The Effects of Guided Breathing at a Fixed Frequency on Untreated Mild Hypertension
David O Hare
Department of Medicine, Ellasanté Health Center, supported by the Pileje Foundation, Paris, France, Urgotech
Submission: June 13, 2024; Published: June 24, 2024
*Corresponding author: Elodie Levert, clinical research associate, Department of Medicine, Ellasanté Health Center, Paris, France
How to cite this article: David O. The Effects of Guided Breathing at a Fixed Frequency on Untreated Mild Hypertension. J Complement Med Alt Healthcare. 2024; 12(5): 555849. DOI: 10.19080/JCMAH.2024.12.555849
Abstract
This is a minimally risky interventional pilot study, single center, conducted in Paris, France, describing the use of a smartphone application to guide patients in the practice of Fixed Frequency Guided Breathing (FFGB) with the aim of evaluating the effects of FFGB on a population of adults with untreated mild hypertension (HTN). Patients were asked to perform their guided breathing exercises at home, following the breathing guide on their smartphones, 3 times a day at a frequency of 0.10Hz with a breathing rate of 6 cycles per minute for 5 minutes over a period of 6 weeks.
Keywords: Hypertension, Breathing, Exercise, heart, Fixed frequency
Abbreviations: FFGB: Fixed Frequency Guided Breathing; HTN: Hypertension; BP: Blood Pressure; PSS: Perceived Stress Scale; PP: Pulse Pressure; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; PP : Pulse Pressure; HR: Heart Rate; ITT: Intent-to-Treat; ITTM: Modified Intent-to-Treat Population; PP: Protocol Population; GBE: Guided Breathing Exercise
Introduction
Hypertension (HTN) is one of the most common disorders, affecting about one-third of the adult population worldwide [1]. The treatment of HTN includes the use of pharmacological and non-pharmacological treatments. Non-pharmacological treatments include meditation, yoga, acupuncture, biofeedback, etc. These techniques have the advantage of being low-cost and having few or no side effects. Randomized controlled trials have been conducted on the use of non-pharmacological methods to help reduce blood pressure (BP) in hypertensive patients. Numerous studies have been conducted on the RESPeRATE device for reducing BP in hypertensive diabetic patients and hypertensive patients [2-4]. A randomized controlled trial evaluating the BIM (Breathe with Interactive Music) device in hypertensive patients demonstrated a statistically significant reduction of about 4 mmHg [5]. A general review on the non-pharmacological treatment of hypertension is provided in the American Heart Association’s scientific statement [6]. However, these recommendations have been refuted following a general review of 365 patients using device-guided breathing [7]. The authors found that studies with acceptable methodological quality demonstrate no evidence of a short-term beneficial effect on BP using device-guided breathing. Furthermore, no benefit could be demonstrated in a double-blind randomized controlled trial initiated by an independent investigator [8]. The practice of Fixed Frequency Guided Breathing (FFGB) has been used under various names (e.g., cardiac coherence or neuropsychological coherence) for about twenty years by healthcare professionals as a tool for stress management. The French Federation of Cardiology [9] recommends this practice through the so-called 365 method (Respiratory Cardiac Coherence: 3 times a day, 6 breaths per minute, for 5 minutes) as a first approach in the prevention of cardiovascular risks through stress prevention. This minimally risky interventional pilot study describes the use of a smartphone application to guide patients in the practice of FFGB. This study was designed to estimate the effects of FFGB on a population of adults with untreated mild HTN. The results of this study will help formulate hypotheses for the implementation of a future comparative study.
Objectives
The primary objective of this study was to evaluate the effects of regular guided breathing practice at a frequency of 0.10Hz (3 sessions per day with a breathing rate of 6 cycles per minute for 5 minutes) on the blood pressure readings of a population of adults with untreated mild hypertension at the time of study inclusion. The secondary objective was to evaluate the subjective effects of Fixed Frequency Guided Breathing (FFGB) practice on perceived stress using the Perceived Stress Scale (PSS10).
Methodology
This is a minimally risky interventional pilot study, single center, conducted in France with a cohort of patients with untreated mild hypertension at the time of study inclusion. Hypertensive patients attending the Ellasante Health Center (29bis Rue d’Astorg, 75008 Paris) were invited to participate in the study. The plan was to include at least 30 patients in the study. Patients could be included in the study if they met the following criteria: male or female aged 18 to 80 years, hypertension with a diastolic blood pressure (DBP) between 90 mmHg and 100 mmHg, and/or a systolic blood pressure (SBP) between 140 mmHg and 160 mmHg; hypertension observed during at least 3 successive consultations over a period of 3 to 6 months; possession of a smartphone or equivalent (for installation of the Urgo Feel breathing guidance application).
Exclusion criteria for the study: History of occlusive cardiovascular disease; respiratory pathology preventing FFGB; pathology or psychological condition preventing understanding of the study recommendations; participation in another clinical trial or being in an exclusion period from another study.
Primary Outcome Analysis
The primary outcome was the change in blood pressure (BP) between the inclusion visit and the end-of-study visit at 6 weeks. The three BP measurements: SBP, DBP, and pulse pressure (PP) were presented at inclusion, mid-study visit, and end-of-study visit (6 weeks). A Wilcoxon signed-rank test was performed to verify the significance of the BP decrease during the study.
Secondary Outcome Analysis
The secondary outcome was the change in the Perceived Stress Scale (PSS10) between the inclusion visit and the end-ofstudy visit at 6 weeks. The 10 items of the PSS questionnaire are described as qualitative variables. The PSS, which is the sum of the scores of each of the 10 questionnaire items, is presented as a quantitative variable and in three classes as a qualitative variable. The score was not calculated if at least 3 items (30%) were not completed. The difference between the PSS10 score at 6 weeks and at inclusion is presented. A Wilcoxon signed-rank test was performed to verify the significance of the PSS10 change during the study.
Number of Subjects Needed
This study, being a pilot study, did not require a sample size calculation. However, to obtain relevant results, it was estimated that it was necessary to recruit a minimum of 30 patients (27 patients with an anticipated dropout rate of 10%) (Figure 1).
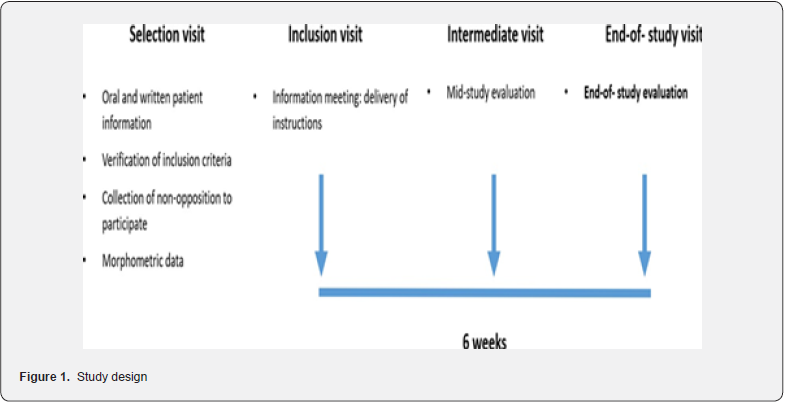
Inclusion Visit
The study was explained, and patients were instructed to practice guided breathing exercises at home using a respiratory guide on their smartphone, 3 times a day (morning, afternoon, and evening) at a frequency of 0.10 Hz with a breathing rate of 6 cycles per minute for 5 minutes over a period of 6 weeks. At baseline, patients were required to assess their perceived stress using the PSS10 questionnaire (10 items measuring the extent to which life situations are perceived as threatening, unpredictable, uncontrollable, or overwhelming). Systolic blood pressure (SBP), diastolic blood pressure (DBP), Pulse Pressure (PP), and heart rate (HR) were also measured. Weight, risk factors, comorbidities, and previous/concomitant treatments were also recorded.
Mid-Study Visit (at 3 weeks)
A visit to the health center where SBP, DBP, PP, and weight were measured. Patient compliance with the practice of GBE was assessed (“Yes”, “Moderately”, “No”).
End of Study Visit (at 6 weeks)
At the health center for the measurement of SBP, DBP, PP, and weight, and evaluation of perceived stress using the same PSS10 questionnaire. Patient compliance with guided breathing exercise practice was reported during the visit.
Results
Study Populations:
i. Intent-to-Treat Population (ITT): All 30 patients participated in the study.
ii. Modified Intent-to-Treat Population (ITTm): 23 patients (76.7%) who completed the end-of-study visit and for whom the primary criterion was evaluable
iii. Per Protocol Population (PP): 15 patients (50.0%) from the ITT Population who participated throughout the study without protocol violations.
Demographics at Inclusion:
i. Gender: 52.2% females (12 patients) and 47.8% males (11 patients).
ii. Age (mean (SD)): 43.4 (14.0) years.
iii. Height (mean (SD)): 174.0 (8.8) cm.
iv. Weight (mean (SD)): 81.8 (17.9) kg.
v. BMI (mean (SD)): 26.9 (5.2) kg/m².
Average Weight Change (SD) (Compared to Inclusion):
i. 3 weeks: -0.2 (0.5) kg.
ii. 6 weeks: -0.8 (3.5) kg.
Adherence (ITT Population):
i. 3 weeks:
a. Adherent (GBE 3 times a day): 58.3% (14 patients).
b. Moderately adherent (GBE 2 times a day): 33.3% (7 patients).
c. Not adherent (GBE less than 2 times a day): 8.3% (2 patients).
ii. 6 weeks:
a. Adherent (GBE 3 times a day): 43.5% (10 patients).
b. Moderately adherent (GBE 2 times a day): 34.8% (8 patients).
c. Not adherent (GBE less than 2 times a day): 21.7% (5 patients).
SBP (mean (SD): ITTm Population):
i. Inclusion: 151.7 (9.0) mmHg.
ii. 3 weeks: 138.9 (12.1) mmHg: -12.7 (8.7) mmHg compared to Inclusion (p<0.0001).
iii. 6 weeks: 141.4 (12.2) mmHg: -10.2 (14.0) mmHg compared to Inclusion (p=0.0003).
DBP (mean (SD): ITTm Population):
i. Inclusion: 95.8 (5.9) mmHg.
ii. 3 weeks: 93.7 (8.0) mmHg: -2.3 (6.9) mmHg compared to Inclusion (p=0.1854).
iii. 6 weeks: 93.9 (10.5) mmHg: -1.9 (9.0) mmHg compared to Inclusion (p=0.2116).
PP (mean (SD): ITTm Population):
i. Inclusion: 55.8 (9.3) mmHg.
ii. 3 weeks: 45.3 (11.6) mmHg: -10.4 (9.5) mmHg compared to Inclusion (p=0.0001).
iii. 6 weeks: 47.5 (9.3) mmHg: -8.3 (10.9) mmHg compared to Inclusion (p=0.0006).
Heart Rate (mean (SD): ITTm Population):
i. Inclusion: 75.5 (12.0) bpm.
ii. 3 weeks: 75.2 (9.7) bpm: -1.9 (10.7) bpm compared to Inclusion (p=0.6170).
iii. 6 weeks: 77.0 (10.3) bpm: 1.4 (10.1) bpm compared to Inclusion (p=0.4168).
PSS10 Score (mean (SD): ITTm Population):
i. Inclusion: 22.4 (5.2).
ii. 6 weeks: 21.4 (3.5): (-0.8 (4.4) compared to Inclusion, p=0.4825).
Adverse Effects: No adverse effects were reported during the study.
Discussion
The majority of patients demonstrated good adherence during the study, indicating the ease and feasibility of practicing Guided Breathing Exercise (GBE) for these patients with mild hypertension, supported by positive guidance. The study shows a statistically significant reduction in SBP and PP at both 3 and 6 weeks, and a non-significant decrease in DBP with GBE practice. The analysis of the PSS10 score suggests an improved stress management (though not statistically significant) by the end of the study. A slight reduction in weight was also demonstrated. This pilot study provides a solid foundation for future research to understand the underlying mechanisms of guided breathing at fixed frequency and to evaluate its long-term efficacy in managing mild hypertension. Randomized controlled trials are necessary to confirm these preliminary findings and explore further the clinical implications of this innovative approach.
Conclusion
In conclusion, preliminary results from this study suggest that guided breathing at fixed frequency could represent a promising non-pharmacological strategy to lower blood pressure in individuals untreated for mild hypertension. These findings encourage further research to better understand and refine this approach as a potential complement to conventional treatments.
References
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, et al. (2005) Global burden of hypertension: analysis of worldwide data. Lancet 365(9455): 217-223.
- Schein MH, Gavish B, Baevsky T, Kaufman M, Levine S, et al. (2009) Treating hypertension in type II diabetic patients with device-guided breathing: a randomized controlled trial. H Hum Hypertension 23(5): 325-331.
- Sharma M, Frishman WH, Gandhi K (2011) RESPeRATE: non-pharmacological treatment of hypertension. Cardiol Rev 19(2): 47-51.
- Cernes R, Zimlichman R (2017) Role of Paced Breathing for Treatment of Hypertension. Curr Hypertens Rep 19(6): 45.
- Schein MH, Gavish B, Herz M, Rosner-Kahana D, Naveh P, et al. (2001) Treating hypertension with a device that slows and regularizes breathing: a randomized, double-blind controlled study. J Hum Hypertens 15(4): 271-278.
- Brooke RD, Lawrence JA, Melvyn R, Gbenga O, John DB, et al. (2013) American Hypertension Association. Beyond medications and diet: alternative approaches to lowering blood pressure. Hypertension 61(6): 1360-1383.
- Van Hateren KJJ, Landman GWD, Logtenberg SJJ, Bilo HJG, Kleefstra N (2014) Device-guided breathing exercises for the treatment of Hypertension. World J Cardiol 6(5): 277-282.
- Landman GWD, Drion I, van Hateren KJJ, van Dijk PR, Logtenberg SJJ, et al. (2013) Device-guided breathing as treatment for hypertension in type 2 diabetes mellitus: a randomized, double-blind, sham-controlled trial. JAMA Intern Med 173(14): 1346-1350.
- (2023) Brochure "Coeur et Stress" from the French Federation of Cardiology.






























