Use of Crude Glycerin as an Energy Source for Goat Diets: A Review
Pin Chanjula*
Department of Animal Science, Prince of Songkla University, Thailand
Submission: March 18, 2017; Published: April 12, 2017
*Corresponding author: Pin Chanjula, Department of Animal Science, Faculty of Natural Resources, Prince of Songkla University, Songkhla 90112, Thailand, Tel:+66-74-558805; Email: pin.c@psu.ac.th
How to cite this article: Pin C. Use of Crude Glycerin as an Energy Source for Goat Diets: A Review. Dairy and Vet Sci J. 2017; 2(1): 555578. DOI: 10.19080/JDVS.2017.02.555578
Abstract
Crude glycerin (CG) is a by-product of the biodiesel production, which has been shown to be a good source for ruminants. There is variability in the energy available from crude glycerin, which is partially explained by the glycerin content of the crude glycerin. Recent efforts have evaluated the effects of the inclusion of crude glycerin (above 85% of glycerin) in diets on intake, performance, carcass and meat quality traits of goat and sheep. It was found that can be used crude glycerin as energy source up to 20% of dry matter, without negative effects on performance and main carcass traits.
Keywords: Crude glycerin; Energy source; Goat diets
Introduction
At present, biodiesel industry has grown rapidly along with the increasing demand of renewable and sustainable energy source, it has lower power pollution. The government incentives given to biodiesel agribusiness has led to a huge growth in the production of this biofuel, which in turn also increases the availability of its by-products such as cakes, meals, and especially crude glycerin (CG) or glycerol. CG is the major by-product of the conversion of vegetable oils, animal fats (first-use oil) and/ or waste greases (second-use oil) into biodiesel [1]. Generally, CG production is up to 10 to 20% from the total volume of biodiesel produced. Rapid growth in biofuel production of Thailand has led to increasing feedstocks of CG, with a subsequent price reduction, making glycerin a potential high energy feed source for ruminants [2-4]. The use of CG as a substitute for energetic ingredients in animal feed has shown promising results for livestock in many species including swine [5], poultry [6], beef cattle [4], dairy cattle [7], goats [2,3,8], and sheep [9]. The inclusion of glycerin in ruminant diets modifies ruminal fermentation such as the acetate: propionate ratio, because CG is preferentially converted to propionate in the rumen [10], absorbed directly by ruminal epithelium or goes directly to the small intestine and is then converted to glucose in the liver [11].
The value of CG in this regard may be further amplified with increasing diversion of corn and other grains to ethanol production. Although there is supporting evidence for use of glycerol for many species, however, to our knowledge, there is little information that examines the use of glycerin as a macro-ingredient in rations for small ruminant, particularly in goats. Moreover, there is scarce information regarding the rate of fermentation and its fermentation products. There is some controversy regarding to the biochemical pathway and the end products of glycerol fermentation by ruminal microbes. Some authors have proposed that propionic acid is the main volatile fatty acid derived from glycerol, supporting the glycogenic role of glycerol in ruminants [12]. Many inconsistent outcomes between individual studies may be resulted from spespecific differences in experimental conditions. Therefore, this review will explore some of the attributes and issues pertinent to glycerin as a feed for small ruminants and highlight results from a recent research study at Prince of Songkla University where the value of CG was examined as a replacement for corn grain.
Glycerin production and quality concerns of glycerin
In an attempt to diversify fuel sources and to decrease dependency on petroleum, the world is currently turning to production of biodiesel as an alternative fuel. Biodiesel is made from renewable biological sources such as vegetable oils and animal fats; even recycled frying oil is used as a substrate [13]. Most biodiesel is currently produced by a reaction that utilizes a base catalyzed trans esterification of the oil, is reacted with an equal weight of a short chain alcohol (methanol or ethanol) in the presence of a catalyst (sodium hydroxide; caustic soda or potassium hydroxide; potash) to yield biodiesel and crude glycerin (Figure 1). The biodiesel is separated from the glycerol by gravity separation or by centrifugation. Alcohol is removed from biodiesel and glycerol phases by flash evaporation or by distillation to recover, and re-use it [14].
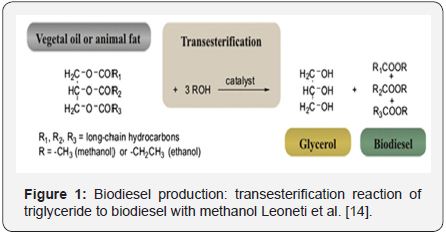
CG obtained form this process, is mainly composed of glycerol (80-88%) and trace amounts of water and methanol depending on the grade of purity [15]. The overall equation and the approximate proportions of the reaction are shown below:
100 liters of oil+10 liters of methanol=100 liters of Biodiesel+10 liters of glycerin
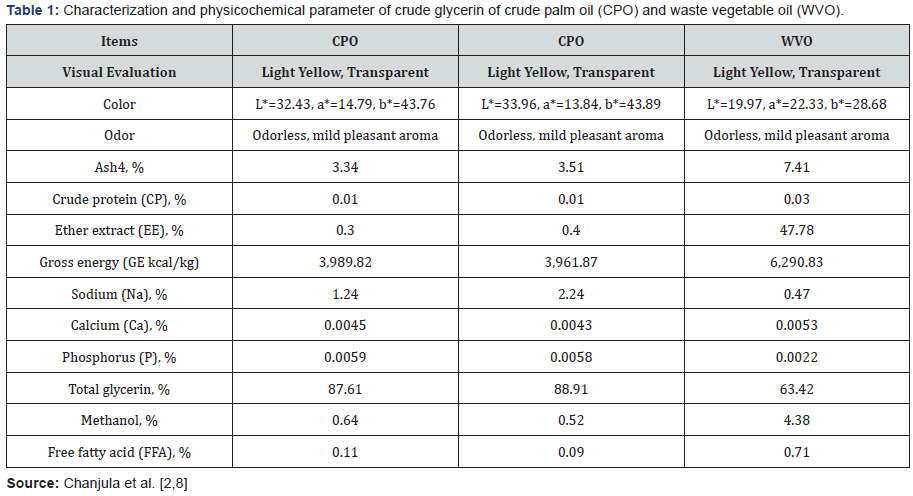
Recent evaluation of crude glycerin from palm biodiesel production (CPO) or waste vegetable oil (WVO) are presented in Table 1, indicates a glycerin content of 87-89%, 0.3-0.4% fat, and 3.3-3.5 ash [3,4]. CG is a colorless, odorless, hygroscopic, and sweet-tasting viscous liquid. Whereas the WVO was produced from waste vegetable oils containing 63.42% glycerin, 47.78% EE, 0.47% sodium, 4.38% methanol, and less of other compounds [8]. Howerver, the chemical compositions of glycerin is dependent on its purity, source of oil and has been reported by Thompson & He [1] reported that evaluation of crude glycerin from soy biodiesel production indicates a glycerol content of 76.2% and as much as 7.98% fat, 0.05% protein, and 2.73% ash. The latter was composed of 11ppm Ca, 6.8ppm Mg, 53ppm P, and 1.2% Na.
The energy content of glycerin is dependent on its purity and has been reported to be between 1.98 and 2.27Mcal NEL/kg when fed in combination with a high and a low starch concentrate, respectively [15]. These values are close to that of corn which is approximately 2.0Mcal NEL/kg [16], and therefore glycerin can be compared with corn as an ingredient by itself, considering of course, that it is not a source of protein. Glycerin is recognized as safe substance, it is classified as a general purpose food additive; therefore CG or glycerol is generally recognized as safe for use in animal feed [17], especially for ruminant. However, it should be noted that the presence of impurities substances are at the acceptable level, such as the residual of methanol should not exceed 150ppm, un saponifiable matter not to exceed 2% [17], and negligible amount of mineral salts, catalysts, and other impurities so that it has no negative influence on animal health [18]. However, impurities like methanol, are a concern and it has been established it that it must not exceed 1% of DM in diets for ruminants. Methanol toxicity in humans and animals is characterized by central nervous system depression, weakness, headaches, vomiting, metabolic acidosis and optic disc oedema with the clinical consequences being blindness and/or Parkinsonian-like motor disease [19].
Methanol concentrations can vary widely according to the manufacturing processes and should be monitored. It has also been reported that methanol in excess of what can be metabolized in the rumen, can have severe effects in ruminants, resulting in inhibition of milk synthesis, anorexia, dullness and sudden death in some cases. However, Methanol is normally produced in the rumen from hydrolysis of methyl esters from pectins by bacteria and protozoa, and is found in concentration close to 28μg/ml [20,21]. Methanol is not likely to accumulate in ruminal fluid since it can be readily used by methylotrophic organisms and converted to methanol or acetate and butyrate [22]. Shröeder & Südekum [15] evaluated glycerins of different purities (0 to 26.7 % methanol) and found no differences in nutrient digestibilities or dry matter intake when fed at relatively high doses (1.1kg/d) in diets for steers. Other impurities found in low purity glycerin are high contents of minerals like phosphorus, potassium and sodium, as well as ether extract.
Rumen Metabolism of Glycerin
When entering the rumen, an important fraction of glycerol can be directly absorbed through the rumen wall [11], from where it is transported to the liver and converted to glucose. Alternatively, glycerol can be fermented to volatile fatty acids (VFA), where propionate, which is in turn converted to glucose, accounts for more than 40% of total VFA [11,23,24]. The glucogenic pathway in ruminants is shown in Figure 2. Due to its high solubility, a small fraction of glycerol is evacuated to the lower gut flowing with the liquid fraction of rumen contents and recovered in the duodenal digesta from where it is redirected to the liver [11].
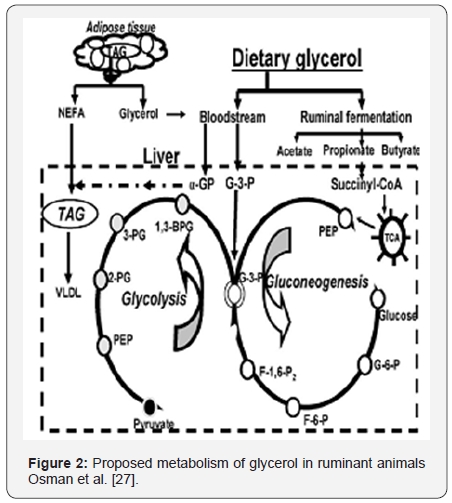
Glycerol is an important precursor of sn-glycerol 3-phosphate (G3P) which is in turn a carbon skeleton for gluconeogenesis [25].
Early reports of glycerol fermentation indicated that glycerol was almost entirely fermented to propionate (C3) [12]. It had been observed that glycerol supplementation improved glucose status in ruminants by acting as a gluconeogenic precursor that increased blood glucose level [24] and/or converted to glucose in the liver [11]. Similar, studies using 14 °C labeled glycerol indicate that that most of the glycerol was found in propionate [26].
Several studies, both in vivo and in vitro, have shown glycerol fermentation to increase molar proportions of propionic (C3) and butyric (C4) acid, and to increase total VFA production, while molar proportions of acetate decrease [10,11,15,27]. Using ruminally cannulated steers, Wang et al. [10] fed increasing doses of glycerol (0, 100, 200 and 300g/animal/day) and reported a linear decrease in acetate to propionate ratio due to increased propionate production. The clearly resembled data is typically acetate to pionate ratio which is being lowered with the increasing level of glycerol in the diet [11,28-30]. Even if glycerin does not bypass the rumen to serve directly as a glucogenic substrate, propionic acid is an important glucogenic precursor by itself, and has been estimated to supply 32 to 73% of the glucose demands in the ruminant animal [31].
Microbial populations and Rumen environment
Dietary changes in ruminants require an adaptation period to facilitate the shift in ruminal microbial populations. The capacity of the bacteria to degrade glycerol also increases with increasing amount of glycerin fed [11]. Glycerol has been reported to be metabolized by Megasphaera elsdenii, Streptococcus bovis and Selenomonas ruminantium species [32]. Morover, in vitro fermentation of glycerol has shown that growth of cellulolytic bacteria like Ruminococcus flavefaciens and Fibrobacter succinogenes can be inhibited when glycerol concentrations are 2 to 5% in a medium with cellobiose as the main substrate [33]. In vitro fermentation suggests that species of Selenomonas could be the major fermenters of glycerol, and that the main products of its fementation are propionate, lactate, succinate and acetate [32,34]. Megasphaera elsdenii, usually utilises lactate, an end product of S. bovis and Selenomonas spp. to form propionate and butyrate [35]. Thus, feeding glycerol is usually associated with an increase in the molar proportion of propionate and to a lesser extent butyrate, at the expense of acetate.Ruminal pH has also been reported to be affected by glycerol. Kijora et al. [36] fed glycerol at 10% of DMI and reported a drop in pH which was not explained by increased VFA production. Feeding 8% (600 g/d) of the concentrate DM as glycerin in high concentrate diets for Holstein bulls, Mach et al. [37], reported lower ruminal pH, however these results may have been confounded by a tendency for higher concentrate intake in this treatment group. Similarly, Wang et al. [10] reported a linear decrease in ruminal pH when increasing doses (100, 200 and 300g/d) of glycerin were fed to steers.Use of crude glycerin as a feed ingredient for goats

The use of CG as a substitute for energetic ingredients in animal feed has shown promising results for livestock in many species. Several researchers have reported that glycerin is an acceptable feed ingredient for ruminants (dairy cattle, beef cattle, sheep, and goat). In goats, studied by Chanjula et al. [2,4,8] determined the suitability of glycerin as an energy source in goat diets (Table 2). Earlier work by Chanjula et al. [2,3] conducted to evaluate the effects of increasing concentrations of CG (0, 5, 10, and 20% of diet DM, 87.7% glycerin) replacing corn in diets on nutrient utilization, ruminal fermentation characteristics, and nitrogen utilization of goats. It was reported that no significant differences among treatment groups regarding DM intake and digestion coefficients of nutrients (DM, OM, CP, EE, NDF, and ADF). Likewise, mean serum glucose, BHBA, and PCV concentrations were not affected by dietary treatments, whereas serum insulin concentration linearly increased (L, p=0.002) with increasing the amount of CG supplementation. Ruminal pH, NH3-N, and BUN concentration were unchanged by dietary treatments, except for 20% of CG, NH3-N, and BUN were lower than for the diets 10% of CG, while the difference between the diets 0, 5, and 20% of CGLY were not significant. The amount of N absorption and retention were similar among treatments.
Similarly, no significant effect of CG inclusion was observed in any of the growth performance, carcass, and meat quality traits studied. Likewise, no apparent effects on FA composition were detected, except for C15:0, C16:0, C16:1, and C22:5n-3 were affected by CG level. We conclude that crude glycerin can be used as substitution for corn grain up to the level of approximately 20% of dry matter in the diets of finishing goats, and this study was a good approach in exploiting the use of biodiesel production for goat production [3]. Moreover, Chanjula et al. [4] also reported that the steers received dietary treatments containing 0, 7, 14, and 21% of CG (88.9% pure) on a DM basis. The diets were offered ad libitum as total mixed rations twice daily. No significant effect of CG inclusion was observed in any of the growth performance and carcass characteristics traits studied. Also, there were no apparent effects of diets on meat quality (pH, water holding capacity, drip losses, and cooking losses). The study concluded that CG could be used as a substitute for corn grain up to the level of approximately 21% of DM in the diets of finishing steers.
A recent most of the CG used in those studies were derived from vegetable oils (first-use oil) of castor bean, soybean, cottonseed, sunflower, rapeseed, canola, and palm oil. A limit number of studies have evaluated the effects of CG originated from waste vegetable oils (CGWVO) that (second-use oil) contained with high crude fat contents in diets fed to animal diets. Thompson & He [1] mentioned that nutritional data generated for the glycerin of the first-use oil samples showed that it was mostly carbohydrate and could reasonably be mixed with high protein meal and used as a feed supplement. They also found that giving the higher fat content of CGWVO could be used as a supplement for energy or fat in animal diets particularly in goats. According Chanjula et al. [8], recent attempts have been made to evaluate of the effects of increasing concentrations of crude glycerin from CGWVO in diets on feed intake, digestibility, ruminal fermentation characteristics, and nitrogen balance of goats. The dietary treatments contained 0, 2, 4, and 6% of dietary DM of CGWVO. Based on this experiment, DM intake and digestion coefficients of nutrients, ruminal pH, NH3-N, and blood urea nitrogen (BUN) concentration were unchanged by dietary treatments, except 6% of CGWVO supplementation, NH3-N, and BUN were lower than for the diets 0% of CGWVO while the difference between the diets 0, 2, and 4% of CGWVO were not significant. Likewise, the amounts of N absorption and retention were similar among treatments, except that for 6% of CGWVO which N absorption was lower than among treatments while the difference between the diets 0, 2, and 4% of CGWVO were not significant. Similarly, there were no effects of CGWVO on carcass length, carcass width, LM area, WBSF, pH and color of LM at 45min after slaughter, as well as on other carcass cut and muscle chemical composition. In conclusion, the addition of up to 4% of dry matter in the diets for crossbred finishing goats seems to be the most interesting strategy, since it promotes greatest animal performance [38]. Moreover, this study was a suitable approach in exploiting the use of biodiesel production from waste vegetable oil for goat production.
Conclusion
Based on this review, it can be concluded that CG is a byproduct of the biodiesel production, which has been shown to be a good source for ruminants. There is variability in the energy available from crude glycerin, which is partially explained by the glycerin content of the CG. Recent efforts have evaluated the effects of the inclusion of CG (above 85% of glycerin) in diets on intake, performance, carcass and meat quality traits of goat and sheep. It was found that can be used crude glycerin as energy source up to 20% of dry matter, without negative effects on performance and main carcass traits.
References
- Thompson JC, He BB (2006) Characterization of crude glycerin from biodiesel production from multiple feedstocks. Applied Engineering in Agriculture 22(2): 261-265.
- Chanjula P, Pakdeechanuan P, Wattanasit S (2014) Effects of Dietary Crude Glycerin Supplementation on Nutrient Digestibility, Ruminal Fermentation, Blood Metabolites, and Nitrogen Balance of Goats. Asian Australas J Anim Sci 27(3): 365-374.
- Chanjula P, Pakdeechanuan P, Wattanasit S (2015) Effects of feeding crude glycerin on feedlot performance and carcass characteristics in finishing goats. Small Rumin Res 123: 95-102.
- Chanjula P, Raungprim T, Yimmongkol S, Poonko S, Majarune S, et al. (2016) Effects of elevated crude glycerin concentrations on feedlot performance and carcass characteristics in finishing steers. Asian Australas J Anim Sci 29(1): 80-88.
- Schieck SJ, Kerr BJ, Baidoo SK, Shurson GC, Johnston LJ (2010) Use of crude glycerol, a biodiesel coproduct, in diets for lactating sows. J Anim Sci 88: 2648-2656.
- Min YN, Yan F, Liu FZ, Coto C, Waldroup PW (2010) Glycerin a new energy source for poultry. Int J Poult Sci 9(1): 1-4.
- Ezequiel JMB, Sancanari JBD, Machado Neto OR, Silva ZF, Almeida MTC, et al. (2015) Effects of high concentrations of dietary crude glycerin on dairy cow productivity and milk quality. J Dairy Sci 98(11): 8009-8017.
- Chanjula P, Pongprayoon S, Kongpan S, Cherdthong A (2016) Effects of crude glycerin from waste vegetable oil supplementation on feed intake, ruminal fermentation characteristics, and nitrogen utilization of goats. Trop Anim Health and Prod 48(5): 995-1004.
- Carvalho VB, Leite RF, Almeida, MTC, Paschoaloto JR, Carvalho EB, et al. (2015) Carcass characteristics and meat quality of lambs fed high concentrations of crude glycerin in low-starch diets. Meat Sci 110: 285- 292.
- Wang C, Liu Q, Huo WJ, Yang WZ, Dong KH, et al. (2009) Effects of glycerol on rumen fermentation, urinary excretion of purine derivatives and feed digestibility in steers. Livest Sci 121: 15-20.
- Rémond B, Souday E, Jouany JP (1993) In vitro and in vivo fermentation of glycerol by rumen microbes. Anim Feed Sci Technol 41(2): 121-132.
- Garton GA, Lough AK, Vioque E (1961) Glyceride hydrolysis and glycerol fermentation by sheep rumen contents. J Gen Microbiol 25: 215-225.
- Friedrich S (2004) A world wide review of the commercial production of biodiesel - a technologial, economic and ecological investigation based on case studies. In: Shriftenreihe U, Ressourcenö K (Eds.), 41: Institute für Technologie und nachhaltiges Produktmanagment. Wirtschaftsuniversität, Vienna, Austria.
- Leoneti AB, Aragão-Leoneti V, Borges de Oliveira SVW (2012) Glycerol as a by-product of biodiesel production in Brazil: Alternatives for the use of unrefined glycerol. Renewable Energy 45: 138-145.
- Schröder A, Südekum KH (1999) Glycerol as a by-product of biodiesel production in diets for ruminants. In: Wratten N, Salisbury PA (Eds.), New Horizons for an Old Crop Canberra. Australia, p. 241.
- NRC (2001) Nutrient requirement of dairy cattle. (7th edn), National Research Council, National Academy of Science, Washington, USA.
- FDA (Food and Drug Administration) (2006) Code of Federal Regulations. 21(6): 21CFR582.1320.
- Dasari M (2007) Crude glycerol potential described. Feedstuffs 79(43): 1-3.
- Dorman DC, Dyy JA, Nassise MP, Ekuta J, Bolon B, et al. (1993) Acute methanol toxicity in minipigs. Fundam Appl Toxicol 20: 341-347.
- Vantchev Z, Pradhan K, Hemken RW (1970) Rumen methanol in-vivo and in-vitro. J Dairy Sci 53(10): 1511-1514.
- Pol A, Demeyer DI (1988) Fermentation of methanol in the sheep rumen. Appl Environ Microbiol 54(3): 832-834.
- Neumann L, Weigand E, Most E (1999) Effect of methanol on methanogenesis and fermentation in the rumen simulation technique (RUSITEC). J of Anim Physiol and Anim Nutr 82: 142-149.
- Lee SY, Lee SM, Cho YB, Kam DK, Lee SC, et al. (2011) Glycerol as a feed supplement for ruminants: In vitro fermentation characteristics and methane production. Anim Feed Sci Technol 166-167: 269-274.
- Chung YH, Rico DE, Martinez CM, Cassidy TW, Noirot N, et al. (2007) Effects of feeding dry glycerin to early postpartum Holstein dairy cows on lactational performance and metabolic profiles. J Dairy Sci 90(12): 5682-5691.
- Lin EC (1977) Glycerol utilization and its regulation in mammals. Annu Rev Biochem 46: 765-795.
- Bergner H, Kijora C, Ceresnakova Z, Szakacs J (1995) In vitro studies on glycerol transformation by rumen microorganisms. Arch Tierernahr 48(3): 245-256.
- Osman MA, Allen PS, Mehyar NA, Bobe G, Coetzee JF, et al. (2008) Acute metabolic responses of postpartal dairy cows to subcutaneous glucagon injections, oral glycerol or both. J Dairy Sci 91(9): 3311-3322.
- Abo El-Nor S, Ghazaleh AA, Potu RB, Hastings D, Khattab MSA (2010) Effects of different levels of glycerol on ruminal fermentation and bacteria. Anim Feed Sci Technol 162: 99-105.
- Krueger NA, Anderson RC, Tedeschi LO, Callaway TR, Edrington TS, et al. (2010) Evaluation of feeding glycerol on free-fatty acid production and fermentation kinetics of mixed ruminal microbes in vitro. Bioresour Technol 101: 8469-8472.
- Avila-Stagno J, Chaves AV, Ribeiro Jr GO, Ungerfeld EM, McAllister TA (2014) Inclusion of glycerol in forage diets increases methane production in a rumen simulation technique system. British J Nutr 111: 829-835.
- Seal CJ, Reynolds CK (1993) Nutritional implications of gastrointestinal and Liver metabolism in ruminants. Nutr Res Reviews 6: 185-208.
- Stewart CS, Flint HJ, Bryant MP (1997) The rumen microbial ecosystem. In: Hobson PN (Ed.), The rumen bacteria, Elsevier Applied Sciences, New York, USA, pp. 10-72.
- Roger V, Fonty G, Andre C, Gouet P (1992) Effects of glycerol on the growth, adhesion, and cellulolytic activity of ruminal cellulolytic bacteria and anaerobic fungi. Curr Microbiol 25(4):197-201.
- Hobson P, Mann SO (1961) The isolation of glycerol-fermenting and lipolytic bacteria from the rumen of the sheep. J gen Microbiol 25: 227- 240.
- Shin JH, Wang D, Kim SC, Adesogan AT, Staples CR (2011) Effects of feeding crude glycerin on performance and ruminal kinetics of lactating Holstein cows fed corn silage or cottonseed hull-based, low-fiber diets. J Dairy Sci 95(7): 4006-4016.
- Kijora, C, Bergner H, Gotz KP, Bartlelt J, Szakacs J, et al. (1997) Research Note: Investigation on the metabolism of glycerol in the ruminal of bulls. Arch Tierernahr 51(4): 341-348.






























