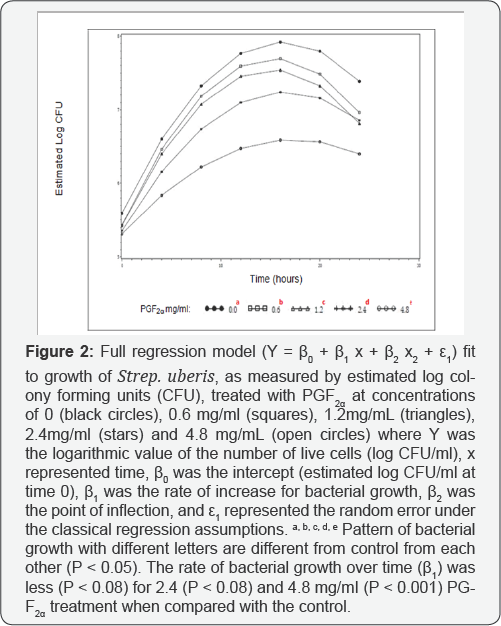
Bacterial growth curves were evaluated in growth media containing PGF2α at concentrations of 0, 0.6, 1.2, 2.4mg/ ml and 4.8, with 0 mg/ml referring to the control with no PGF2α. Based on bacterial growth curves, PGF2α, in the form of dinoprosttromethamine, has inhibitory effects on growth of Strep. uberis (Figure 1). Overall, growth of Strep. uberis decreased with increasing concentrations of PGF2α, with 4.8 mg/ml of PGF2α being the most inhibitory (Figure 2).
There was an effect of treatment and treatment by time interaction on log CFU/ml (P < 0.05), providing evidence that bacterial growth of Strep. uberis over time was not similar among PGF2α treatments. Preplanned contrasts were conducted to compare the mean log CFU/ml values between treatments at 12 and 24h. At 0h the mean log CFU/ml values were not different among treatments and control, and averaged 5.6±0.02 log CFU/ ml (Figure 1). Mean log CFU/ml values at 12 and 24h for each PGF2α treatment dose, however, were different (P < 0.05) from the control (Figure 1). At 12h after incubation, the bacterial growth for each PGF2α treatment reached its maximum (Figure 1). Mean log CFU/ml for 0.6 mg/ml (7.7±0.06 log CFU/ml), 1.2 mg/ml (7.6±0.06 log CFU/ml), 2.4 mg/ml (7.3±0.06) and 4.8 mg/ ml (6.7±0.06) were all different (P < 0.05) from 0mg/ml (control, 8.0±0.06). At 24h, mean logs CFU/ml for 0.6 mg/ml (6.9±0.06 log CFU/ml), 1.2 mg/ml (6.8±0.06 log CFU/ml), 2.4 mg/ml (6.8±0.06) and 4.8 mg/ml (6.4±0.06) were also different (P < 0.05) when compared with 0 mg/ml (control, 7.5±0.06) (Figure 1) . Interestingly, PGF2a at the greatest dose (4.8 mg/ml) had the greatest effect on bacterial growth as log CFU/ml never reached above 6.4 log CFU/ml.
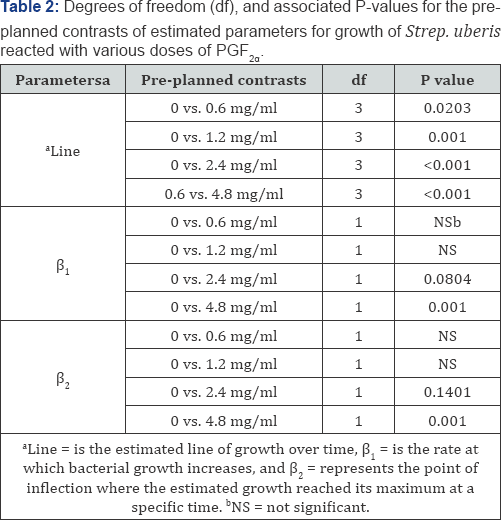
The reduced and full dummy variable models were carried out to evaluate the effects of different PGF concentrations on 2a the growth pattern of the bacteria concentrationson the growth pattern of the bacteria over time. The parameter estimates of the reduced model for all treatment doses of PGF were2 a significant (P < 0.05), indicating that the reduced model fit the data well for each of those treatments and that all parameters are required (Table 1, Figure. 2). The preplanned contrasts carried out using the dummy variable regression model (Table 2) indicated that the overall line of growth over a 24h period was different (P<0.05) for treatments 0.6, 1.2, 2.4, and 4.8 mg/ ml, when compared with the control (0 mg/ml), implying that the bacterial growth pattern for these treatments were different from control and lending support to results found through the repeated measure analysis. The rate of bacterial growth over time (β1 was less (P < 0.08) for 2.4 (P < 0.08) and 4.8 mg/ml (P < 0.001) PGF2α treatment when compared with the control, providing evidence that the rate of bacterial growth over time was different between those treatments in a dose dependent manner (Table 2). In addition, the rate of bacterial growth was slower in 4.8 than with 1.2, 2.4mg/ml PGF2α (data not shown). Each growth curve had an estimated point of inflection, where the estimated maximum log CFU/ml was reached at a specific time. The estimate parameter point of inflection (P2 the estimate of maximum bacterial growth) for 4.8 mg/ml PGF2α treatment was different from the control (P < 0.05, Table 2) and all other PGF2α treatment groups (P < 0.05, data no shown).
Discussion
This research addresses the question whether the fatty acid PGF2α is inhibitory to growth of Strep. uberis. The results support the hypothesis that PGF2α, in the form of dinoprosttromethamine has inhibitory effects on growth of Strep. uberis . These findings support the results from our previous research in which PGF2α, inhibited growth of gram positive bacteria, S. aureus [14] as well as Mycoplasma bovis [15]. The antimicrobial properties of fatty acids on bacteria have been studied for years. The effectiveness of fatty acids in inhibiting growth of several gram-positive bacteria have also been demonstrated and reviewed [11,13,16].
Arachidonic acid, a fatty acid originally derived from linoleic acid, has been shown to inhibit gram-positive bacteria such as Streptococcus faecalis and Staphylococcus epidermidis, and S. aureus [13]. The authors hypothesized those bactericidal effects on S. aureus mediated by peroxidation of arachidonic acid. Because both linoleic acid and arachidonic acid are precursors to PGF2α, it is plausible that PGF2α, synthesized from these fatty acids, has similar antibacterial properties. The results supported our hypothesis that commercially available PGF2o (dinoprosttromethamine) inhibited the growth of Strep. uberis in vitro in a dose dependent manner (Figure 3), resembling the actions of linoleic acid on growth of S. aureus Novel as previously described [11].
The mechanism by which PGF2α affected Strep. uberis cannot be determined from the current study. The inhibitory properties of fatty acids were more noticeable with increased chain length and degree of un-saturation [10,13,17]. Zheng et al [13] found differences in antibacterial activity between unsaturated fatty acids and saturated fatty acids in that saturated fatty acids had less or no antibacterial activity against S. aureus and S. pyogenes. Dinoprosttromethamine contains two double bonds and consists of 24 carbons. These features may be important factors in its antibacterial properties.
One potential mechanism of action centers on the ability of fatty acids to penetrate and disruptthe phosphor lipid bi-layer of the plasma membrane of bacteria and ultimately increases the negative charge on the bacterial membrane surface [10]. Zheng et al. [13] proposed that antibacterial action of unsaturated fatty acids is mediated by inhibition of bacterial enoyl-acyl carrier protein reductase which is an essential component of bacterial fatty acid biosynthesis. Another proposed mechanism involves the hindering of bacterial growth via an interaction with lipid bi-layer of the cell membrane, resulting in a change in membrane permeability, or the interference with transduction cascades leading to cell lysis [10, 18]. In summary, the current in vitro results provide evidence that the fatty acid PGF2α, in the form of dinoprosttromethamine, has inhibitory effects on the growth of Strep. uberis in a dose dependent manner. The potential use of PGF2α, as an anti-bacterial fatty acid, for treatment of mastitis requires more research.
Acknowledgments
This study was made possible by the support of Zoetis Animal Health, Florham Park, NJ, in providing pure prostaglandin F2α(dinoprosttromethamine), Idaho Dairymen's Association, and the Idaho Agricultural Experiment Station.
References
- Philpot WN (1984) Economics of mastitis control. The Vet Clinics of North America. Large Anim Practice, 6(2): 233-245.
- Zepeda L, Buelow KL, Nordlund KV, Collins MT, Goodger WJ, et al. (1998) A linear programming assessment of the profit from strategies to reduce the prevalence of Staphylococcus aureus mastitis. Preventive Vet Med 33(1-4): 183-193.
- Degraves FJ, Fetrow J (1993) Economics of mastitis and mastitis control. Vet Clin North Am Food Anim Pract, 9(3): 421-434.
- Verbeke J, Piepers S, Supre K, De Vliegher S (2014) Pathogen-specific incidence rate of clinical mastitis in Flemish dairy herds, severity, and association with herd hygiene. J Dairy Sci, 97(11), 6926-6934.
- Sampimon O, Barkema HW, Berends I, Sol J, Lam T (2009) Prevalence of intramammary infection in Dutch dairy herds. J Dairy Res 76(2): 129136.
- Barkema HW, Schukken YH, Zadoks RN (2006) Invited review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J Dairy Sci 89(6): 1877-1895.
- Milne MH, Biggs AM, Barrett DC, Young FJ, Doherty S, et al. (2005) Treatment of persistent intramammary infections with Streptococcus uberis in dairy cows. Vet Rec 157(9): 245-250.
- Ruegg PL (2004) Lactation therapy and milk quality in Natl. Mastitis Counc. Reg. Mtg. Proc., Charlotte, NC. Natl. Mastitis Counc., Inc, USA.
- Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods-a review. Int J Food Microbiol 94(3): 223-253.
- Desbois AP, Smith VJ (2010) Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol 85(6), 1629-1642.
- Kelsey JA, Bayles KW, Shafii B, McGuire MA (2006) Fatty acids and monoacylglycerols inhibit growth of Staphylococcus aureus. Lipids 41(10) 951-961.
- Mayes PA, Botham KM (2003) Metabolism of unsaturated fatty acids and eicosanoids. Harper's Illustrated Biochemistry, 26th Edn., Lange Medical Books, New York, pp 190-196.
- Zheng CJ, Yoo JS, Lee TG, Cho HY, Kim YH, et al. (2005) Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett 579(23): 5157-5162.
- Autran CA, Shafii B, Dalton JC, McGuire MA, Carnahan KG, Ahmadzadeh A (2013) Effect of prostaglandin F2α on growth of Staphylococcus aureus associated with bovine mastitis. J Vet Sci Technol 4(1): 129-134.
- Ahmadzadeh A, Fox LK, McGuire MA, Carnahan KG (2015) Effect of prostaglandin F2α growth of Myco plasma bovis associated with bovine mastitis. Adv Dairy Research 10: 1-3.
- Hogan JS, Pankey JW, Duthie AH (1987) Growth inhibition of mastitis pathogens by long-chain fatty acids. J Dairy Sci 70(5): 927-934.
- Sun CQ, Connor OCJ, Roberton AM (2003) Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol Med Microbiol 36(1-2): 9-17.
- Chamberlain NR, Mehrtens BG, Xiong ZH, Kapral FA, Boardman JL, et al. (1991) Correlation of carotenoid production, decreased membrane fluidity, and resistance to oleic acid killing in Staphylococcus aureus 18Z. Infect Immun 59(12): 4332-4337.






























