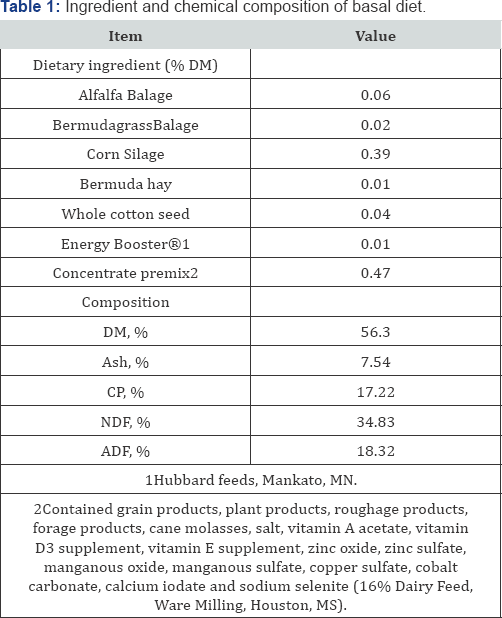
Calcium Montmorillonite Clay for the Reduction of Aflatoxin Residues in Milk and Dairy Products
C R Maki1, S Allen2, M Wang1, SH Ward2, BJ Rude2, HR Bailey2, RB Harvey3 and TD Phillips1*
1College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, USA
2Department of Animal and Dairy Sciences, Mississippi State University, USA
3Department of Agriculture, Agricultural Research Service, USA
Submission: April 13, 2017; Published: April 28, 2017
*Corresponding author:T D Phillips, VMA Building 107 MS 4458, College Station, TX 77843, USA, Tel: 979- 865-6414; Fax: -979- 847-8981; Email: tphillips@cvm.tamu.edu
How to cite this article: C R Maki, S Allen, M Wang, SH Ward. Calcium Montmorillonite Clay for the Reduction of Aflatoxin Residues in Milk and Dairy Products. Dairy and Vet Sci J. 2017; 2(3): 555587. DOI: 10.19080/JDVS.2017.02.555587
Abstract
In this study, dairy cows were treated with calcium montmorillonite clay (NSP; BASF Corp., Ludwigshaven, Germany) in a replicated 5x5 Latin square design. The primary objectives were to determine if milk composition was altered following ingestion of NSP, and to investigate the ability of NSP to reduce aflatoxin (AF) transfer to milk with the inclusion of low doses in the diet (concentrations equal to 0.125 and 0.25% w/w). The experiment was conducted at the Bearden Dairy Research Center at Mississippi State University. Cows were housed in a free-stall barn with sand bedding and were fed and milked twice daily. The experiment consisted of 5 10-d periods, where cows were randomly assigned to 1 of 5 dietary treatments (n=3 for each treatment):
- absolute control (CON), basal total mix ration (TMR) with no AF or NSP;
- AF Control (AFC), basal TMR plus 50 ppb AF;
- NSP Control (NSPC), basal TMR plus 0.5% estimated dry matter intake (DMI) NSP;
- low-dose clay with AF (NSP-0.125%), basal TMR plus 0.125% estimated DMI NSP and 50 ppb AF;
- Or high-dose clay with AF (NSP-0.25%), basal TMR plus 0.25% estimated DMI NSP and 50 ppb AF.
All additions to the basal TMR were top dressed and mixed into the top of feed offered. Dry matter intake and nutrient intake did not differ among dietary treatments (P>0.05). Milk yield and feed efficiency (FE) were similar throughout all treatments, and no treatment effects were observed for fat yield, lactose, protein yield, solids, or somatic cell count (SCC). Furthermore, vitamin A and riboflavin concentration in milk were similar across all treatments and averaged, 0.30±0.03 and 1.54±0.13μg/mL, respectively. A dose dependent reduction (P<0.01) in concentration of aflatoxin Ml (AFM1) in milk with the inclusion of NSP was shown. Feeding the AFC diet resulted in 0.75±0.05μg AFM1/L; this value was reduced by 13.3% (0.65±0.05μg/L) with the inclusion of NSP at 0.125% of DMI and by 22.7% (0.58±0.05μg/L) when NSP was fed at 0.25% of DMI. Specifically, transfer rate was reduced from 1.66% with the AF diet to 1.43% and 1.28±0.16% with the inclusion of NSP at 0.125% and 0.25% of DMI. Due to reduced transfer rate, total excretion of AFM1 was also reduced (P<0.01) in a dose dependent manner. This study was part of a multistate dairy project. When compared to other studies in this project, NSP resulted in a linear decrease in AFM1 ranging from 13% (at the smallest dose of clay) to 71% (at the greatest dose of clay). At all doses, DMI, milk yield, milk composition, minerals, vitamin A, and riboflavin concentrations were unaffected by the dietary treatments. The inclusion of NSP in contaminated dairy feeds may help mitigate AF problems without affecting milk production or composition. The results of this study will aid in determining the appropriate dosage of NSP needed to decrease AFM1 below allowable concentrations.
Keywords: Aflatoxin; Food safety; Milk vitamins; Mycotoxins; Calcium montmorillonite clay
Abbreviations: AF: Aflatoxins TMR: Total Mix Ration; CON: Absolute Control; SCC: Somatic Cell Count; AFC: AF Control; NSPC: NSP Control; FE: Feed Efficiency; AFM1: Aflatoxin M1; DMI: Dry Matter Intake; AFB1: Aflatoxin B1; AFB2: Aflatoxin B2; FDA: Food and Drug Administration; NSP: NovaSil Plus
Introduction
Aflatoxins (AF) are secondary metabolites produced by the fungi Aspergillus flavus and Aspergillus parasiticus and are immunosuppressive, anti-nutritional, and mutagenic Kurtzman et al. [1]. Additionally, they are potent carcinogens in a variety of species including humans Linsell & Peers [2]; Peers et al. [3]. The four naturally occurring aflatoxins are aflatoxin B1 (AFB1), B2 (AFB2), G1 (AFG1), and G2 (AFG2), named for their characteristic florescent properties Binder & Krska [4]. Of these, AFB1 is the most toxic and carcinogenic Food and Drug Administration [5] and is classified as a group 1 carcinogen World Health Organization and International Agency for Research on Cancer[6] .
Contamination of food with AF is a global problem and typically occurs in regions that experience elevated temperatures and frequent drought. This includes areas of Sub-Saharan Africa, Southeast Asia, Central America, and the southern United States Williams et al. [7]. In addition to direct consumption of AF, humans and animals may also be exposed to toxic metabolites of AF. One such metabolite is aflatoxin M1 (AFM1), the hydroxylated derivative of AFB1. When lactating animals consume AFB1- contaminated diets, the toxin is metabolized through cytochrome P450-mediated oxidation and excreted into milk as AFM1 Van Egmond et al. [8].
Feed contamination with AF may occur during pre- or postharvest contamination of crops. Pre-harvest contamination increases in periods of drought stress and elevated temperatures during the growing season of crops Cotty & Jaime-Garcia [9], whereas post-harvest contamination may occur from improper storage conditions that promote fungal growth Cavallarin et al. [10]. Although AFM1 is less carcinogenic than the parent AFB1 molecule, it is toxic and considered to be a risk factor for aflatoxicosis in vulnerable populations and is classified as a group 2 carcinogen [6]. Young children and animals are more susceptible to the effects associated with AF exposure. Because milk is a major nutrient source for the young, AF concentrations are strictly regulated in milk and milk products. The United States Food and Drug Administration (FDA) has established Action Limits of 0.5 and 20ppb for AFM1 and AFB1 in milk and feed for lactating dairy animals, respectively [5]. To keep the concentration of AF below legal limits, postharvest treatments are often considered. Efforts to mitigate AFM1 may be ineffective due to its resistance to pasteurization and processing Stoloff et al. [11]; Stoloff & Trucksess [12]; Yousef & Marth [13].
For this reason, various approaches have been developed to address the issue of AF contamination in feeds. These methods include various mechanical methods and density separation procedures Dickens & Whitaker [14]; Henderson et al. [15], thermal inactivation Yazdanpanah et al. [16]; Bagley [17], treatment with ammonia and ozone Allameh et al. [18]; McKenzie et al. [19], and the potential use of various adsorbents, Diaz et al. [20]; Firmin et al. [21]; Queiroz et al. [22]; Huwig et al. [23].
One of the most promising strategies used to mitigate exposure is the inclusion of high affinity AF adsorbents in the diet. For example, montmorillonite clays including NovaSil (NS) and NovaSil Plus (NSP) have been reported to be effective in reducing AF exposures Harvey et al. [24]; Kutz et al. [25]; Smith et al. [26]. These clays are able to bind AF in the gastrointestinal tract, effectively reducing its bioavailability and distribution throughout the body Phillips [27]; Phillips et al. [28]; Phillips et al. [29]. Importantly, studies with clay in animals and humans have shown that it does not interfere with vitamin or nutrient uptake and utilization Afriyie-Gyawu et al. [30]; Afriyie-Gyawu et al. [31]; Mitchell et al. [32]; Phillips et al. [33].
NovaSil Plus (at concentration between 0.5 and 2.0% w/w in the diet) has been shown to reduce AFM1 concentrations in milk from dairy cows without altering the nutritional quality or causing overt toxicity Harvey et al. [34]. This study focused on low dose inclusion of clay. Further work is needed to establish safety and efficacy of montmorillonite clay at a wider range of dose to facilitate its inclusion as an aflatoxin binder in dairy animal feed.
In a recent multi-state project, where lactating cows at each research site were fed AF or NSP clay or both, the primary objectives were to determine whether milk quality and composition changed following ingestion of clay by the animals, and to further validate the efficacy of NSP clay to reduce AF transfer to milk. Studies conducted at Tarleton State University in Texas Maki et al. [35] and the University of Georgia Maki et al. [36] have shown that NSP significantly reduces AF carry-over, measured by AFM1 metabolite concentrations in milk from AF treated cows, without altering the composition of milk.
The final study (of a multistate project) is presented in this paper, and was conducted at Bearden Dairy Research Center at Mississippi State University (MSU). The objectives were to assess the carryover of AF into milk and its effects on milk quality and milk composition at doses of clay lower than have been tested previously.
Materials and Methods
Animal care, housing and feeding
This study was conducted at the Mississippi Agriculture and Forestry Experiment Station, Bearden Dairy Research Center (Starkville, MS) under the approval of the Mississippi State Institutional Animal Care and Use Committee. The study consisted of fifteen lactating Holstein cows housed in free-stalls with sand bedding. Cows were trained to use individual feeding gates (Calan Broadbent Feeding System, American Calan, Northwood, NH) prior to treatment and were individually fed at 0530 and 1730h, allowing for ad libitum intake. Cows were milked at 0400 and 1600h in a double eight parallel milking parlor. Treatment was received once daily during the 0530 feeding.
Animals, experimental design, and treatments
Cows were placed in a triplicate 5 x 5 Latin square study design consisting of five 10-d periods. Treatment was applied on days 1 through 5, whereas days 6 through 10 were used as a washout period to prevent carry-over effects. NovaSil Plus was tested at different concentrations. Cows were predicted to consume 25kg of DM, and NSP was fed at 0.125, 0.25, and 0.5% (NSP control) of predicted DMI. The AF supplement was produced from rice fermentation by A. parasiticus NRRL 2999 as described by Shotwell et al. [37] and modified by West et al. [38]. Rice Powder containing 758mg AFB1/kg was obtained from the Food and Feed Safety Research Facility, USDA/ARS (College Station, TX). The concentration of AF was verified by the Office of The Texas State Chemist, Texas A&M University (College Station, TX). Cows were randomly assigned to 1 of 5 dietary treatments (n=3):
Absolute control (CON), basal total mix ration (TMR) with no AF or NSP;
AF Control (AFC), basal TMR plus 50 ppb AF;
NSP Control (NSPC), basal TMR plus 0.5% DMI NSP;
low-dose clay with AF (NSP-0.125%), basal TMR plus 0.125% estimated DMI NSP and 50 ppb AF;
Or high-dose clay with AF (NSP-0.25%), basal TMR plus 0.25% estimated DMI NSP and 50 ppb AF.
All additions to the basal TMR were top dressed and mixed into the top portion of feed offered. Basal TMR and individual orts were sampled on d 4 of each period.
Sampling and data collection
Feed samples were dried at 15.5 °C to determine air DM, ground through a 2 mm screen in a Thomas Wiley mill (model 4, Thomas Scientific, Swedesboro, NJ), and stored at room temperature. Subsamples of orts were taken and combined by treatment and period. All feed samples were subjected to proximate analysis for total DM (method 934.01; AOAC International [39]), ash (method 942.05;[39]), CP (method 2001.11;[39]), NDF (method 973.18;[39]), and ADF (method 2002.04;[39]). Milk samples were taken at both milkings on d4 and 5 of treatment periods. Samples from the 0400h milking both days were analyzed for fat, protein, solids, and SCC AOAC International [40] by Mid-South DHIA (Missouri), and results were averaged. Mid-South utilized aBently FTS Combi (Chaska, MN) to analyze SCC and components. Somatic cell counts were analyzed using flow cytometry, and components were analyzed using Fourier Transform Spectrometer (infrared spectroscopy).
Vitamin A was determined using official methods of the AOAC (Method 992.06; [40]) with modifications in milk described by Jakobsen [41]. The analysis was performed by HPLC (Waters, Milford, MA). An aliquot of 10mL milk was added to a 150mL centrifuge tube. 30mL of antioxidant solution (1 % pyrogallol) and 5mL of saponification solution (10.5M potassium hydroxide) were added to the test tubes. Tubes were capped and swirled briefly to mix. The tubes were placed in a shaking H2O bath at 70 °C set to 60oscillations/min for 25min. After mixing, samples were allowed to cool to room temperature and transferred to a 125mL separatory funnel; complete transfer was ensure by rinsing the centrifuge tubes with 30mL of H2O into the funnel.
30mL of extraction solvent (hexane-methylene chloride (3+1 v/v)) were added into the funnel and gently mixed for 2min. The aqueous layer was then removed, and 30mL of Wash solution (water ethanol (3+2 v/v)) was added; this last step was performed in triplicate. A 20mL aliquot was evaporated to dryness under nitrogen and resuspended in 5mL of mobile phase. The analysis was performed by HPLC (Waters Milford, MA, USA). In this protocol, 100μL of sample were injected in a mobile phase consisting of hexane-isopropyl alcohol (100+0.25, v/v) with a flow rate of 1.5±0.2mL/min at ambient temperature. Peaks were detected via UV absorbance at 325nm with a sensitivity 0.1 AUFS. The column was a 4.6mm id x 15cm stainless steel reversed-phase C-18 column with a 5m particle size.
Concentration of riboflavin was determined according to AOAC official methods (Method 985.31; [5]) with modifications for milk described by Ndaw et al. [42]. Fifty mL of 0.1M hydrochloric acid were added to 5mL of milk in a 250mL conical flask. Samples were autoclaved at 121 °C for 30min and then allowed to cool to room temperature. The pH was adjusted to 4.5 with 2.5M sodium acetate, followed by addition of 100mg of Takadiastase (Pfaltz& Bauer, Waterbury, CT).
The solution was incubated for 18h in an oven at 45 °C. After incubation the solution was diluted to 100mL with 0.01M HCl and filtered with a 0.2m filter. Analysis was performed by HPLC using an injection volume of 50 μL of the filtrate at a flow rate of 1mL/min. The HPLC-system was equipped with a 250 x 4.6mm id reversed phase column with a 5m particle size. Samples were run in isocratic mode using methanol: 0.0 5M sodium acetate buffer (30:70) as the mobile phase. Riboflavin was measured directly with fluorescence detection using excitation and emission wavelengths set at 422nm and 522nm, respectively.
AFM1 was extracted from milk samples according to official methods of the AOAC (Method 2000.08; [40]). Samples were analyzed by LC-MS/MS (Waters H-class UPLC-MS/MS with ESI capability) in the positive mode using the method of Warth et al. [43]. Briefly, samples were warmed to 37 °C, centrifuged for 20min at 2000xg and defatted. The samples were passed through a coffee filter to remove any residual fat. A 10mL aliquot of the defatted fraction was passed through an immunoaffinity column (Afla WB, Vicam, Milford, MA) at a steady gravity controlled flow rate (approximately 1mL/min).
Columns were washed twice with 10mL of double distilled, deionized water (MilliQ 18.2Mflcm) and eluted with 4mL of acetonitrile. Samples were evaporated to dryness under constant nitrogen and were then re-suspended in 1mL of 1:1 MeOH water solution and analyzed by LC-MS/MS (Waters H-class UPLC-MS/ MS with ESI capability) in the positive mode for AFM1 (mol. wt. 328). The mobile phase consisted of an isocratic gradient of 30% water, 70% acetonitrile and 0.1% formic acid at a flow rate of 0.325mL/min. The column temperature and injection volume were 40 °C and 10μL, respectively
Aflatoxin standards were purchased from Sigma Chemical Co. (St. Louis, MO). Aflatoxin concentrations were quantified with the instrument software (Empower 2, Waters Corporation, Milford, MA). Aflatoxin excretion was calculated by multiplying the concentration of AFM1 by the milk yield based on milk production the day of collection. Aflatoxin transfer was calculated by dividing AFM1 excretion by AFB1 intake and multiplying by 100. As shown by the following equations:
AF transfer=((AF excretion)/(AF intake))x100........................(1)
Statistical analysis
Data were analyzed as a triplicated 5x5 Latin square design using JMP Pro software 11.0.0 (SAS Institute Inc., Cary, NC) following methods described by Littell et al. [44] for repeated measures. Aflatoxin M1 and vitamin concentrations from each treatment period were represented by milk samples collected on d 4 and 5. The mean values of DMI and milk yield from d1 through 5 were used to represent the experimental period. All AFM1, DMI, milk yield, milk composition, vitamin A, and riboflavin data are expressed as mean±standard error of the mean (SEM).
Means were separated for DMI, milk yield, milk composition, and feed composition using LSMEANS. A Tukey's test was used to assess differences between treatment means for AFM1 variables. Vitamin concentrations were analyzed using a oneway analysis of variance (ANOVA) to compare treatment groups by experimental period, followed by a Tukey's test to assess differences between treatment means.
Statistical significance for all treatment effects was declared at P≤0.05; trends are discussed at P≤0.15. All mean results are presented as least square means±the largest standard error of the mean unless stated otherwise.
Results
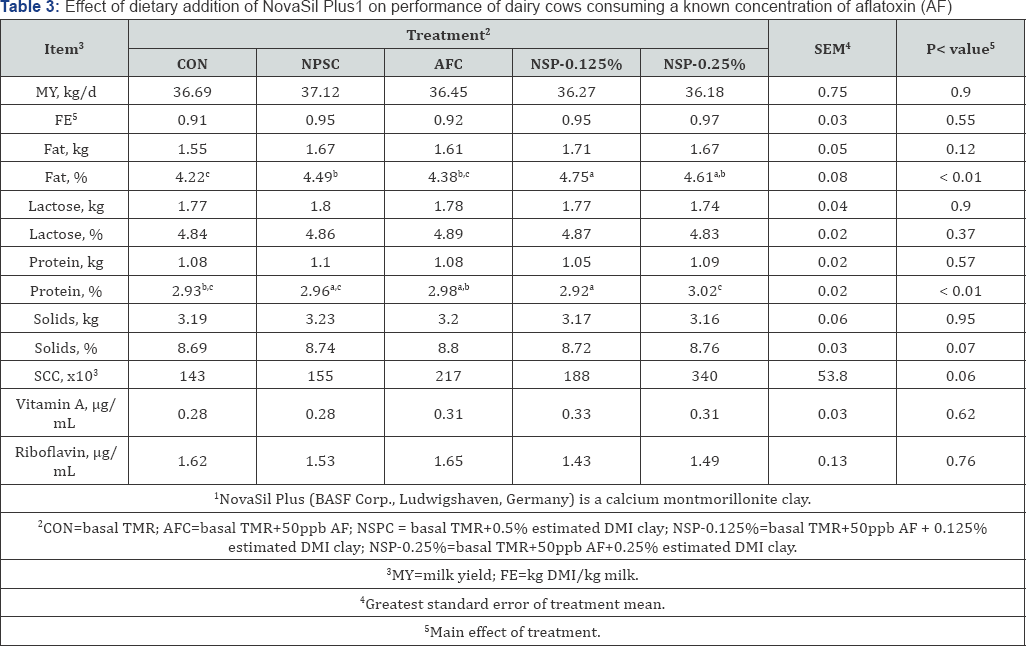
Diet composition averaged 56.3% DM, 17.2% CP, 7.54% Ash, 34.8% NDF, and 18.3% ADF (Table 1). Dry matter intake and nutrient intake did not differ among the 5 dietary treatment groups (P > 0.05), averaging 33.52 kg/d and 20.72 kg/d, respectively (Table 2). Data on milk performance are presented in Table 3. Milk yield and FE were similar throughout treatments, and no treatment effects were observed for fat yield, lactose, protein yield, solids, or SCC. Milk fat content was 4.22, 4.49, 4.38, 4.75, and 4.61 (%) for CON, NSPC, AFC, NSP-0.125%, and NSP-0.25%, respectively. Milk protein content was 2.93, 2.96, 2.98, 2.92, and 3.02 for CON, NSPC, AFC, NSP-0.125%, and NSP- 0.25%, respectively. Furthermore, vitamin A and riboflavin concentration in milk were similar across the 5 treatment groups and average, 0.30 0.03 and 1.54±0.13 g/mL, respectively.
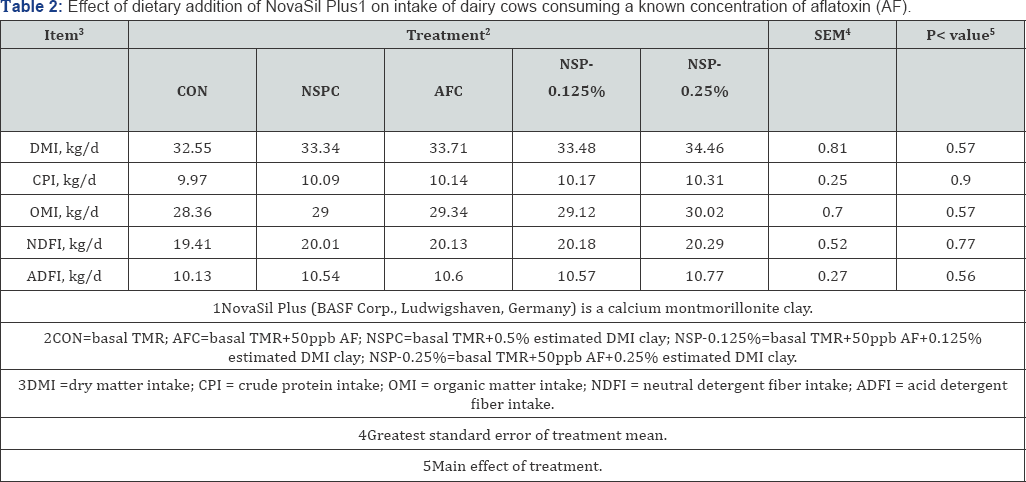
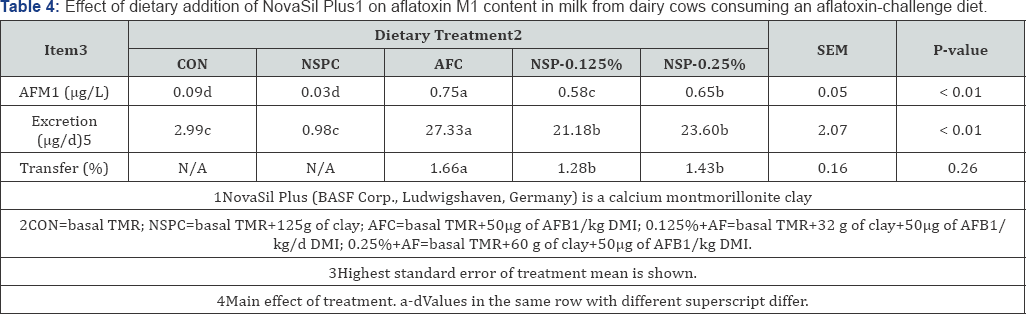
Data for AFM1 content in milk are presented in Table 4; a dose dependent reduction (P<0.01) in concentration of AFM1 in milk was observed with the inclusion of low concentrations of NSP. Feeding AFC diet resulted in 0.75±0.02 µg/L AFM1in milk; this value was reduced by 117.3% (0.62±0.02µg/L) with the inclusion of NSP at 0.125% of DMI and by 22.7% (21.3%(0.59±0.02µg/L) when NSP was fed at 0.25% of DMI. Specifically, the transfer rate from AFB1 intake to AFM1 excretion was reduced from 1.78% in the AF diet treatment to 1.50% and 1.46±0.16% with the inclusion of NSP at 0.125% and 0.25% of DMI, respectively.
Due to a reduced transfer rate, the total excretion of AFM1 was also reduced (P<0.01) in a dose dependent manner. Cows that consumed AFC excreted AFM1 at 29.45µg/d, whereas cows consuming the NSP-0.125% and NSP-0.25% excreted AFM1 at 24.69µg/d and 23.89±2.07µg/d; equivalent to 16.1% and 19.0% reduction, respectively.
Discussion
Excretion of AFM1 in bovine milk occurs when AF- contaminated feed is consumed by lactating dairy cows, resulting in increased risk of exposure to contaminated milk and dairy products. Therefore, reducing the carryover of AF into milk through the inclusion of a toxin adsorbent, such as NSP, is an effective way to reduce AFM1 content in milk. This study investigated the effects of NSP on milk yield and composition from lactating dairy cows fed an AF-contaminated diet and indicated that doses as low as 0.125 and 0.25% significantly decreased the AFM1 concentrations in milk without affecting milk quality and composition.
Cows did not demonstrate any abnormal behavior or clinical signs that would be associated with aflatoxicosis. Dry matter intake, milk yield, vitamin A, and riboflavin across the entire experimental period were similar among dietary treatments (P>0.05). These results are consistent with the previous work at Tarleton State University [35] and the University of Georgia [36] with the exception of milk composition, including milk fat and protein reported from previous studies [25]; [35,36]. Queiroz et al. [22] reported a suppression of milk fat yield and protein percent in animals consuming feed contaminated with 75 ppb AFB1, however there were no differences in animals treated with a clay additive compared to control animals. This differs from the current results showing an increase in milk protein content in NSP-0.125% and an increase in milk fat content in NSP-0.25% cows compared to CON cows. The tendency for increase in milk solids content can most likely be attributed to the increase in fat and protein content. In addition, the tendency for an increase in SCC is possibly due to cow variation and normal incidence of disease in the herd. Animals consuming NSP-0.25% diets tended to have increased SCC; however NSPC cows were similar to CON and AFC cows were similar across all treatments.
The tendency for this increase does not appear to be attributed to AF or inclusion of NSP in the diets of lactating cows. This work also agrees with previous studies in other animals and humans showing that ingestion of NS and similar clays, at concentrations ranging from 2.5g/kg to 20.0g/kg of the diet, did not interfere with serum vitamins and minerals Afriyie-Gyawu et al. [45]; [31,28]. Maki et al. [35] also investigated mineral content in milk from animals that were treated with NSP at concentrations as great as 1.0% w/w and showed no difference among treatments.
Novasil Plus has been reported to effectively sorb AFB1at pH 6.5 and bind it to active surfaces within its interlayer pores in vitro Marroquin-Cardona et al. [46]. This pH is close to the mean ruminal pH of dairy cows. Because of this, the significant reduction (P<0.01) in AF transfer to milk may be explained by binding and sequestration of AF in the rumen, resulting in decreased bioavailability and transfer to the milk. It is important to note that NSP was still active as a binder for aflatoxins at the very low inclusion rate of 0.125%. This is the first report of efficacy of NSP clay at these low concentrations.
The transfer rates in this study were similar to transfer rates reported for dairy cows consuming AF contaminated diets Harvey et al. [16]; Xiong et al. [47]. It is important to note that background AFM1 concentrations were detected in this study (and in similar studies in Texas and Georgia) in milk from cows consuming control diets. This finding confirms the presence of naturally occurring AF in the basal TMR and further supports the critical need for practical strategies to more effectively mitigate this toxin in milk.
As part of the multistate dairy project, in recent studies in Texas and Georgia, NSP was introduced at doses of 0.5% (w/w) and 1.0% (w/w) in the feed. The study in Texas Maki et al. [35] resulted in a reduction of AFM1 by 47.3% and 70.9% when NSP was included in the diet at 0.5% and 1.0%, respectively Likewise, the study in Georgia Maki et al. [36] demonstrated a similar reduction of AFM1 by 55.3% and 68.2% when NSP was included in the diet at 0.5% and 1.0%, respectively. When the data from these independent studies is combined with data from the current study in Mississippi, a dose-dependent, decrease in AFM1 in milk is observed in a linear manner. This correlation is illustrated in Figure 1 with an R2=0.8914. The equation is represented as:
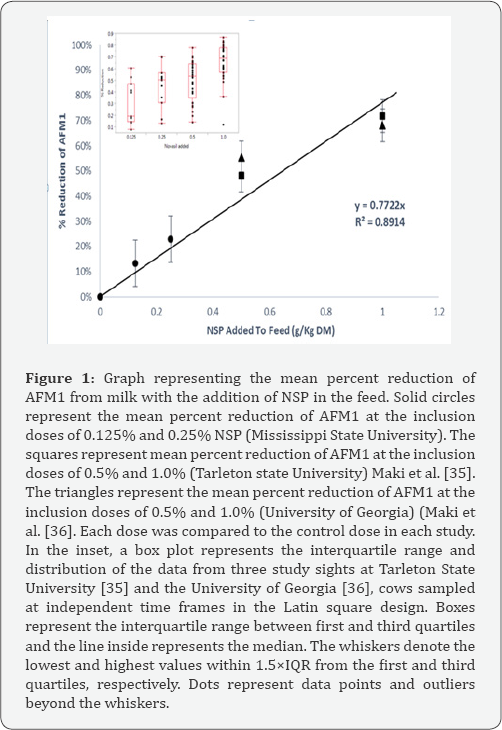
y=0.7722x..............(2)
Where y = percent reduction of AFM1 in milk and x = NSP added to the feed at a w/w ratio
Based on the association between dose of NSP and the percentage reduction of aflatoxin in milk, it is possible to use this algorithm to derive an estimate of the amount of clay inclusion needed to maintain a concentration below 0.5ppb. This equation, does not take into account other potentially confounding factors that may affect AFM1 transfer, such as DIM, milk yield, breed, or TMR. However, the association is strengthened by the fact that it was derived from different dairy cows on different diets at different research sites in different states at different times. Only the sources of clay and aflatoxin were the same.
Each of these three studies was performed using the same 5x5 design, but allowed the dairies to utilize their normal routines without the inconvenience of additional equipment or tasks. This suggests that similar feed treatments may be successfully employed at other dairies without the need for expensive equipment or special circumstances. The viability of these results is reflected in its ability to reduce the concentration (50-100ppb) of AF, which may allow the dairy industry to intervene in times of drought when AF in feed can frequently exceed 20ppb. Inclusion of clay can decrease potential adverse effects in cows and reduce the carryover of toxins into the milk.
Feed contaminated with AF is of special concern in dairy animals due to the inherent risk of increased AFM1 in dairy products intended for human consumption. The current data demonstrates that feeding NSP is a safe and effective strategy to reduce AFM1 in milk. NovaSil Plus did not affect milk quality and composition when included at 12.1g/kg and 6.0g/kg of DM in contaminated feed.
Conclusion
This work indicates that NSP clay was able to significantly decrease AFM1 concentrations in milk at doses lower than 0.5% (the lowest dose tested prior to this study). Importantly, the efficacy and safety of NSP was consistent throughout recent studies despite the differences in cows, feed, and location. NovaSil Plus reduced the concentration of AFM1 even when fed at the smallest dose. When all studies were compared, NSP resulted in a linear decrease in AFM1 ranging from 13% (at the smallest dose of clay) to 71% (at the greatest dose of clay). At all doses, DMI, milk yield, milk composition, minerals, vitamin A, and riboflavin concentrations were unaffected by the various dietary treatments.
NovaSil Plus has favorable characteristics for AFB1 sorption as well as negligible interactions with nutrients. The inclusion of NSP in contaminated dairy feeds may help to mitigate AF problems without affecting milk production or composition. Importantly, the results of this study will aid in determining the appropriate dosage of NSP needed to decrease AFM1 below allowable concentrations.
Acknowledgment
This work was supported by funding through the Aflatoxin Mitigation Center of Excellence Research Program (National Corn Growers Association), M1403049.
References
- Kurtzman CP, Horn BW, Hesseltine CW (1987) Aspergillus-Nomius, a New Aflatoxin-Producing Species Related to Aspergillus-Flavus and Aspergillus-Tamarii. A Van Leeuw J Microb 53(3): 147-158.
- Linsell CA, Peers FG (1977) Aflatoxin and Liver-Cell Cancer. T Roy Soc Trop Med H 71(6): 471-473.
- Peers F, Bosch X, Kaldor J, Linsell A, Pluijmen M (1987) Aflatoxin Exposure, Hepatitis-B Virus-Infection and Liver-Cancer in Swaziland. Int J Cancer 39(5): 545-553.
- Binder, E. & Krska, R. (2012) Romer labs guide to mycotoxins. Leicester: Anytime Publishing Services, UK.
- Food and Drug Administration (2012) Pathogenic Escherichia coli group , In: Keith A (Eds.), Lampel Bad bug book, foodborne pathogenic microorganisms and natural toxins, (2nd edn), pp. 1-292.
- World Health Organization & International Agency for Research on Cancer (1993) Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monographs 56: pp. 599.
- Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, et al. (2004) Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr 80(5): 1106-1122.
- Van Egmond HP, Eaton D, Groopman J (1994) Aflatoxins in milk. Academic Press, USA.
- Cotty PJ, Jaime-Garcia R (2007) Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int J Food Microbiol 119(1-2): 109-115.
- Cavallarin L, Tabacco E, Antoniazzi S, Borreani G (2011) Aflatoxin accumulation in whole crop maize silage as a result of aerobic exposure. J Sci Food Agr 91(13): 2419-2425.
- Stoloff L, Trucksess M, Hardin N, Francis OJ, Hayes JR, et al. (1975) Stability of Aflatoxin-M in Milk. J Dairy Sci 58(12): 1789-1793.
- Stoloff L, Trucksess MW (1981) Effect of Boiling, Frying, and Baking on Recovery of Aflatoxin from Naturally Contaminated Corn Grits or Cornmeal. J Assoc Off Ana Chem 64(3): 678-680.
- Yousef A, Marth E (1989) Stability and degradation of aflatoxin M1. Mycotoxins in dairy products: 127-161.
- Dickens J, Whitaker T (1975) Efficacy of Electronic Color Sorting and Hand Picking to Remove Aflatoxin Contaminated Kernels from Commercial Lots of Shelled Peanuts 1. Peanut Science 2(2): 45-50.
- Henderson JC, Kreutzer SH, Schmidt AA, Smith CA, Hagen WR (1989) Flotation separation of aflatoxin-contaminated grain or nuts. Google Patents.
- Yazdanpanah H, Mohammadi T, Abouhossain G, Cheraghali AM (2005) Effect of roasting on degradation of aflatoxins in contaminated pistachio nuts. Food and Chemical Toxicology 43(7): 1135-1139.
- Bagley E (1978) Detoxification of aflatoxin-contaminated corn by roasting. Cereal Chemistry, USA.
- Allameh A, Safamehr A, Mirhadi SA, Shivazad M, Razzaghi-Abyaneh, M et al. (2005) Evaluation of biochemical and production parameters of broiler chicks fed ammonia treated aflatoxin contaminated maize grains. Animal feed science and technology 122(3): 289-301.
- McKenzie KS, Sarr AB, Mayura K, Bailey RH, Miller DR, et al. (1997) Oxidative degradation and detoxification of mycotoxins using a novel source of ozone. Food and Chemical Toxicology 35(8): 807-820.
- Diaz DE, Hagler WM, Blackwelder JT, Eve JA, Hopkins BA, et al. (2004) Aflatoxin binders II: Reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia 157(2): 233-241.?
- Firmin S, Morgavi DP, Yiannikouris A, Boudra H (2011) Effectiveness of modified yeast cell wall extracts to reduce aflatoxin B1 absorption in dairy ewes. J Dairy Sci 94(11): 5611-5619.
- Queiroz OC, Han JH, Staples CR, Adesogan AT (2012) Effect of adding a mycotoxin-sequestering agent on milk aflatoxin M 1 concentration and the performance and immune response of dairy cattle fed an aflatoxin B 1-contaminated diet. J Dairy Sci 95(10): 5901-5908.
- Huwig A, Freimund S, Käppeli O, Dutler H. (2001) Mycotoxin detoxication of animal feed by different adsorbents. Toxicology letters 122(2): 179-188.
- Harvey RB, Kubena LF, Elissalde MH, Corrier DE, Phillips TD (1994) Comparison of two hydrated sodium calcium aluminosilicate compounds to experimentally protect growing barrows from aflatoxicosis. J Vet Diagn Invest 6(1): 88-92.
- Kutz RE, Sampson JD, Pompeu LB, Ledoux DR, Spain JN, et al. (2009) Efficacy of Solis, NovasilPlus, and MTB-100 to reduce aflatoxin M-1 levels in milk of early to mid lactation dairy cows fed aflatoxin B-1. J Dairy Sci 92(8): 3959-3963.
- Smith EE, Phillips TD, Ellis JA, Harvey RB, Kubena LF, et al. (1994) Dietary Hydrated Sodium-Calcium Aluminosilicate Reduction of Aflatoxin M(1) Residue in Dairy Goat Milk and Effects on Milk- Production and Components. J Anim Sci 72(3): 677-682.
- Phillips TD (1999) Dietary clay in the chemoprevention of aflatoxin- induced disease. Toxicol Sci 52(2): 118-126.
- Phillips TD, Kubena LF, Harvey RB, Taylor DR, Heidelbaugh ND (1988) Hydrated sodium calcium aluminosilicate: a high affinity sorbent for aflatoxin. Poult Sci 67(2): 243-247.
- Phillips TD, Lemke SL, Grant PG (2002) Characterization of clay-based enterosorbents for the prevention of aflatoxicosis. Adv Exp Med Biol 504: 157-171.
- Afriyie-Gyawu E, Ankrah NA, Huebner HJ, Ofosuhene M, Kumi J, et al. (2008a) NovaSil clay intervention in Ghanaians at high risk for aflatoxicosis. I. Study design and clinical outcomes. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25(1): 76-87.
- Afriyie-Gyawu E1, Wang Z, Ankrah NA, Xu L, Johnson NM, et al. (2008b) NovaSil clay does not affect the concentrations of vitamins A and E and nutrient minerals in serum samples from Ghanaians at high risk for aflatoxicosis. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25(7): 872-884.
- Mitchell NJ, Kumi J, Aleser M, Elmore SE, Rychlik KA, et al. (2014) ShortTerm Safety and Efficacy of Calcium Montmorillonite Clay (UPSN) in Children. Am J Trop Med Hyg 91(4): 777-785.
- Phillips TD, Afriyie-Gyawu E, Williams J, Huebner H, Ankrah NA, et al. (2008) Reducing human exposure to aflatoxin through the use of clay:A review. Food Addit Contam 25(2): 134-145.
- Harvey RB, Phillips TD, Ellis JA, Kubena LF, Huff WE, et al. (1991) Effects on aflatoxin M1 residues in milk by addition of hydrated sodium calcium aluminosilicate to aflatoxin-contaminated diets of dairy cows. Am J Vet Res 52(9): 1556-1559.
- Maki CR, Thomas AD, Elmore SE, Romoser AA, Harvey RB, et al. (2016b). Effects of calcium montmorillonite clay and aflatoxin exposure on dry matter intake, milk production, and milk composition. J Dairy Sci 99(2): 1039-1046.
- Maki CR, Monteiro APA, Elmore SE, Tao S, Bernard JK, et al. (2016a) Calcium montmorillonite clay in dairy feed reduces aflatoxin concentrations in milk without interfering with milk quality, composition or yield. Animal Feed Science and Technology 214: 130135.
- Shotwell OL, Hesseltine CW, Stubblefield RD, Sorenson WG (1966) Production of Aflatoxin on Rice. Appl Microbiol 14(3): 425-428.
- West S, Wyatt RD, Hamilton PB (1973) Improved Yield of Aflatoxin by Incremental Increases of Temperature. Appl Microbiol 25(6): 10181019.
- AOAC International (2009) Official Methods of Analysis. AOAC Int., USA.
- AOAC International (2000) Official methods of analysis. Association of Analytical Communities. USA.
- Jakobsen J (2008) Optimisation of the determination of thiamin, 2-(1-hydroxyethyl)thiamin, and riboflavin in food samples by use of HPLC. Food Chem 106(3): 1209-1217.
- Ndaw S, Bergaentzle M, Aoude-Werner D, Hasselmann C (2000) Extraction procedures for the liquid chromatographic determination of thiamin, riboflavin and vitamin B-6 in foodstuffs. Food Chem 71(1): 129-138.
- Warth B, Sulyok M, Fruhmann P, Mikula H, Berthiller F, et al. (2012) Development and validation of a rapid multi-biomarker liquid chromatography/tandem mass spectrometry method to assess human exposure to mycotoxins. Rapid Commun Mass Sp 26(13): 1533-1540.
- Littell RC, Henry PR, Ammerman CB (1998) Statistical analysis of repeated measures data using SAS procedures. J Anim Sci 76(4): 12161231.
- Afriyie-Gyawu E1, Mackie J, Dash B, Wiles M, Taylor J et al. (2005) Chronic toxicological evaluation of dietary NovaSil clay in Sprague- Dawley rats. Food Addit Contam 22(3): 259-269.
- Marroquín-Cardona A, Deng Y, Garcia-Mazcorro J, Johnson NM, Mitchell N, et al. (2011) Characterization and safety of uniform particle size NovaSil clay as a potential aflatoxin enterosorbent. Appl Clay Sci 54(3- 4): 248-257.
- Xiong JL, Wang YM, Nennich TD, Li Y, Liu JX (2015) Transfer of dietary aflatoxin B1 to milk aflatoxin M1 and effect of inclusion of adsorbent in the diet of dairy cows. J Dairy Sci 98(4): 2545-2554.






























