Determination of Phospholipids in Milk by HPLC with Evaporative Light Scattering Detector: Optimization and Validation
Ferreiro T, Gayoso L and Rodríguez-Otero JL*
Universidade de Santiago de Compostela, Spain
Submission: February 13, 2017; Published:March 03, 2017
*Corresponding author: Rodríguez-Otero JL, Instituto de Investigación e Análises Alimentarias, Universidade de Santiago de Compostela, Facultade de Veterinaria, 27002 Lugo, Spain, Tel:+34 982822408; Email: jlr.tero@usc.es
How to cite this article: Ferreiro T, Gayoso L, Rodríguez-Otero J. Determination of Phospholipids in Milk by HPLC with Evaporative Light Scattering Detector: Optimization and Validation. Dairy and Vet Sci J. 2017; 1(3): 555562. DOI: 10.19080/JDVS.2017.01.555562
Abstract
Phospholipids are part of the milk fat globule membrane. Functional and technological properties make them interesting for the development of functional foods or as ingredients of technological interest. In this study an analytical procedure for determination of major phospholipids in milk (phosphatidyl Ethanolamine, phosphatidylinositol, phosphatidyl Choline, phosphatidyl Serine and sphingomyelin) by HPLC with evaporative light scattering detector was optimized and validated. The best calibrations were achieved when an exponential model was applied; the squared correlation coefficients show a satisfactory linearity ranging from 0.975 to 0.993. The limit of detection for the different phospholipids ranged from 0.17μg for phosphatidyl Serine to 0.61μg for phosphatidyl Ethanolamine; and the limit of quantification from 0.40μg for phosphatidyl Serine to 1.26μg for phosphatidyl Ethanolamine. The intra-day and inter-day precision of the method was below 10% for all the compounds except phosphatidylinositol in the intra-day assay with a value of 12.4%. However, the values of intra-day and inter-day repeatability show that the variations, due to the extraction procedure, do not depend whether the assays were performed after short intervals of time or not. The recovery ranged from 74% for phosphatidil Serine to 112% for phosphatidilcoline. The addition of formic acid to the mobile phase in order to achieve an acidic buffer extends the column life considerably.
Keywords: Laser microdissection; mammary tissue; carcinoma; immunolabelled cell; transcriptomics; proteomics
Abbreviations: ELSD: Evaporative Light Scattering Detector; PC: Phosphatidyl Choline; PE: Phosphatidyl Ethanolamine; PI: phosphatidyl Inositol; PS: phosphatidyl Serine; SM: Sphingo Myelin; IDF: International Dairy Federation; vol: volume; G: Gravitational field intensity; LOD: Limit Of Detection; LOQ: Limit of Quantification; SD: Standard Deviation; RSD%: Relative Standard Deviation
Introduction
Phospholipids are present in all living organisms and are the main structural and functional compounds of cellular membranes. They are amphipathic molecules with a hydrophobic moiety and a hydrophilic head group. The glycerophospholipids are characterized by a diglyceride that is covalently bonded to a phosphate group by an ester linkage, and different organic groups (choline, serine, ethanolamine and inositol) may be bound to the phosphate group. The sphingophospholipids consist of a sphingoid base on which a fatty acid is bound to form a ceramide and on this ceramide unit is linked an organophosphate group.
Phospholipids are recently been taken more into consideration because of their nutritional and technological characteristics [1]. Their inhibitory effect on some types of cancer [2-5], their ability to reduce blood cholesterol levels [5,6] and enhance brain functioning [5,7], their anti-bacterial and anti-inflammatory activity [5,8] and their protective effecton gastric mucosa [9] have been studied. Additionally, their emulsifying properties can be used in several applications in the food, pharmaceutical and cosmetic industry [10].
High-performance liquid chromatography (HPLC) coupled to an evaporative light scattering detector (ELSD) is a useful method for the determination of phospholipids in food matrices [11]. Most of the published HPLC-ELSD methods are normal-phase, with a silica gel or chemically modified silica gels (diol, cyanopropyl, aminopropyl phases) stationary phase and a gradient or isocratic elution, with different solvents; particularly, chloroform: methanol: ammonium hydroxide [12,13] and hexane: isopropanol: water/acids/bases [14]. Recently Pimentel et al. [15] reviewed the analysis procedures of phospholipids in dairy products including sample extraction and analysis by GC or HPLC using ELSD, CAD and MS detectors.
The aim of this work was to optimize a HPLC-ELSD method for qualitative and quantitative determination of the major phospholipids present in milk: phosphatidyl Ethanolamine (PE), phosphatidylinositol (PI), phosphatidyl Choline (PC), phosphatidyl Serine (PS) and Sphingo Myelin (SM).
Material and Methods
Chemicals
For sample extraction, all the reagents were of analytical grade, chloroform and methanol from Sigma-Aldrich (St. Louis, USA), HCl (25%) and NaCl from Panreac (Barcelona, Spain). For HPLC analysis, HPLC-grade chloroform, HPLC-grade methanol and ammonium hidroxide (max. 33%) were supplied by Sigma- Aldrich. Formic acid (98%) and HPLC-grade water were from Panreac.
Phospholipids standards PE and PC (from bovine brain) and PI (from bovine heart) were obtained from Larodan (Malmö, Sweden) and PS and SM (from bovine brain) from Sigma-Aldrich.
Samples for analysis
Raw cow milk samples were stored at -42°C prior to the extraction procedure.
Major components analysis
The fat content was determined by the Röse-Gottlieb method in accordance with IDF International Standard 1 [16]. All analyses were made in duplicate.
Extraction
The extraction procedure was a modification of the method described by Rombaut et al. [17]: 5 g of quark or 10 g of milk were mixed with 20mL of distilled water. The mixture was transferred into a separatory funnel and 80mL of chloroform: methanol (2:1vol/vol) was added. After shaking for 2 minutes, the mixture was separated into two layers, and the lower chloroform layer was released. Sometimes, it was necessary to centrifuge (318 x G, for 15-30 minutes at 10 °C to break the emulsion. Then, 40mL of chloroform: methanol (20:1vol/vol) was added to the upper phase. This step was repeated and the two chloroform phases were separated. In a fourth step, 40mL of chloroform: methanol: water with 1M HCl and 0.9% (w/v) NaCl (86:14:1 vol/vol/vol) was used and the lower phase was pooled with the other three, and then evaporated using a rotary vacuum evaporator at 35ºC. The lipid sample was redissolved in 10mL of chloroform: methanol (88:12vol/vol), filtered with a syringe filter (15mm×0.20μm), transferred into an amber vial and stored at -42°C until HPLC analyses. All samples were extracted and injected in duplicate.
Chromatographic analysis
Phospholipid separations were carried out using a Shimadzu HPLC system (Kyoto, Japan) composed of the following units: degasser, solvent delivery module, controller module, column oven, Rheodyne (Shimadzu) manual injector and an interfacemodule. The detector was a Shimadzu ELSD-LTII. The optimal parameters stabilised for the detector were: pressure of gas nebulizer (N2) 3.5 bar, temperature 50 °C and gain 3. A Prevail silica column (150×3mm) with 3 μm particle diameter (Grace Davison, Deerfield, IL, USA) and a precolumn with the same packing was used.
The gradient elution was a linear gradient of chloroform: methanol: buffer (0.5% formic acid with ammonium hydroxide until pH 6) 80:19.5:0.5 (vol/vol/vol) at t=0 minutes to 60:33:7 (vol/vol/vol) at t=17 minutes. The initial conditions were restored at t=20 minutes and the time required to reequilibrate the column was 15 minutes. The flow rate of the mobile phase was 0.5mL/minute, the temperature of the column oven was 35°C and the injection volume was 20μL.
The phospholipids were identified
Results and Discussion
Chromatography conditions
In the first stage of optimization of the chromatographic conditions, a mobile phase gradient of chloroform, methanol and 1M formic acid buffer, adjusted to pH 3 with triethylamine, as described by Rombaut et al. [17] was used. By this procedure the separation of the five major phospholipids (PE, PI, PS, PC and SM) was achieved. However, the resolution between PS and PI peaks was not acceptable. Therefore, several modifications were made with the aim of achieving a total separation.
Since the addition of organic ions to the mobile phase improves the resolution of acidic phospholipids such as PS and PI [18], pH and buffer concentration were changed. Buffers of different pH (3.5 and 4.0) and at different concentrations (1M and 2M formic acid) were tested, but without changing the gradient described in the method [17].
By the buffer modification the PS and PI separation was not improved. Therefore, it was decided to vary the gradient and maintain the initial buffer (1M formic acid, pH 3)
Finally, the conditions that achieved the separation of all compounds were:
Time 0 min: 87.5:12:0.5 (vol / vol / vol) chloroform: methanol: buffer
Time 20 min: 28:63:9 (vol / vol / vol) chloroform: methanol: buffer
Time 30 min: mobile phase returns to initial conditions.
Under these conditions an acceptable separation was achieved; but a prolonged use of triethylamine caused significant fluctuations in the baseline and ghost peaks appeared after minute 15, preventing the quantification of the phospholipids. According to other authors [19] another drawback of the use of modifiers like triethylamine and formic acid is the gradual deterioration in the PS peak shape.
After that, tests were performed without the use of buffer (chloroform: methanol) and substituting the buffer by water (chloroform: methanol: water). In both cases, as expected, the separation of all peaks was not achieved, since triethylamine and formic acid increase the ELSD response [20].
Then it was decided to seek an alternative to triethylamine. Before testing mobile phase mixtures in gradient, a test in isocratic with isopropanol, hexane and water was made, but the results were not satisfactory.
Subsequently, different gradients of chloroform: methanol: ammonia, mobile phases used by other authors for the separation of phospholipids [13,21] were tested, and the separation of PE, PI, PS, PC and SM was acceptable. But the use of ammonia has a major drawback, since its basic pH dissolves silica and the column life diminishes [12]. To solve this problem, formic acid was added to the solution of ammonia in order to achieve an acid pH. Different mixtures of formic acid and ammonia, and different gradients were tested to optimize the separation conditions, for the identification and quantification of the five major phospholipids present in milk. The details of the final chromatographic method were described in section 2.5.
The resolution of the peaks was 4.5 for PE-PI, 4.7 for PIPC, 2.7 for PC-PS and 1.2 for PS-SM. In all cases the resolution is higher than 1.5, except between the peaks of PS and SM. A resolution of 1.5 indicates the complete separation of the two components; whereas with a resolution of 1.0 the non-separated area is about 4% [22].
Each peak matches with a type of phospholipid, itself made up of molecular species with different fatty acids. This fact may explain the existence of peaks with shoulders and also double peaks. For the specific case of SM, when a standard of bovine origin was analyzed separately from the other phospholipids, 3 sub-peaks were detected, while in combination with the other phospholipids, it was observed that SM eluted as 2 subpeaks (Figure 1). Double or triple peak of the SM has been described in several studies [19, 23-25]; this has been attributed to the presence or absence of an extra hydroxyl group or due to the different fatty acids or sphingoid groups.
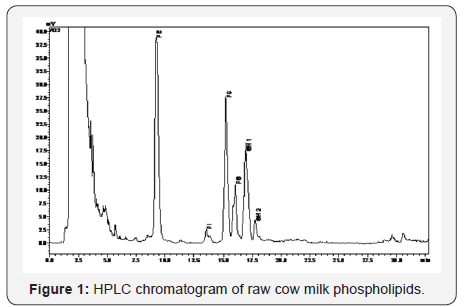
In relation to the light scattering detector parameters, the gain can be modified from 1 to 12, and it is important to choose a gain that does not saturate the detector. The nitrogen pressure should be fixed at an optimum flow of gas to produce an adequate signal to noise ratio. The evaporating temperature is another important factor; at higher temperature, the baseline shows more noise; but the choice temperature has to be enough to evaporate the mobile phase, avoiding the analyte volatilization. Different temperatures (50 °C, 55 °C, 60 °C, 65 °C and 80 °C) were tested and it was noted that when temperature increases, the response of SM decreases. According to other authors [14] this could be due to the evaporation of some free fatty acids of low boiling point. These values were finally established as follows: gain 3, nitrogen pressure 3.5 bar and evaporation temperature 50 °C.
After the chromatographic conditions and the detection parameters were established, the method validation was carried out.
Calibration and detection limits
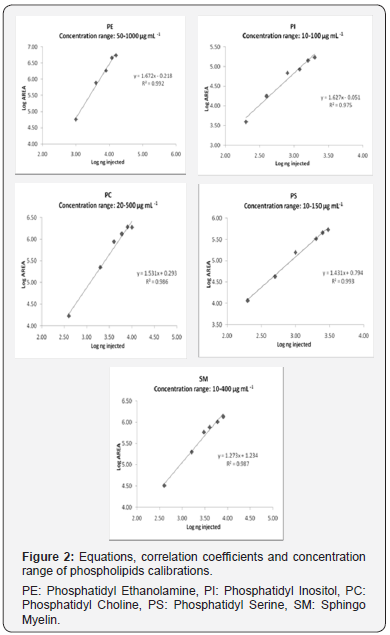
To evaluate linearity of the ELSD, two different equations were applied: linear and exponential. The response of the ELSD has been described as linear [13,17] and non-linear [26,27]. In the present study, the best results were obtained with the exponential model, which was plotted on a logarithmic scale to obtain a linear calibration. The calibration curves were calculated by applying logarithms to the area values and the mass of lipid standard injected on column. Six levels of concentration were used for the calibration of each compound. The calibration equations, correlation coefficients (R2) and concentration range for PE, PI, PS, PC and SM are shown in Figure 2. The R2 values show a satisfactory linearity, ranging from 0.993 to 0.975.
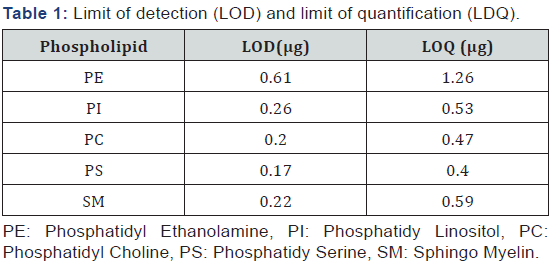
The limits of detection (LOD) and quantification (LOQ) were determined as the amount injected that provided a signal to noise ratio of 3 and 10 respectively. The LOD for the different phospholipids ranged from 0.17 μg for PS to 0.61 μg for PE and the LOQ from 0.40 μg for PS and 1.26 μg for PE (Table 1). Similar values were previously reported [19,28].
Intra-day and inter-day precision
For the determination of intra-day precision, the same sample (raw milk) was extracted 5 times, and each extraction was injected in duplicate on the same day. To evaluate the interday precision, 5 extractions were performed on 5 different days and each injected twice.
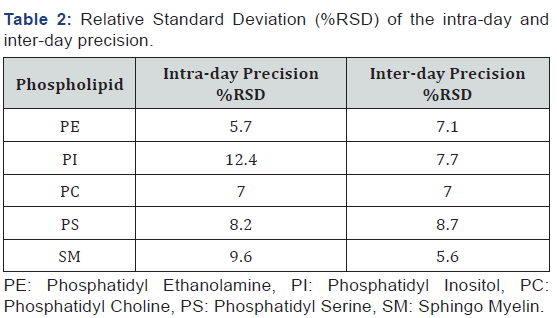
For all the compounds, the RSD% was below 10%, except for PI in the intra-day assay with a RSD% of 12.4, and there is not a higher tendency for RSD% in inter-day assays (Table 2). These results show that the variations due to the extraction procedure are quite constant and they do not depend on the time factor. The RSD% obtained was in accordance with the results reported by other authors [13,14].
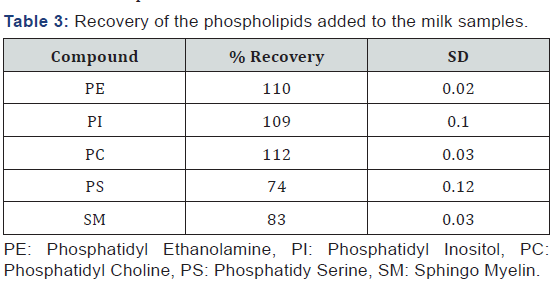
Recovery
To establish the efficiency of the extraction procedure, a mixture of the five phospholipids was added to the milk sample. The results range from 74% for PS to 112% for PC (Table 3). Taking into account the complexity of the extraction process, the results are acceptable.
Conclusion
The HPLC-ELSD is an adequate technique for the analysis of the main phospholipids present in milk. The best calibrations were achieved when an exponential model was applied. The square correlation coefficients, limits of detection, limits of quantification, and intra-day, inter-day precision and recoveries were acceptable. However, the values of intra-day and inter-day repeatability show that the variations, due to the extraction procedure, do not depend whether the assays were performed after short intervals of time or not.
Since the basic pH of the ammonia used in the mobile phase dissolves silica, the addition of formic acid to the mobile phase in order to achieve an acidic buffer, extends the column life considerably.
Acknowledgment
This study was made possible thanks to financial support the INCITE programme of the Xunta de Galicia (07TAL056E).
References
- FDewettinck K, Rombaut R, Thienpont N, Le TT, Messens K, et al. (2008) Nutritional and technological aspects of milk fat globule membrane material. Int Dairy J 18(5): 436-457.
- Lemonnier LA, Dillehay DL, Vespremi M J, Abrams J, Brody E, et al. (2003) Sphingomyelin in the suppression of colon tumors: prevention versus intervention. Arch Biochem Biophys 419(2): 129-138.
- Kuchta AM, Kelly PM, Stanton C, Devery RA (2012) Milk fat globule membrane-a source of polar lipids for colon health? A review. Int J Dairy Technol 65(3): 315-333..
- Castro-Gómez P, Rodríguez-Alcalá LM, Monteiro KM, Ruiz AL, Carvalho JE, et al. (2016) Antiproliferative activity of butter milk lipid fractions isolated using food grade and non-food grade solvents on human cancer cell lines. Food Chem 212: 695-702.
- Verardo V, Gómez-Caravaca AM, Arráez-Román D, Hettinga K (2017) Recent advances in phospholipids from colostrum, milk and dairy by products. Int J Mol Scie 18(1): 173.
- Duivenvoorden I, Voshol PJ, Rensen PC, van Duyvenvoorde W, Romijn JA, et al. (2006) Dietary sphingolipids lower plasma cholesterol andtriacylglycerol and prevent liver steatosis in APOE*3Leiden mice. Am J Clin Nutr 84(2): 312-321.
- McDaniel M, Maier S, Einstein G (2003) Brain-specific nutrients: a memory cure? Nutrition 19(11-12): 957-975.
- Vesper H, Schmelz E, Nikolova-Karakashian MN, Dillehay DL, Lynch DV, et al. (1999) Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J Nutr 129(7): 1239-1250.
- Kivinen A, Tarpila S, Salminen S, Vapaatalo H (1992) Gastroprotection with milk phospholipids: a first human study. Milchwissen schaft 47(11): 694-696.
- Leser ME, Sagalowicz L, Michel M, Watzke HJ (2006) Self-assembly of polar food lipids. Adv Colloid Interface Sci 123-126: 125-136.
- Rombaut R, Dewettinck K, Van Camp J (2007) Phospho- and sphingolipid content of selected dairy products as determined by HPLC coupled to an evaporative light scattering detector (HPLC–ELSD). J Food Comp Anal 20: 308-312.
- Vaghela MN, Kilara A (1995) Quantitative analysis of phospholipids from whey protein concentrates by high-performance liquid chromatography with a narrow-bore column and evaporative lightscattering detector. J Am Oil Chem Soc 72(6): 729-733.
- Avalli A, Contarini G (2005) Determination of phospholipids in dairy products by SPE/HPLC/ELSD. J Chromatogr A 1071(1-2): 185-190.
- Descalzo AM, Insani EM, Pensel NA (2003) Light-scattering detection of phospholipids resolved by HPLC. Lipids 38(9): 999-1003.
- Pimentel L, Gomes A, Pintado M, Rodríguez-Alcalá LM (2016) Isolation and analysis of phospholipids in dairy foods. J Anal Methods Chem 9827369.
- International Dairy Federation (2010) Milk-Determination of fat content-gravimetric method (Reference method). International Standard 1, International Dairy Federation, Brussels, Belgium.
- Rombaut R, Camp JV, Dewettinck K (2005) Analysis of phospho- and sphingolipids in dairy products by a new HPLC method. J Dairy Scie 88(2): 482-488.
- Christie WW (1986) Separation of lipid classes by high-performance liquid chromatography with the mass detector. J Chromatogr 361: 396- 399.
- Fagan P, Wijesundera C (2004) Liquid chromatographic analysis of milk phospholipids with on-line pre concentration. J Chromatogr A 1054(1-2): 241-249.
- Deschamps FS, Gaudin K, Lesellier E, Tchapla A, Ferrier D, et al. (2001) Response enhancement for the evaporative light scattering detection for the analysis of lipid classes and molecular species. Chromatographia 54(9): 607-611.
- Becart J, Chavalier C, Biesse, JP (1990) Quantitative analysis of phospholipids by HPLC with light scattering detector: application to raw materials for cosmetic use. J High Resolut Chromatogr 13(2): 126- 129
- Skoog DA, Holler FJ, Nieman TA (2003) Principios de análisis instrumental. (5th edn), McGraw-Hill DL, Madrid, Spain, pp. 745-746.
- Christie W, Noble R, Davies G (1987) Phospholipids in milk and dairy products. Int J Dairy Technol 40(1): 10-12.
- Rombaut R, Camp JV Dewettinck K (2006) Phospho- and sphingolipid distribution during processing of milk, butter and whey. Int J Food Sci Tech 41(4): 435-443.
- Lopez C, Briard-Bion B, Menard O, Rousseau F, Pradel P, et al. (2008) Phospholipid, sphingolipid, and fatty acid compositions of the milk fat globule membrane are modified by diet. J Agr Food Chem 56: 5226- 5236.
- Bünger H, Pison U (1995) Quantitative analysis of pulmonary surfactant phospholipids by high-performance liquid chromatography and lightscattering detection. J Chromatogr B Biomed Sci Appl 672: 25-31.
- Megoulas NC, Koupparis MA (2005) Twenty years of evaporative light scattering detection. Crit Rev Anal Chem 35(4): 301-316.
- Rodríguez-Alcalá LM, Fontecha J (2010) Major lipid classes separation of buttermilk, and cows, goats and ewes milk by high performance liquid chromatography with an evaporative light scattering detector focused on the phospholipid fraction. J Chromatogr A 1217(18): 3063- 3066.






























