Trade-Off between Lactation Effort and Gastrointestinal Nematode Infection in a Resistant and a Susceptible Breed of Domestic Sheep
Lucas Gruner 1, Guy Bechet2 and Jacques Cabaret1*
1INRA and F Rabelais University, France
2INRA, Unité Mixte de recherche sur les Herbivores, France
Submission: March 15, 2017; Published: April 28, 2017
*Corresponding author: Jacques Cabaret, NRA and F Rabelais University, France, Email: jacques.cabaret@inra.fr/ jcabaret37@gmail.com
How to cite this article: Lucas G, Guy B, Jacques C. Trade-Off between Lactation Effort and Gastrointestinal Nematode Infection in a Resistant and a Susceptible Breed of Domestic Sheep. Dairy and Vet Sci J. 2017; 2(2): 555584. DOI: 10.19080/JDVS.2017.02.555584
Abstract
The effect of natural infection by digestive-tract nematodes in two breed of ewes-susceptible Romanov and resistant Merinos d'Arles, were studied in relation to lactation status (dry, lactating one or two lambs). Lactating one lamb is the common situation for Mérinos d'Arles whereas Romanov usually lactates twins. Lactation is one of the most demanding in terms of protein (and energy) among the reproduction traits. Mérinos d'Arles are less infected than Romanov when not lactating as previously recorded. Merinos d'Arles are as infected as Romanov when lactating one lamb. Merinos d'Arles lactating two lambs are more infected the first month of lactation than Romanov ewes with two lambs. The two breeds had similar infection at the end of lactation. Modifications in grass intake explain in part this increasing nematode infection of the resistant Merinos d'Arles as the lactation effort increases whereas no obvious difference was observed in the susceptible and prolific Romanov in relation to lactation status. It is the first report on the interaction between feeding (here grazing), genetic susceptibility to nematode infection and lactation status (breeding one or two lambs) in natural conditions.
Keywords: Ewe; Lactation; Grass intake; Parasitic nematode
Introduction
Gastrointestinal nematodes are present in all grazing ruminants. The hosts become infected by ingesting infective larvae on herbage; the larvae develops into male and female adults in the digestive-tract in three weeks, female lay eggs which are shed in faeces. These eggs will develop into infective larvae on pastures in two or three weeks depending on climatic conditions. The intensity of infection is often evaluated on the eggs found in faeces, as it is statistically a good reflect of the actual infection [1]. It has been shown that nematode infection in sheep may affect adversely reproduction performances in bighorn sheep [2] and domestic sheep [3,4]. Many ovine studies indicate that protein supply is a key determinant of the ability to mount a strong immunity associated protection to gastrointestinal nematodes. Infection may result in a loss of 12g protein per day. This loss from the infection may account for about 0.71-, 0.77-fold the protein requirement for pregnancy and lactation, respectively, and creates strong competition for protein [5]. Energy requirements are also important: lactation accounted for over 50% of the daily energy requirements in Corriedale ewes on the Peruvian Puna [6]. A possible ordering of the priorities given by a reproducing animal to its various body functions when partitioning a scarce food resource was proposed from highest to lowest priority [7]. Maintenance of body protein, reproductive effort, expression of immunity and attainment of desired fatness. The total demand cannot be met by pastures during the reproductive season in most Mediterranean environments, and it may be nutrient deficiency relative to the demand of sheep, which may result in weak protection against parasites. This weaker protection can act on establishment of new infective larvae and hence on the egg-laying capacity of nematodes. It is a documented phenomenon that in lactating ewes the egg excretion of parasitic nematodes increases [8,9]. It is one of the most important seasonal variation in the nematode egg output, responsible of the infection of the pastures, and, consequently, of the lambs. The proposed explanation is a relaxation of the resistance of the ewe during the first weeks of lactation [10] permitting the growth of the existing worms, the facilitated establishment of newly acquired ones, and possibly an increase of worm fecundity.
Lactation effort is different in ewes with single or twin suckling lambs. The lactation effort may have a different impact on ewes depending on their fertility: it was shown in field conditions, that dry Mérinos d'Arles ewes had lower faecal egg excretions than dry Romanov ewes, but the difference was inverted in lactating ewes with two suckling lambs [11]. The differences recorded could be ascribed in dry ewes to breed susceptibility to infection [12] whereas the differences shown in lactating ewes could be due to better resilience to the lactation effort in the more prolific breed (Romanov) or to differences in infective larvae ingestion in relation to modified grazing behaviour The aim of present work was to explore the interaction between breed and lactation effort on nematode egg excretion with a study of grazing behaviour.
Material and Methods
Experimental animals and pastures Figure 1
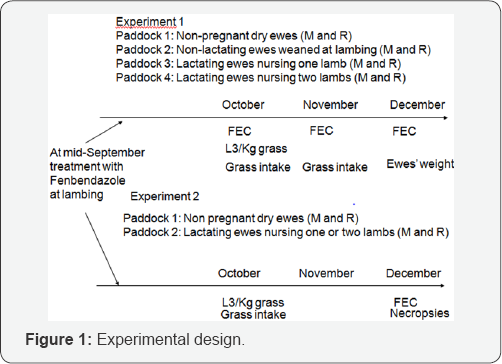
A flock composed of Romanov (R), Mérinos d'Arles (M) ewes spent summertime on Alpine pastures. They returned for lambing at the beginning of September in the low lands (Experimental farm of Le Merle, south of France). Ewes were allocated into different flocks. On the first experiment, four flocks were constituted:
- 43 dry non pregnant ewes (M and R).
- 25 non lactating ewes weaned at lambing (M).
- 42 lactating ewes suckling one lamb (M and R).
- 31 lactating ewes with twins (M and R).
All ewes were treated with an anthelmintic (fenbendazole) at lambing, in order to commence the experiment with noninfected hosts. Each flock grazed its own irrigated pastures at the same stocking rate from mid-September to end of November These pastures were grazed during the preceding months by a contaminating flock, and 6 tracer lambs of the same breed (M) were added to each flock to compare the level of infection between them. In the second experiment, two flocks composed of M and R ewes were constituted one with 51 non lactating dry ewes and the other with 69 lactating ewes with one (44) or two lambs (25 ewes). All ewes were regularly weighed in order to evaluate indirectly the lactation effort. They were grazed from mid September to the end of November on irrigated pastures. The botanical composition of the pastures (in dry matter) was distributed into gramineous plants 55-64%, Plantago lanceolatum 7-10%, clover (Trifolium spp.) 9-17% and dandelion (Taraxacum dens-leonis) 15-19%; the amount of available grass in October was about 1.4-1.5 tons dry matter per hectare at the entrance of the flocks on the paddocks. Pastures were flooded a night every 2-3 weeks in spring, summer and autumn.
Parasitological measures
Individual egg counts were performed on each ewe and tracer lambs on 3 occasions in the first experiment (October, November and December, reflecting 4, 6 and 10 weeks of grazing duration, respectively), and on one occasion at the end of grazing season in the second experiment (December), reflecting 10 weeks of grazing time. Group faecal cultures were assessed for each group of ewes (breed x flock) to identify the nematode genera that were present. The availability of infective larvae (L3) on the grass within a day was established in October in each experiment on a part of one paddock (2000 square meters) grazed by the lactating ewes. Herbage was sampled by picking 200 pinches of grass three times every three hours during three consecutive days. Herbage was soaked during 24 hours in water before sieving [13], and L3 retained on the sieved identified and counted after centrifugal-floatation in magnesium sulphate. Nematode species were identified and counted on necropsied ewes (6M and 6R) on December (experiment 2).
Feeding behaviour
Visual observations. On two occasions in the first experiment (mid and end of grazing period), 16 ewes (8 R and 8 M) were marked with a large number painted on each side in dry ewes flock and in lactating ewes with twins flock. The grazed paddocks were divided in 10 to 12 squared with sticks. Every 30 mn during the daylight of two consecutive days, the location of the marked ewes was noted on the map of the paddock as well as their behaviour (grazing, walking, standing up but inactive, and resting). The between breed distribution of the observations of the grazing ewes was studied in space (in terms of distance to the resting area on the paddock) and time (duration of grazing). In addition, the number of prehensile bites per min. was estimated by observing four to five grazing M and R ewes in each dry and twin-suckling ewes flocks and counting the head movements in 4-6 periods of more than one minute.
Automatic recording. Jaw movements of several ewes from each group (3R and 3M dry and lactating ewes with twins) were recorded during 4 consecutive days twice in the first experiment and once in the second experiment (only in lactating ewes) using electronic recorders. The recorders were made of an elastic conductor surrounding ewes' mouths and whose resistance varied when elongated by spacing of jaws. Each 2.5 seconds, elementary data were stored in a RAM memory: 0 if the animal had kept its mouth closed during the previous time unit; 1 if it had opened it once or more. The memory was emptied each fourth day on a computer, data were analysed and transformed in terms of feeding activity [14]. The parameters used for the description of the animals' feeding behaviour were the duration of meals or rumination periods and the time spent grazing or ruminating. The true duration of intake was the duration of meals in minutes excluding the pauses.
Statistical analysis
Faecal egg counts were log-transformed (log egg count + 1) to stabilise the variance. General Linear Model procedure of the SAS library (SAS, Cary, USA) was used to analyse the fixed effect of factors as breed, flock, number of suckled lambs, age (in 5 classes of years). The weight of ewes were analysed with the same procedure.
Results
Nematode community
The gastro-intestinal nematodes were identified to species on 12 ewes (6M and 6R) that were necropsied at the end of the grazing season in December in the twin reared groups: no significant difference in the proportions of species were evidenced between the two breeds. The following species were found: Trichostrongylus vitrinus (48% of the worms), T. colubriformis (30.5%), Teladorsagia circumcincta (18%), Nematodirus spp. (2.1%; N. spathiger, N. filicollis, N. chabaudi and N. abnormalis), and Chabertia ovina, Oesophagostomum venulosum and Trichuris ovis being recovered at a lower frequency.
Merinos of Arles non-lactating and non pregnant are equivalent to non-lactating after lambing
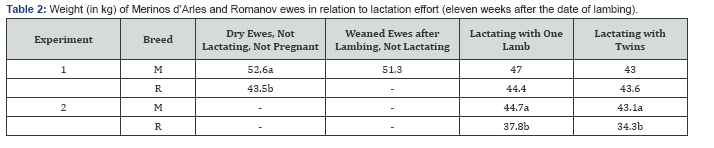
Ewes that were pregnant and from whom the lambs were taken away at birth (“weaned” ewes) were very similar to the ewes that were not pregnant and not lactating (“dry” ewes) in faecal egg counts during 3 months of grazing in autumn (Table 1). They also reached a similar weight at the end of grazing season (Table 2), and the increase from four weeks to 11 weeks post-lambing date were similar: 3.5 and 4.9%, respectively.
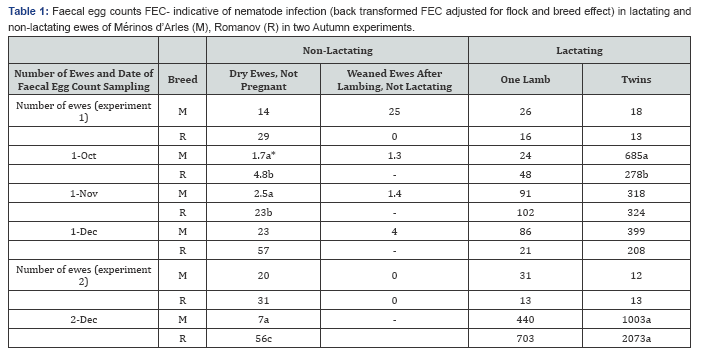
Mérinos d'Arles are less infected than Romanov when not lactating
The FEC were significantly lower in Mérinos d'Arles, for all periods and both experiments (Table 1). The weight after 11 weeks grazing were higher in Merinos d'Arles (Table 2) as it is a larger breed, but the weight increases 3.5 vs 2.4%, respectively, were not significantly different. Merinos d'Arles are as infected as Romanov when lactating one lamb.
No significant differences were seen in both experiments and periods of samplings (Table 1). The weight of Romanov ewes were similar to Mérinos d'Arles in the first experiment and smaller in the second one (Table 2). The weight increase was higher for Romanov ewes (3.0 vs 0.6%).
Merinos d’Arles lactating two lambs are more infected the first month of lactation than Romanov ewes with two lambs
The FEC were significantly higher only in the October sampling corresponding to the first month of lactation. The Romanov ewes remained significantly smaller than Merinos d'Arles, but the weight decreased from 4 to 10th week after lambing by 0.2 and 3.7%, respectively.
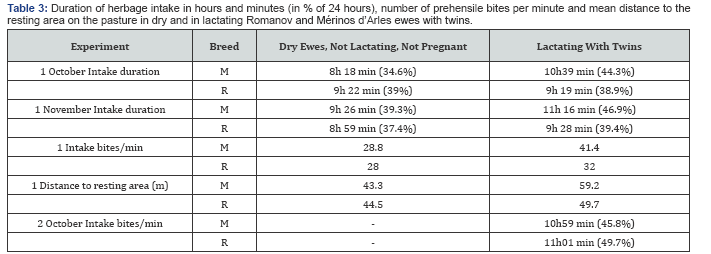
Modifications in grazing behaviour in relation to breed and lactation status
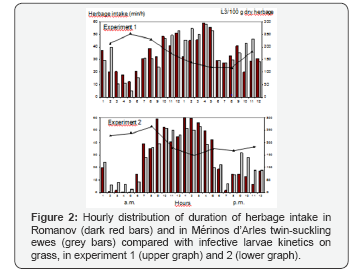
The modification were much more important in Mérinos d'Arles than in Romanov when dry ewes were compared with ewes breeding two lambs (Table 3). Merinos, when confronted with lactating two lambs increased its intake duration, the number of bites /min and the distance to resting area. By comparing the hourly distribution of the herbage intake between M and R twins-suckling ewes (Figure 2), M ones grazed more time during the night, hours during which the level of infective larvae populations on the grass was a little higher If we consider that ewes ingested 1kg dry matter of grass in 24 hours (Lapeyronie et al. 1989), this corresponded to intake of 1775 and 1819 larvae for R and M twin-suckling ewes respectively when taking into account the hourly variations in experiment 1-October. In experiment 2- October, these differences were even smaller
Discussion
The intensity of infection of the different flocks increased during autumn grazing as previously observed in several Mediterranean areas. The species richness was relatively high as already noted in these irrigated pastures [11]. The situation of the two years of experiment was apparently within the usual frame of previous digestive-tract infections.
Life-history traits of host may change under the parasitic infection [15], even with sublethal parasites such gastrointestinal nematodes (they do not cause outright the death of their host). The processes of feeding, growth and reproduction are closely interrelated, and many trade-off may be investigated, in particular in wild animals. These trade-off are somewhat limited in domestic animals as feeding is under breeder control, The host, according to the dynamic energy budgets [16] can only distribute the reserves into a fixed fraction into somatic maintenance (growth) and maturation-reproduction (maturity maintenance). This fixed fraction may be very different in a susceptible or a resistant breed of sheep, since we may think that the latter will invest more in somatic maintenance. It may even differ within a host population, up to the point of invalidating more general conclusions (see [16] in mice and [2] in bighorn sheep) Digestive-tract nematodes interfere clearly with reproduction success in domestic sheep [3] although it is difficult to partition effect between gestation and lactation effort [17,18].
In our conditions gestation was apparently poorly influent on infection, at least in the resistant breed Merinos d'Arles. This may be explain as protein requirements in sheep are not substantially increased during gestation (0.69-fold) whereas the increase is substantial in lactating animals (2.59-fold) compared to dry ewes [5]. The most striking result was the infection of ewes in relation to absence of lactation, lactating one or two lambs in Mérinos d'Arles: it was less, equivalently, and more infected than Romanov , respectively. The explanation can be found in part in grass intake. Merino twin-suckling ewes spent 140 min more ingesting grass than dry ones, at their 40th lactating day, data which correspond to the 130 min found in Ile de France ewes [19]. A similar increase (112 min) was found Ile de France twin- suckling ewes compared to single-suckling ones [14]. This intake compensation not only concerned the duration but also the speed in bites per minute [20]. By comparing Merino ewes with twins to dry ones, the intake duration increased by 128.3%, the number of bites/min by 143.8%, so the total intake increased by 184.5 %, which corresponded to other estimate [18] (1.77 kg dry matter of grass per day on day 29 of lactation compared with 1 kg for dry Merino ewes) in the same type of pastures. With 1792 L3/kg dry herbage, the ewes with twins ingested 3306 L3/day whereas dry ewes would ingest only 1792 L3/day, this being due to the sole supplementary intake. The comparison of the hourly durations of herbage ingestion and the kinetics of populations of infective larvae on the grass explained an extremely small part of the difference of ingestion between Mérinos d'Arles and Romanov., and the key factor explaining higher infection in twin-lambs Mérinos d'Arles is the increase of grass intake and hence larval ingestion. The cost of lactation in the infection resistant breed Mérinos d'Arles, is increasing gradually with lactation effort (see data of ewes with one or two lambs); this cost is particularly high when Mérinos d'Arles breed two lambs (which is not very common), whereas the cost seems inexistent in Romanov (which is a prolific breed with usually two lambs).
Our work shows that there is a strong interaction between lactating effort and infection of ewes by a sub lethal internal parasite, mostly dependant on grass intake. Life-traits are not independent and it would be interesting to evaluate other traits such as morbidity and survival in ewes. The success of the offspring would also be a useful complement to lactation studies.
Acknowledgement
We are grateful to J. Cortet and C. Sauvé for their technical assistance and to P. Bosc and M. Vincent for the management of the flocks at the Le Merle experimental farm.
References
- Cabaret J, Gasnier N, Jacquiet P (1998) Faecal egg counts are representative of digestive-tract strongyle worm burdens in sheep and goats. Parasite 5(2): 137-142.
- Festa-Bianchet M (1989) Individual differences, parasites, and the cost of reproduction for Bighorn ewes (Ovis Canadensis). J Anim Ecol 58(3): 785-795.
- Pandey V S, Cabaret J, Fikri A (1984) The effect of strategic anthelmintic treatment on the breeding performance and survival of ewes naturally infected with gastro-intestinal strongyles and protostrongylids. Ann Rech Vet 15(4): 491-496.
- Ceyhan A, Moore K, Mrode R (2015) The estimation of (co)variance components growth, reproduction, carcass, FEC and FECN traits in Lleyn sheep 131: 29-34.
- Liu SM, Masters DG, Adams NR (2003) Potential impact of nematode parasitism on nutrient partitioning for wool production, growth and reproduction in sheep. Aust J Exp Agric 43: 1409-1417.
- Fierro LC, Bryant FC (1990) Grazing activity and bioenergetics of sheep on native range in southern Peru. Small Rum Res 3(2): 135-146.
- Coop RL, Kyriazakis I (1999) Nutrition-parasite-interaction. Vet Parasitol 84(3-4): 187-204.
- Michel JF (1974) The epidemiology and control of some nematode infections in grazing animals, In: Ben Dawes (Ed), Advances in Parasitology, Academic Press, London and New York, pp. 355-397.
- Courtney CH, Gessner R, Sholz SR, Loggins PE (1986) The periparturient rise in fecal egg counts in three strains of Florida Native ewes and its value in predicting resistance of lambs to Haemonchus contortus. Int J Parasitol 16(3): 185-189.
- Cizauskas CA, Turner WC, Pitts N, Getz WM (2015) Seasonal Patterns of Hormones, Macroparasites, and Microparasites in Wild African Ungulates: The Interplay among Stress, Reproduction, and Disease. PLOS One 10(4): e0120800.
- Gruner L, Bouix J, Cabaret J, Boulard C, Cortet J, et al. (1992) Effect of genetic type, lactation and management on helminth infection of ewes in an intensive grazing system on irrigated pasture. Int J Parasitol 22(7): 919-925.
- Gruner L (1991) Breeding for Helminth resistance in sheep and goats, In: Owen JB, Axford RFE (Eds.), Breeding for disease resistance in farm animals, CAB International, Wallingford, UK, pp. 187-200.
- Gruner L, Raynaud JP (1980) Lightened techniques for routine sampling of herbage for cattle so as to appreciate pasture contamination by nematode parasite larvae. 131(7): 521-529.
- Bechet G, Theriez M, Prache S (1989) Feeding behaviour of milk-fed lambs at pasture, Small Rumin. Res 2(2): 119-132.
- Hochberg ME, Michalakis Y, Meeus DT (1992) Parasitism as a constraint on the rate of life-history evolution. J Evol Biol 5: 491-504.
- Kooijman SA (2001) Quantitative aspects of metabolic organization: a discussion of concepts. Phil trans Royal Soc London B 356(1407): 331349.
- Kristan DM (2004) Intestinal nematode infection affects host life history and offspring susceptibility to parasitism. J Anim Ecol 73: 227238.
- Lapeyronie P, Béchet G, Molenat G, Vincent M (1989) Feeding behaviour of the Arles Merino breed when grazing irrigated pastures in Crau, In: Proceedings of the 15th International Grassland Congress, 4-11,Nice, France, pp. 1283-1284.
- Dulphy JP, Raymond B, Theriez M (1979) Ingestive behaviour and related activities in ruminants, Proc. 5th Int, Symp. Rumin. Physiol, Clermont-Ferrand, France, pp. 103-122.
- Roguet C, Prache S, Petit M (1998) Development of a methodology for studying feeding behaviour of grazing ewes. Appl Anim Behav Sci 55: 307-316.






























