Nutrition and Immune System in Livestock's: Mini Review
Bagath M1*, Sejian V1, Krishnan G1, Vidya MK1,2, Prathap Pragna1,3,4, Archana PR1,3,4 and Joy Aleena1,3,4
1ICAR-National Institute of Animal Nutrition and Physiology, India
2Karnataka Veterinary Animal and Fisheries Sciences University, India
3Kerala Veterinary and Animal Sciences University, India
4Academy of Climate Change Education and Research, India
Submission: January 30, 2017; Published: April 28, 2017
*Corresponding author: Bagath M, Animal Nutrition Division, National Institute of Animal Nutrition and Physiology, India, Tel: +91-9035865536; Fax: +91-080-25711420; Email: bbagath@gmail.com
How to cite this article: Bagath M, Sejian V, Krishnan G, Vidya M, Prathap P, Archana P, Joy A. Nutrition and Immune System in Livestock's: Mini Review. Dairy and Vet Sci J. 2017; 2(2): 555582. DOI: 10.19080/JDVS.2017.02.555582
Introduction
The relationship between nutrition and immunity are interlinked to each other that nutrient as a single or in a group affects immunity either direly or indirectly [1]. Directly nutrients affect the immune system by triggering the immune cell activation or altering immune cell interaction and indirectly by changing the substrates of DNA synthesis, changing, the physiological integrity of the cell, altering energy metabolism, or by altering the signals or hormones [2]. Specific nutrients like vitamins, minerals, antioxidants, etc. play a vital role in augmentation of immunity while deficiencies cause immune suppression [3]. The types of immunity, the role of different cells, their components, mechanism of action, measuring the immunity are described in brief to have a better understanding the importance of the relationship between nutrition and immunity.
Types of Immunity
The immune system has evolved over a period of time to protect the host against the invading pathogens like bacteria, virus, protozoa, or fungus. Both innate and adaptive immune systems are interlinked and work together to provide immunity. The innate immunity acts as fist line of defence followed by the action of adaptive immunity during the site of entry of the pathogen. The phagocytes, NK cells, and granulocytes play a major role in innate immunity, while the lymphocytes, antigen- presenting cells (APC) play an important role in adaptive immunity. Phagocytes APC functions act as a linkage between the innate and adaptive immunity by presenting the antigens to the lymphocytes [4].
Innate immunity
The innate immune system is the primitive of the two immunities and is capable of destroying the pathogens non-specifically. This immunity is present in vertebrates, invertebrates, and plants also [5].
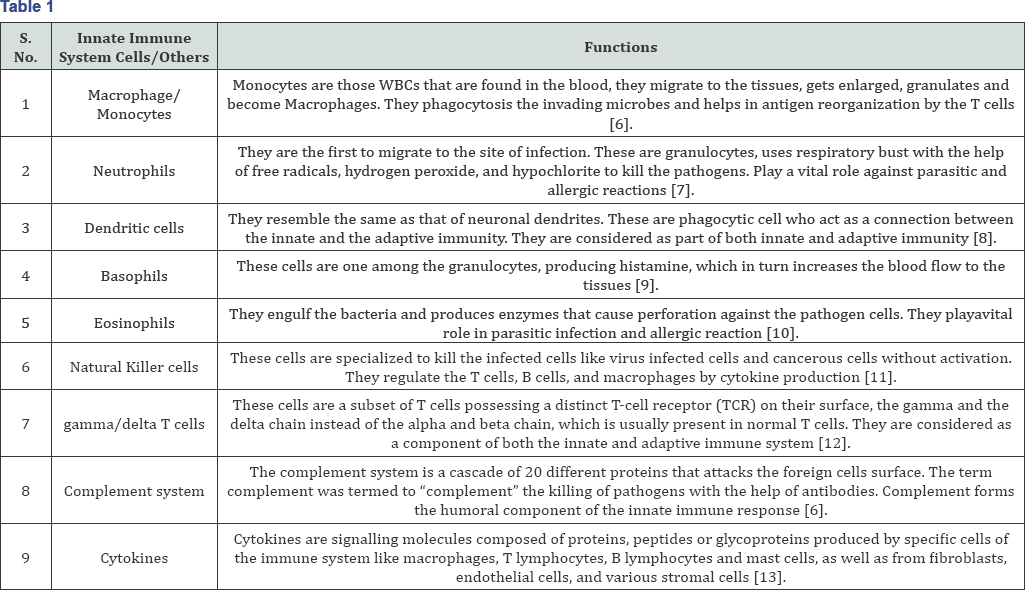
The innate cells includes the NK cells, mast cells, basophils, eosinophil, and the phagocytic cells including the macrophages, neutrophils, and dendritic cells while complement system and cytokines from other components of innate immunity and function within the immune system by identifying and by eliminating the pathogens that might cause infection [6]. Monocytes, macrophages, and neutrophils form the primary component of the innate immune system, in addition, the skin barrier, the mucous secretion etc. come under this immune system (Table 1) [14].
Adaptive/Acquired immune system
Adaptive immunity is the second line of defence which develops once antigen is presented to both T and B cells. Both T and B cells play a vital role in adoptive/acquired immune system, where B-cell plays a vital role in humoral immune system whereas the T-cells play a vital role in cell-mediated cell- mediated immune response [15].
B cells: Antibody produced by B cells recognizes and bind to pathogens or destroy them with the help of macrophages. Such response is called humoral immunity [15].
T cells: Various T-cell sub groups exists and each has its own roles in adaptive immunity. The table below describes each subGroups of T cells (Various For instance, cytotoxic T cells (killer T cells) directly attack and kills the infected cells, while helper T cells aid in the function of other lymphocytes and enhance the responses. Regulatory T cells, sometimes called suppressor T cells, suppress immune responses. T cells possess T cell receptors on their surface which help them to get distinguished from B and NK cells [16].
Types of T cells (Table2)

Measuring the Immunity
Immunity can be assessed for both humoral as well as the cell mediated immunity. In humoral immunity, the basic principle is to measure the antibody titters whereas cell mediated immunity involves more complex techniques to measure the specific cell surface markers, specific memory T cells and lymphocyte proliferation etc [17].
Measuring humoral immunity
Humoral immunity is measured based on quantification of immunoglobulin (Ig) concentrations. Various types of immunoglobulins include IgG, IgM, IgA and IgE, their level varies with nutrition and immunity [18].
Assessing the Immunoglobulin concentration: Assessing the Immunoglobulin IgM and IgG concentrations helps to know the humoral immunity status. The presence of antibody against the specific pathogen provides information if immunity was developed or not against the specific pathogen. Their tier level will give substantial information about the immunity status.
Titre: Titre provides information about the concentrations of antibodies (whether IgM or IgG) produced towards a specific antigen.
Lymphocyte proliferation assay: Rate of cell division by T and B cell by using substances like pokeweed mitogen and oligosaccharide respectively provides an index of the ability of the humoral immune system to boost following an infection [19].
Measuring cellular immunity
Measurement of cell-mediated immunity can be undertaken by both in vitro and in vivo methods. It is, however, more problematic than humoral assessment as assays are plagued by difficulties in standardization, biological variability, imprecision and technical complexity [20].
Flow cytometry
The evaluation of cell-mediated immunity is to quantify circulating immune cells and their subsets by flow cytometric analysis. Fluorochrome-labelled monoclonal antibodies directed against cell surface molecules and analyzed by a flow cytometer, which measures light scatter and fluorescence emission from individual cells. The amount of fluorescence is directly proportional to the number of cells with the particularly targeted surface molecule. Different cell populations (B cells, and CD4/ CD8T cells and natural killer cells) can be distinguished based on their scatter profile and surface molecule expression [21].
Delayed-type hypersensitivity skin testing
Delayed-type hypersensitivity skin testing provides cellular immunity assessment under in via condition. The test involves inoculation of antigen intradermally leading to local inflammation surrounding the site of injection after 1-2days due to the recruitment of mononuclear cells (lymphocytes, monocytes) and neutrophils. Flow cytometry is usually done to test Mycobacterium and Brucella infection [22].
Cytokine production/concentration
Communication among cells that mediate immunity is carried out by a large number of hormones called "cytokines” Common signalling cytokines include 1L-1, 1L-2, 1L-4, 1L-5, IL- 6, IL-10, tumor necrosis factor-alpha (TNF-α) and interferongamma (IFN-γ). Measuring concentrations of cytokines in biological samples can provide useful information on activities of specific aspects of the immune system [16].
Natural killer cell cytotoxicity assays
Natural killer cell cytotoxicity is assessed by a 51Cr-release assay in which patients' natural killer cells are incubated with 51Cr-labelled target cells. The target cells get lysed by natural killer cells led to the release of radioactivity which can be measured. Natural killer cell dysfunction may occur in patients with CD16 genetic mutations, chronic mucocutaneous candidiasis, severe combined immunodeficiency and other cellular immunodeficiency syndromes. These conditions need to be considered and excluded if natural killer cell dysfunction is confirmed. With respect to T and B lymphocytes, functional natural killer cell deficits can occur though the natural killer cell counts are normal. Natural killer cell assays are complex technically and seldom performed in diagnostic immunology laboratories.
Mechanisms of Immunity and Nutrition
Nutrition and immunity interact in a number of ways, many of which researchers are just beginning to understand. These include:
Nutritional immunity
The body can fight infections by withholding nutrients from pathogens; this concept is named nutritional immunity. For example, iron, biotin, and manganese play a role in depriving pathogens of required nutrients [23].
Hormonal regulation
Certain vitamins, minerals, and macronutrients (substances required in large amounts) provide the molecules needed for hormonal regulation. Glucocorticoids are essential for mediating response towards the pathogens by the host. Hormones also regulate the activities of the lymphocytes. White blood cells play a central role in coordinating immune responses by responding to the presence of foreign material in the body [24].
Fatty acids and vitamins A, D and E
Fatty acids and vitamins have direct effects on the immune system by supporting the manufacture and function of cytokines- -the chemical signalling molecules of the immune response [25,26].
Substrates
These provide the molecules required for metabolism. All nutrients provide the amino acids and energy that help build and maintain the components and the metabolic processes of the immune system. For example, vitamin A helps with the development of B- and T-lymphocytes (key parts of the immune response) [27].
Antioxidants
These are molecules that reverse the chemical process of oxidation by stabilizing the number of unstable oxygen atoms (free radicals) that can cause cell damage over time. At the molecular level, the oxidation that free radicals produced can damage DNA, RNA, and the cell lipids (fat molecules that make up the cell membrane), which are essential for the cell membrane integrity. The weakening of the lipid layers can hasten cell death [28]. Important antioxidants are vitamins E, C, and A, and minerals such as zinc and selenium, as mentioned above.
Vitamins A and E, in particular, have antioxidant effects in the horse. For instance, vitamin A might direct lymphocyte development and maintenance of the mucous membranes, which help fight off challenges from pathogens.
Specific Nutrients and its Effect on Immunity
In non-ruminants, essential amino acids, folic acid, vitamin B6, vitaminB12, vitamin C, vitamin A, vitamin E, linoleic acid, iron, zinc, selenium, and copper affect one or more indexes of immunity. In ruminants, their capability to synthesize amino acids and vitamins with the help of microbial population had refrained scientist to study their effects. But few reported that cobalt, copper, selenium, chromium, vitamin E and A have regulated the immune system in ruminants [29].
Nutrients with antioxidant properties have shown to protect the cell membrane of the immune system as they are susceptible to Reactive Oxygen Species (ROS) - mediated damage. Hence antioxidant properties of vitamin C,E, carotenes and zinc and selenium minerals support the cell membrane which in turn strengthens the immune system as a whole [30].
Carotenoids
Carotenoids include β-carotene, lutein, canthaxanthin, lycopene, and astaxanthin are potent source of vitamin A. In ruminants, supplementation with β-carotene at dry periods reduced mammary gland infections with increased lymphocyte blastogenesis and increased neutrophil killing activity [31].
Vitamin E and selenium (Se)
Feeding elevated levels of Se to ruminant animals reduces the incidence of diseases. Feeding of Se enhanced neutrophil killing activity. Se deficiency increases neutrophil adherence and could affect the ability of neutrophils to attack and sequester pathogen [32]. Vitamin E prevented the peripartum reduction in neutrophil superoxide anion production and impaired 1L-1 production by monocytes and increases lymphocyte proliferation. Injection of Se either alone or in combination with vitamin E significantly improved the production of specific antibodies against E Coli and that the production of specific antibodies was greater after the administration of Se alone [33].
Omega-3and-6 fatty acids (ω-3and ω-6)
Fatty acids affect immunity through the production of cytokines, eicosanoids (e.g., prostaglandins) and leukotrienes. Dietsrich in the ω-6 fatty acids, such as linoleic acid (C18:2), lead to the formation of arachidonic acid, whereas diets rich in the ω-3 fatty acids (such as linolenic acid, C18:3, flaxseed and fish oils) lead to the formation of, eicosapentaenoic acid (EPA). Eicosanoids synthesized from arachidonic acid tend to have stronger inflammatory potential whereas those synthesized from EPA have lesser potential. Hence, feeding fatty acid mixtures which are enriched in the ω-3fattyacids reduces inflammatory reactions and reduces production of proinflammatory cytokines including 1L-1, 1L-6, and TNF-α. Studies on flaxseed increased progesterone and PGE4concentrations increased TNF-α and nitric oxide [34].
Chromium (Cr)
The addition of Crto livestock diets improves immunity. Chromium increased blastogenesis in the lymphocytes recovered from sick calves. However, the same effect was not detected in healthy calves' lymphocytes. A common theme among studies has detected the benefit to Cr supplementation could be due to the presence of a stressor (shipping, parturition, weaning). 1t is possible that stress and consequent immuno suppression can be suppressed with Cr supplementation [35].
Copper (Cu)
Natural Cu deficiency increases the susceptibility of ruminant animals to disease. However, experimental models of Cu deficiency often fail to increase the incidence of disease. Supplementation of Cu-deficient diets augments markers of immune function whereas others do not. Cu or Zn affected the humoral immune response to albumin immunization [36].
Zinc (Zn)
Zn plays an important role in transcriptional control through its action as a Zn-finger motif. Cells deficient in Zn have reduced ability to proliferate. The immune response requires a rapid proliferation of cells (e.g., T- and B-lymphocytes) in response to specific antigens and, therefore, Zn deficiency prevents this aspect of immunity from developing.
Spears (2000) reported that, in contrast to studies with humans and laboratory animals, marginal Zn deficiency has little effect on immune function in ruminant animals while Zn supplementation could be beneficial [37,38].
Conclusion
Nutrition plays an important role in determining the immunity of the animal in its life time. Their role is crucial during early and late stages of the animal's life span. Deficiency of protein-energy tend to affect the immune status of the animals. Deficiency of single or group of vitamins, minerals has influence on immune system. Identification of specific nutritional deficiency and supplementation with the respective deficient nutrition is the key to alter the immune status and to boost the immunity of the animal.
References
- Chandra RK (1983) Nutrition, immunity, and infection: present knowledge and future directions. Lancet 321(8326): 688-691.
- Chandra RK, Newberne PM (2012) Nutrition, immunity, and infection: mechanisms of interactions. Springer Science and Business Media, Netherlands.
- Bendich A (1993) Physiological role of antioxidants in the immune system. J Dairy Sci 76(9): 2789-2794.
- Iwasaki A, Medzhitov R (2010) Regulation of adaptive immunity by the innate immune system. science 327(5963): 291-295.
- Medzhitov R, Janeway CA (2002) Decoding the patterns of self and nonself by the innate immune system. Science 296(5566): 298-300.
- Gasque P (2004) Complement: a unique innate immune sensor for danger signals. Mol Immunol 41(11): 1089-1098.
- Medzhitov R, Janeway CA (1997) Innate immunity: the virtues of a nonclonal system of recognition. Cell 91(3): 295-298.
- McFall-Ngai M (2007) Adaptive immunity: care for the community. Nature 445(7124): 153-153.
- Starr TK, Jameson SC, Hogquist KA (2003) Positive and negative selection of T cells. Annual Rev Immunol 21(1): 139-176.
- Blalock JE (1984) The immune system as a sensory organ. J Immunol 132(3): 1067-1070.
- Tan TT, Coussens LM (2007) Humoral immunity, inflammation and cancer. Curr opin Immunol 19(2): 209-216.
- Gmelig-Meyling F, UytdeHaag AGCM, Ballieux RE (1977) Human B-cell activation in-vitro: T cell-dependent pokeweed mitogen-induced differentiation of blood B lymphocytes. Cellular Immunol 33(1): 156169.
- Wunderlich JR, Canty TG (1970) Cell mediated immunity induced in- vitro. Nature 228(5266): 62-63.
- Caruso A, Licenziati S, Corulli M, Canaris AD, De Francesco MA, et al. (1997) Flow cytometric analysis of activation markers on stimulated T-cells and their correlation with cell proliferation. Cytometry 27(1): 71-76.
- Fine PE, Sterne JA, Ponnighaus JM, Rees RJ (1994) Delayed-type hypersensitivity, mycobacterial vaccines and protective immunity. Lancet 344(8932): 1245-1249.
- Carson RT, Vignali DA (1999) Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay. J immunol Methods 227(1): 41-52.
- Hood MI, Skaar EP (2012) Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10(8): 525-537.
- Beagley KW, Gockel CM (2003) Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol 38(1): 13-22.
- Calder PC (1998) Dietary fatty acids and the immune system. Nutrition Reviews 56(1 Pt 2): S70-S83.
- Mora JR, Iwata M, VonAndrian UH (2008) Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 8(9): 685-698.
- De la Fuente M (2002) Effects of antioxidants on immune system ageing. Eur J Clin Nutr 56(S3): S5-S8.
- Van Soest PJ (1994) Nutritional ecology of the ruminant. Cornell University Press, USA.
- Sies H, Stahl W (1995) Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J clin Nutri 62(6): 1315S-1321S.
- Mayland HF (1994) Selenium in plant and animal nutrition. Marcel Dekker, Inc., New York, USA, pp. 29-46.
- Wintergerst ES, Maggini S, Hornig DH (2007) Contribution of selected vitamins and trace elements to immune function. Annals Nutri Metab 51(4): 301-323.
- Calder PC (2008) Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutri Food Res 52(8): 885-897.
- Moonsie-Shageer S, Mowat D (1993) Effect of level of supplemental chromium on performance, serum constituents, and immune status of stressed feeder calves. J Anim Sci 71(1): 232-238.
- Ward JD, Spears JW, Kegley EB (1993) Effect of copper level and source (copper lysine vs copper sulfate) on copper status, performance, and immune response in growing steers fed diets with or without supplemental molybdenum and sulfur. J Anim Sci 71(10): 2748-2755.
- Spears JW, Kegley EB (2002) Effect of zinc source (zinc oxide vs zinc proteinate) and level on performance, carcass characteristics, and immune response of growing and finishing steers. J Anim Sci 80(10): 2747-2752.
- Aderem A, Underhill DM (1999) Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17(1): 593-623.
- Lee WL, Downey GP (2001) Neutrophil activation and acute lung injury. Curr Opin Crit Care 7(1): 1-7.
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, et al. (2000) Immunobiology of dendritic cells. Ann Rev Immunol 18(1): 767-811.
- Huntley JF (1992) Mast cells and basophils: a review of their heterogeneity and function. J Comp Pathol 107(4): 349-372.
- McEwen BJ (1992) Eosinophils: a review. Vet Res Commun 16(1): 1144.
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S (2008) Functions of natural killer cells. Nature immunology 9(5): 503-510.
- Raulet DH (1989) Antigens for delta T cells. Nature 339: 342.
- Borish LC, Steinke JW (2003) Cytokines and chemokines. J Allergy Clin Immunol 111(2): S460-S475.
- Bluestone JA, Abbas AK (2003) Natural versus adaptive regulatory T cells. Nat Rev Immunol 3(3): 253-257.






























