Evaluation of Anti-Inflammatory and Anti-Arthritic Activities of AV11-03 in Female Rats
Chandrasekhar VM1, Karishma S2, Shivprakash R2 and Pundarikakshudu K2*
1Department of Pharmacology, Hangal Shri Kumareshwar College of Pharmacy, India
2Department of Research and Development, Avance Phytotherapies, India
Submission: January 23, 2023; Published: April 13, 2023
*Corresponding author: Pundarikakshudu K, Department of Research and Development, Avance Phytotherapies, India
How to cite this article: Chandrasekhar VM, Karishma S, Shivprakash R,d Pundarikakshudu K. Evaluation of Anti-Inflammatory and Anti-Arthritic Activities of AV11-03 in Female Rats. J Complement Med Alt Healthcare. 2023; 11(5): 555825.DOI: 10.19080/JCMAH.2023.11.555825
Abstract
Rheumatiod arthritis is a systemic auto-immune disease characterized by chronic inflammation at multiple synovial joints, cartilage destruction and bone erosion. Currently available non-steroidal anti-inflammatory drugs, cortico-steroids and disease modifying anti-rheumatic therapies have severe side effects on prolonged use and thus throw a challenge for development of safe, effective alternatives. Of the many plant drugs claimed to be effective in the treatment of rheumatoid arthritis, in depth studies were carried out on the fruits of Terminalia chebula (Haradae) and the oleo-gum resin of Boswellia serrata (Sallaki). Glucosamine was shown to be chondrio-protective and effective in osteo-arthritis. Hence, we tested ‘AV-11 03’ which is a combination of the extracts of haradae, salliki an maize glucosamine on acute and chronic inflammatory models by studying a battery of physical, biochemical, histological and radiological parameters.
Materials and Methods
In the acute-inflammatory study, 32 female Sprague-Dawley rats (four groups each with 8 rats) weighing from 150 -200 g were injected 0.l mL of 1.0% carrageenan on the right hind paw after oral administration of tween 80 (1% v/v), diclofenac (10 mg/kg), AV-11 03 (200 mg/kg and 400 mg/kg). Paw volumes were measured at 30 minutes intervals up to 5 hours on a digital plethysmometer and per cent inhibition of the inflammation was calculated. Chronic inflammation was induced in female Sprague-Dawley rats (four groups of 8 rats each) by injecting Complete Freund’s Adjuvant (CFA) into the sub plantar region of the right hind paw after anesthesia (45 mg/Kg of Ketamine chloride). Diclofenac (10 mg/kg), AV-11 03 (200 mg/kg and 400 mg/kg) were administered from day one to day28 and various chronic inflammatory parameters like paw odema, development of arthritis, horizontal locomotor activity, grooming and open field exploration, vascular permeability and histamine release were measured on 7th, 14th, 21st and 28th days. At the end of the experiment, X-rays of the inflamed paws were taken, and rats were sacrificed to study serum and liver biochemical parameters and histological changes in the paws.
Results
Significant (p<0.01) reductions in paw odema, development of arthritis, grooming, vascular permeability and histamine release were noted in the AV-11 03 treated rats. The rats in these treatment groups exhibited increased locomotor activity and open field exploration. There was significant amelioration in all the serum and liver biochemical parameters. E.S.R. values significantly decreased in the AV-11 03 treated rats as compared to those in CFA treated rats at the end of day 28. X-ray analysis revealed a reduction in soft tissue swelling, bone deformities and bone erosion in the paws of AV-11 03 treated rats as compared to those in the CFA treated group. Severe inflammation and erosion of cartilage were seen in the micro-sections of the paws of CFA treated rats. These features were found to be mild in the paw sections of AV-11 03 treated rats. AV-11 03 at a dose of 400 mg/kg exhibited optimum activity.
Conclusion
AV-11 03 has significant activity in chronic and acute arthritis at a dose of 400 mg/kg.
Keywords: AV-11 03; Terminalia chebula; Boswellia serrata; Glucosamine; Carrageenan; Complete Freund’s adjuvant
Introduction
Rheumatoid arthritis is an inflammatory condition of synovial tissues leading to the destruction of joints [1]. Over a period, this results in deformity and destruction of the joints due to loosening of ligaments and erosion of cartilage [2]. It is a systemic auto-immune disease characterized by chronic inflammation at multiple synovial joints, cartilage destruction and bone erosion [3,4]. Non-steroidal anti-inflammatory drugs (NSAIDs), cortico-steroids and disease modifying anti-rheumatic drugs are currently the available treatment options. But all of them have mild to severe undesirable effects on the patients [5,6]. Due to this reason, alternative systems of medicines including herbal medicines have been sought after for treatment of various types of arthritis, inflammation, and pain.
AV11-03 is a mixture of standardized extracts of Terminaliachebula Retz, (family: Combretaceae), Boswellia serrata (Family: Burseraceae) and glucosamine derived from Zea mays (Family: Poaceae).
Unripe fruit of Terminaliachebula popularly known as ‘Haritaki’ is employed in the traditional systems of medicine for the treatment of gastrointestinal diseases, chronic fever, anemia, metabolic disturbances, joint pains and rheumatoid arthritis [7,8]. 50% hydro-alcohol extract of T. chebula was shown to significantly reduce arthritic index, synovial hyperplasia and joint narrowing in collagen II induced arthritic (CIA) mice. Production of TNF-α, IL-6 and IL -1β were suppressed while INF-γ production was unaffected. When splenocyte cells stimulated with type II collagen were exposed to this extract, IL-17 levels were also found to be reduced. Hemocytometer and flow cytometer studies on draining lymph and knee joints of CIA mice treated with the extract showed reduction in the number of helper T cells, Cytotoxic T cells, activated helper T cells and activated T cells. The extract was also found to inhibit acetic acid induced writhing in mice [9].
Anti-inflammatory activities of T. chebula were reported in systemic and local anaphylaxis and LPS-stimulated RAW 2647 cells [10,11].
Aqueous extract of T. chebula, when given to osteo-arthritic dogs, significantly ameliorated their condition [12]. Reddy et al. [13] (2009) found inhibition of COX2 and lipoxygenase (5-LOX) by chebulagic acid, a key component of T. chebula fruit. Das et al [14] reported inhibition of NF-κB by the extract of this fruit. The hydro-alcoholic extract of T. chebula fruits when given to Complete Freund’s Adjuvant (CFA) induced arthritic rats, significantly reduced TNF-α and synovial expression of RNF- R1, IL-6 and IL-1 β. Joint swelling was also found to be reduced markedly [15]. In clinical trials involving healthy human volunteers, standardized aqueous extract of T. chebula fruit increased mean pain threshold time and mean pain tolerance time [16].
Glucosamine is a derivative of cellular glucose metabolism found in the cartilage of bones. It has good absorption from gastrointestinal tract on oral administration [17]. It was found to reduce inflammation by inhibiting NF-κB production induced by IL-1 [18,19]. Reduction of prostaglandin E2 by chondrocytes of patients on glucosamine treatment was reported by Nakamura et al. [20].
Gum resin of Boswellia serrata, commonly known as ‘Sallai guggul’ in India, has been used in Ayurvedic system of medicine for the treatment of various inflammatory conditions [21]. It was found to suppress Intrleukin - 1 β (IL- 1β), Tumor necrosis factor -α (TNF-α), Interferon - γ (INF-γ) and promote production of IL-10 in collagen induced arthritis rats [22]. In CFA induced arthritis of rats, Boswellia serrata resin gum extract reduced inflammation, TNF-α levels, cartilage disruption, pannus formation, vascular proliferation and fibrinoid necrosis of the inflamed hind limb [23].
Acidic and non-acidic fractions of Boswellia serrata (Serratrin) showed strong inhibition of 5-lipoxinage activity and production of leukotriene B4 and prostaglandin E2 in human blood derived cells. There was significant improvement in body weight bearing capacity, pain sensitivity threshold to pressure in monoiodoacetate induced osteoarthritis in rats. Structural damage to the cartilage and loss of extracellular matrix was also prevented [24].
In a clinical study involving seventy osteo-arthritis (OA) patiens, 5-loxin, a standardized Boswellia serata extract containing 30% 3-O-acetyl-11-keto-beta boswellic acid (AKBA) reduced pain and improved physical functioning as also prevented enzymatic degradation of cartilage in the OA patients [25].
In a meta-analysis of clinical trials of Boswellia serrata on OA patients, data from seven trials involving 545 patients furnished clear evidence of the potential therapeutic activity of the gum resin in improving the symptoms of the disease [26]. Since AV11-03 is a combination of the above three potential herb extracts, we were interested to study its effect on acute and chronic arthritis and various parameters associated with this disease.
Materials and Methods
Material
AV11-03 is a herbal extract mixture from standardized extracts of Terminaliachebula (35% w/w total phenols calculated as gallic acid), Boswellia serrata (50% w/w of total boswellic acids) and glucosamine (90% derived from Zeamays). The mixture contains150 mg of glucosamine, 50 mg each of Terminalia chebula and Boswellia serrataextracts
Chemicals
Carrageenan, DTNB, TBA, TCA, Epinephrine, DPPH, Griess reagent, (Sigma – Aldrich Chemicals Co., U.S.A.); Sodium Nitroprusside, EDTA, H2O2 (Qualigens Fine Chemicals Pvt. Ltd. India); Diclofenac (Empree Medicaments Ltd., Belgaum, Karnataka, India); Tween 80 (Merck Specialties Pvt. Ltd., Mumbai, India; FeSO4, Methanol (NICE Chemicals Pvt. Ltd. Mumbai); Potassium chloride (SD fine Chemicals Ltd., Mumbai); L-ascorbic acid, Tris free base (Hi-media Laboratory, Mumbai); SGOT, SGPT, ALP, Bilirubin kits (ERBA diagnostic Kits, Germany).
Instruments
UV-Spectrophotometer (UV-1601, Shimadzu, Japan); Auto-analyzer (Star 12 Plus, India); Refrigerated centrifuge (MPW-350 R, South Korea); Deep Freezer (DFC-84CE OPERON, South Korea); Tissue homogenizer (RQ 127A-REMI, India); Blood cell counter (Swelab-alfa, Sweden) ELISA reader (Bio Tech, U.S.A.); Digital Plethysmometer-7140 (UGO Basile, Italy).
Animals
Sprague-Dawley rats of female sex (150-200 grams) were obtained from the central animal house of H.S.K. college of Pharmacy and Research Centre, Bagalkot. In standard propylene cages at 22-28 0C, 65 ± 10% Relative Humidity, 12 h dark light cycle. All animals had free access to standard pellet diet (Pranav Agro Industries, Sangli, Maharashtra, India) and water. All experimental protocols were approved by institutional Animal Ethics Committee (IAEC/HSK/RP/e, 30-4-2011).
Preparation of test sample solutions
Carrageenan: 1% carrageenan in normal saline
Diclofenac: 100 mg diclofenac was dissolved in 10 ml of normal saline (1% w/v)
AV11-03: 1 g was suspended in 5 mL 1% tween 80.
Effect of AV11-03 on Carrageenan induced rat paw Oedema
Rats were fasted overnight and divided into four groups of eight rats in each group. The groups are as under:
Group I: Control group (1% v/v tween 80; po) + Carrageenan (0.1 mL)
Group II: Diclofenac (10 mg/Kg; po) + Carrageenan (0.1 mL)
Group III: AV11-03 actives (200 mg/Kg, po) + Carrageenan (0.1 mL)
Group IV: AV11-03 actives (400 mg/Kg, po) + Carrageenan (0.1 mL)
Oedema was induced by injecting 0.1 ml of carrageenan solution (1% w/v in normal saline) into the sub-plantar region of the right hind paw 30 minutes after the administration of the test drug. Paw volume was measured on a digital plethysmometer at 0, 0.5, 1, 2, 3, and 5 hours after carrageenan injection [27]. The per cent inhibition of paw oedema in each treated group was calculated by the equation:
% inhibition
Where
= Mean paw volume of treated group
= Mean paw volume of control group
Effect of AV11-03 on Complete Freund’s Adjuvant (CFA) induced chronic arthritis in rats
The method of Newbould (1963) [28] was followed.
Rats were divided into four groups of eight animals each as given hereunder:
Group I: Control group (1% v/v tween 80; po) + CFA
Group II: Diclofenac (10 mg/Kg; po) + CFA
Group III: AV11-03 actives (200 mg/Kg, po),) + CFA
Group IV: AV11-03 tablet (equivalent to 400 mg/Kg of actives, p AV11-03 actives (400 mg/Kg , po) + CFA
CFA was injected into the sub planter region of the right hind paw after anesthesia (45 mg/Kg of Ketamine chloride). The animals were administered the test drugs every day from the start of the experiment. Sub-plantar injection of CFA produces local oedema after few hours with a progressive increase reaching to maximum on the 28th day [29]. Hence, the animals were thoroughly inspected for 28 days for various arthritis related parameters.
Paw oedema
Paw oedema volume was measured on a digital plethysmometer on 7th, 14th ,21st and 28th day and the percentage of oedema inhibition was calculated as described above.
Evaluation of Development of arthritis (DOA)
Development of arthritis was evaluated by two blinded observers on 7th, 14th, 21st and 28th days after the injection of CFA. The observations were made on a three-point scale for each paw. 0 = normal joint; 1 = slight inflammation and redness; 2 = severe erythema and swelling affecting the entire paw with inhibition of its use; and 3 = deformed paw or joint with ankylosis, joint rigidity and loss of function [30].
Spontaneous behavioral parameters on open-field test
The method of Parent (2012) [31] was adopted in this observational study. The rat is placed in an open field in the sound-attenuated room. The floor is white polyvinyl with a black grid dividing open field into 100 squares (10 x 10). Illumination is provided by a bulb (60 W) placed above the center of the field, while the rest of the room is kept dark. Initially, the rat was placed in the center of the field and observed for 5 minutes in all behavioral parameters, and these include latency (in sec) time to start exploring the open field, horizontal locomotor activity (grid lines crossed) and grooming (rubbing the nose with its forepaws and preening). All the observations were made between 18.00 and 20.00 hours 0th, 7th, 14th, 21st and 28th day.
Assessment of vascular permeability-Evans blue extravasation test
The method described by Franchis et al (2004) [32] was adopted for this study. Evan’s blue (50 mg/kg) was administered via the jugular vein of the anaesthetized rat. After 4 h, the rats were sacrificed by ether anesthesia, anterior and posterior synovial capsules and fat pad were dissected from each knee joint. The tissue obtained from each knee was weighed and the amount of Evan’s blue in the sample was estimated. The tissue was cut into small pieces and mixed with acetone in 1% Na2SO4 in 7:3 ratio. The samples were gently shaken continuously for 24 h at room temperature and centrifuged for 10 minutes at 2000 rpm. 2.0 ml of the supernatant was taken and absorbance was measured at 620 nm on a UV-sspectrophotometer. The amount of dye recovered from the tissue was calculated from a calibration curve of Evan’s blue. Percentage inhibition of joint infiltration was calculated by the equation.
Percentage Inhibition of Joint infiltration
Where, = mean Evan’s blue infiltration of treated group
= mean Evan’s blue infiltration of control group
Estimation of release of histamine from blood
The method described by Tiligada et al. [33] was adopted. Briefly, 5.0 ml of blood collected from cardiac puncture of rat heart was taken in a test tube and to it 4.5 ml distilled water and 0.5 ml 10 -12 N HClO4 (perchloric acid) were added, After keeping for 10 minutes at room temperature, 9 ml of 0.4N.
HClO4was added and homogenized in a glass homogenizer, kept for 10 minutes and centrifuged. 4.0 ml of the supernatant fluid was shaken with 0.5 ml of 5 N NaOH, 1.5 g of solid sodium chloride and 10 ml of n-butanol. The contents were shaken for 5 minutes and centrifuged. The n-butanol phase was again shaken for one minute with 5.0 ml of salt-saturated 0.1N NaOH to remove any residual amounts of histidine. The contents are centrifuged at 500 -600 rpm , 8.0 ml of butanol phase was taken, 4.5 ml of 0.1 N HCl and 15.0 ml of n-heptane were added, centrifuged and the aqueous phase was collected for the assay.
To 2.0 ml of the aqueous phase 0.4 ml 1 N NaOH was added followed by 0.1 ml of o-phthalaldehyde (o-PΤ) reagent and mixed thoroughly. After 4 minutes, 0.2 ml of 3 N HCl was added and the contents were thoroughly mixed. The fluorescence of the acidified solution was measured at 450 nm in a spectrofluorimeter. The quantity of the histamine in the sample was calculated from a standard curve of histamine.
Estimation of serum biochemical parameters
At the end of the experiment on the 28th day, blood was collected from the animals by retro orbital puncture and centrifuged at 3000 rpm for 10 minutes to separate the serum for the estimation of total proteins, aspartate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), and Creatinine. Total proteins were estimated by the method of Lowry et al. [34]. AST, ALT , ALP and creatinine were analysed as per the instructions given by the supplier of the kits.
Estimation of liver biochemical parameters
After sacrificing he animals, liver was removed and homogenized in cold phosphate buffer (0.1 M pH 7.4). The homogenates were centrifuged at 10000 rpm for 10 minutes at 4ᴼC and post-mitochondrial supernatant (PMS) was used for the estimation of lipid peroxidation (LPO). The supernatant was again centrifuged at 15000 rpm for 1 hour at 4ᴼC and the supernatant obtained was used for further estimation of superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH).
For the estimation of LPO, thiobarbituric acid reactive substances (TBARS) in the homogenate was estimated by the method described by Chandrasekhar et al. [35] and expressed as TBRAS nmoles/mg of protein. SOD activity was determined based on the ability of SOD to inhibit the auto-oxidation of epinephrine to adrenochrome at alkaline pH [36]. Catalase activity was assayed by the method of Claiborne (1985) [37] and was calculated in terms of nM H2O2 consumed/min/mg protein. For the estimation of GSH, the method of Sedak and Lindsay [38] was employed. Total thiols: This assay is based on the principle of formation of relatively stable yellow color by sulfhydryl groups with DTNB[(5, 5’-Dithio-bis (2-Nitrobenzoid Acid)] the experiment was carried out as described by Sedak and Lindsay (1968) [38].
Estimation of hematological parameters and E. S. R.
The blood collected through retro-orbital puncture at the end of the experiment on day 28 was taken into EDTA tubes and shaken thoroughly. The samples were analyzed on a blood cell counter (Swelab-alfa, Sweden) for the determination of White Blood cells (WBCs), Red Blood Cells (RBCs), Haemoglobin (Hb),), Platelets, Lymphocytes and Granulocytes [39]. Determination of Erythrocyte Sedimentation Rate (ESR) was done as described by Jain et al. [40].
X-ray radiographic analysis
The radiographic analysis was carried out as described by Kalpesh et al. [41]. On day 28, the rats were anaesthetized with 45 mg/Kg ketamine hydrochloride and radiographs of CFA injected paws were taken with a Dental X-ray machine and the paw of each rat was evaluated for radiographic changes.
Histopathological assessment
After sacrificing the animals on the 28th day, the right hind paw of each rat was excised and fixed in 10% formalin for 24 hours. The excised hind paw fixed in formalin was decalcified for 10 days in EDTA and embedded in paraffin. Sections of 6.0 µ were taken on a microtome and stained with haematoxylin and eosin [42-45].
The stained sections were observed for histopathological alteration of joints and blindly graded by a pathologist and assigned a score of 1-4 based on the following criteria:
Minimal synovitis, primarily infiltration of inflammatory cells into synovial membrane
Mild synovitis, pannus formation, cartilage degeneration.
Proliferation and infiltration of large amount of inflammatory cells, sub-chondral bone erosion, superficial cartilage damage.
Severe destruction of cartilage and subchondral, complete disorganization of joint space and bony ankyloses.
Results
Effect of AV11-03 on Carrageenan induced paw edema in rats
Carrageenan showed inflammation of the paw which progressively increased till the 5th hour. Diclofenac inhibited the inflammation by 44.71%, 59.18% and 56.12% at 2nd, 3rd, and 5th hours respectively. AV11-03 inhibited inflammation at both the doses tested in the first 0.5, 1.0 and 2.0 hours of carrageenan challenge. There was 69.25% and 64.54% inhibition of edema in the first 30 minutes with 200 mg/Kg and 400 mg/Kg doses respectively (Figure 1).
Effect of AV11-03 on Complete Freund’s Adjuvant (CFA) induced chronic arthritis in rats
Paw oedema
In the CFA injected rats, a steady development of arthritis and swelling of the right paw was noticed from day 7 to day 28. Both diclofenac and AV11-03 exhibited steady reduction in the edema of the right paw from day 14 and by days 21 and 28, there was remarkable reduction in the paw volume in rats treated with diclofenac, and AV11-03 (200mg/Kg and 400 mg/Kg). The results are depicted in Figure 2.
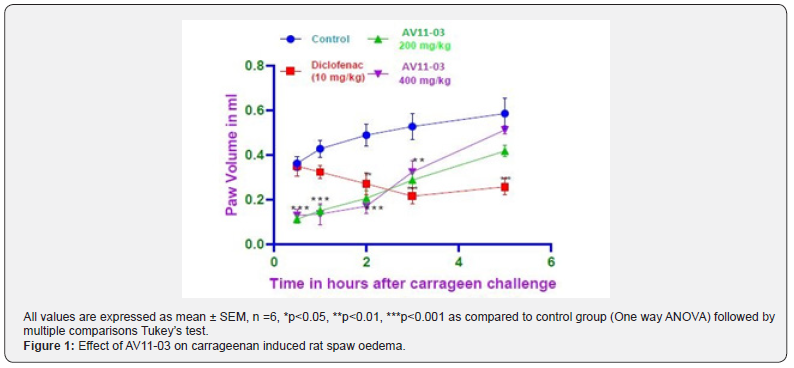
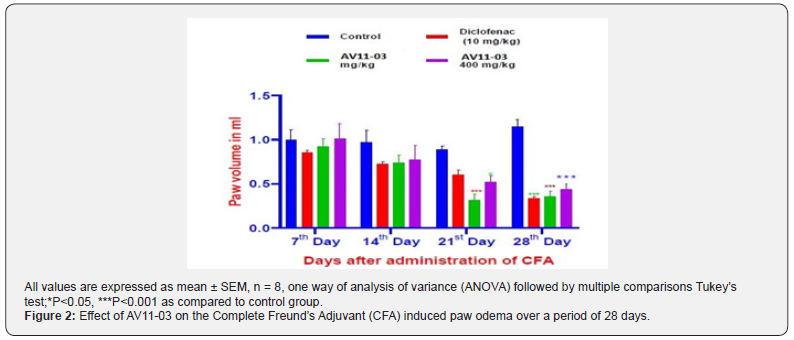
Evaluation of Development of arthritis (DOA)
The development of arthritis in CFA injected rats judged by observations of symptoms and scoring was in harmony with the volume of the arthritis paw. Untreated control rats had significant increase in redness, swelling and erythema at the knee joints while rats treated with either diclofenac / AV11-03 exhibited a significant reduction in all these parameters. The scores of developments of arthritis are graphically represented in Figure 3.
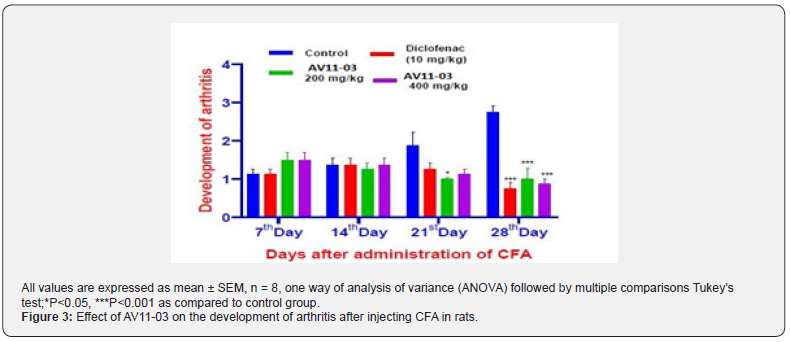
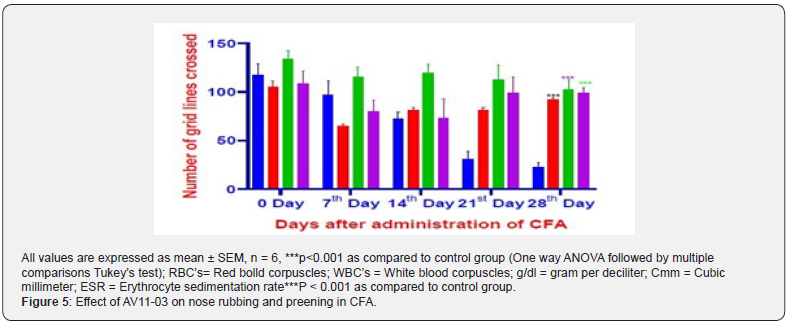
Spontaneous behavioral parameters on open-field test
In the experiments of the spontaneous behavioral parameters on open field test, a significant decrease in the horizontal ambulatory movement and grooming were noticed in the CFA treated control animals. Treatment with diclofenac and AV11-03 brought about significant improvement in the number of grid lines crossed and grooming (Figure 4 & 5). The rats in the control group had virtually stopped showing any vertical locomotor activity due to pain and inflammation after 21st day of CFA injection. Diclofenac treated and AV11-03 treated rats exhibited normal vertical movements on day 28 as they had shown on day 0 (Table 1). The rats in control CFA induced arthritis exhibited delayed response to start exploring the open field, while rats in the diclofenac and AV11-03 treatment groups were instant in exploring the open field from day 7 onwards after CFA challenge (Table 1).
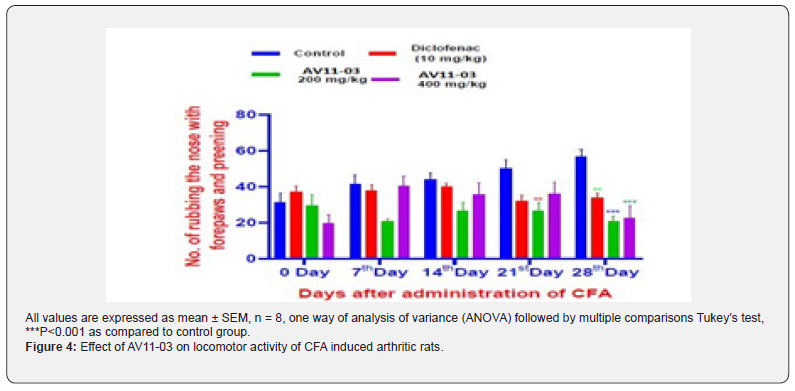
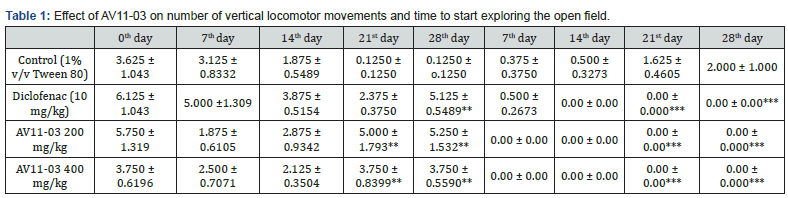
ll values are expressed as mean ± SEM, n =6, *p<0.05, **p<0.01, ***p<0.001 as compared to control group (One way ANOVA) followed by multiple comparisons Tukey’s test.
Assessment of vascular permeability-Evans blue extravasation test
There was a clear infiltration of the dye into the joints in the untreated group. Treatment with AV11-03 brought about 42.67% (200 mg/Kg) and 55.37% (400 mg/Kg) inhibition of joint infiltration in the treated groups (Table 2).

All values are expressed as mean ± SEM, n = 6, **p<0.01, ***p<0.001 as compared to control group (One way ANOVA followed by multiple comparisons Tukey’s test
Estimation of release of histamine from blood
There was an elevated release of histamine in the blood of untreated CFA arthritis rats which was significantly inhibited by AV11-03. There was 46.33%, 25.42% and 43.74% reduction in the release of histamine in diclofenac, AV11-03 (200 mg/Kg) and AV11-03 (400 mg/Kg) treated animals respectively as compared to that from the control CFA arthritis rats (Table 2).
Effect of AV11-03 on serum biochemical parameters
ALP, AST, ALT and creatinine were significantly elevated in the CFA treated control group animals due to the development of arthritis. Treatment with AV11-03 brought about a significant reduction of these values in the animals. The reduction in ALP and ALT was found to be very prominent (Table 3).
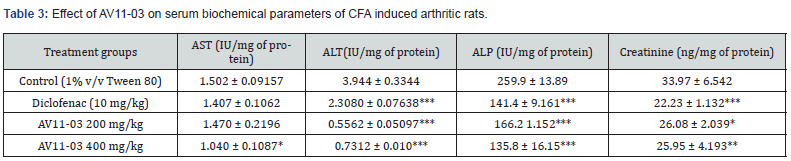
All values are expressed as mean ± SEM, n =6, *p<0.05, **p<0.01, ***p<0.001 as compared to control group (One way ANOVA followed by multiple comparisons Tukey’s test
Estimation of liver biochemical parameters
In the liver homogenates of CFA treated control animals, LPO levels significantly elevated and CAT, GSH, SOD and total thiols significantly reduced as compared to normal control animals. Treatment with AV11-03 ameliorated these biomarkers in the animals. There was significant reduction in LPO levels and very remarkable improvement in the levels of CAT, GSH, SOD and Total thiols (Table 4).
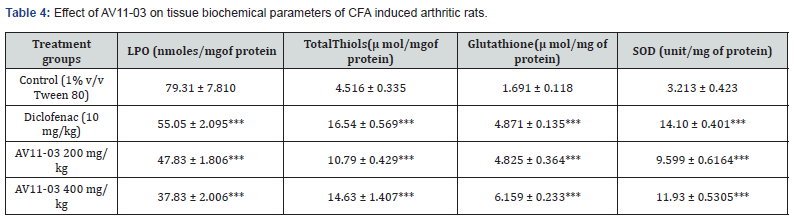
All values are mean ± SEM, n = 6, ***p<0.001 as compared to control group (One way ANOVA followed by multiple comparisons Tukey’s test
Estimation of hematological parameters and E. S. R.
The hematological parameters were estimated in the untreated and treated animals. There was no significant difference in the values of RBCs, hemoglobin, MCHC, HCT, Granulocytes, WBCs and lymphocytes. However, a significant reduction in ESR values were noted in the AV11-03 and diclofenac treatment groups (Table 5).
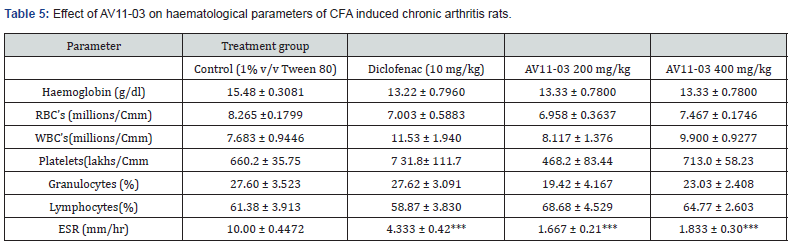
All values are expressed as mean ± SEM, n = 6, ***p<0.001 as compared to control group (One way ANOVA followed by multiple comparisons Tukey’s test); RBC’s= Red bolld corpuscles; WBC’s = White blood corpuscles; g/dl = gram per deciliter; Cmm = Cubic millimeter; ESR = Erythrocyte sedimentation rate.
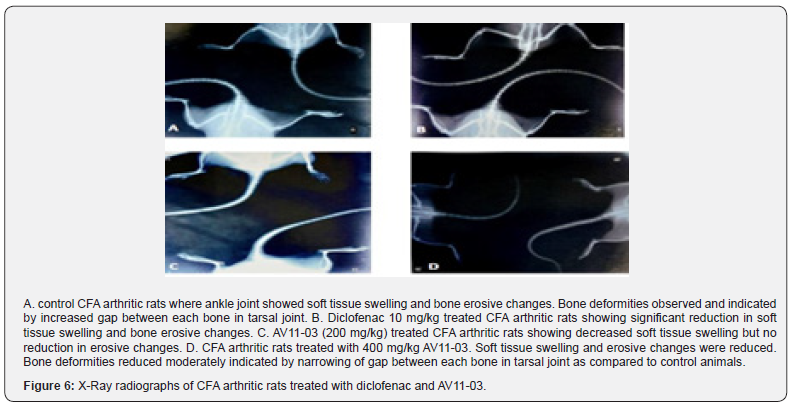
X-ray radiographic analysis
X-ray analysis of the joints in the arthritis control group showed soft tissue swelling, bone erosive changes and bone resorption. The deformities were less evident in the radiographs of AV11-03 treated rats (Figure 6).
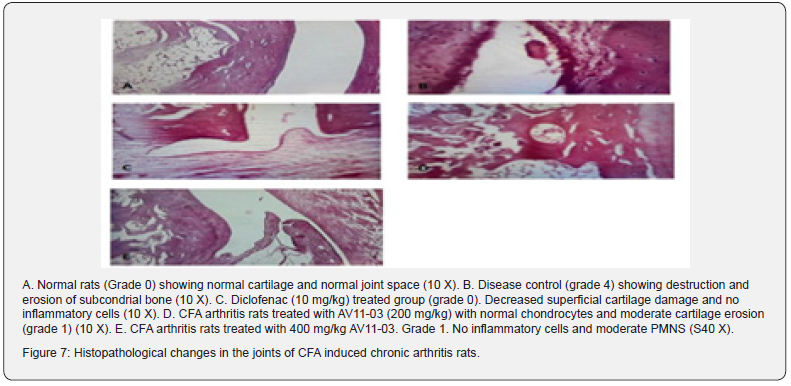
Histopathological assessment
In the normal rats, there was no inflammation and no change in cartilage. In the CFA arthritis control group severe inflammation and cartilage erosion was observed. In the AV11-03 treated groups mild inflammation with mild cartilage erosion could be seen (Table 6 & Figure 7).
Discussion
There was significant reduction of carrageenan induced inflammation by AV11-03 at both the doses tested. Carragenan induced inflammation is bi-phasic in nature. Release of histamine and serotonin is responsible for the initial phase and the later phase is due to kinin. AV11-03 exhibited very sharp activity in the first 0.5 and 1.0 hours thereby indicating that it might be inhibiting the release of histamine and serotonin. Complete Freund’s Adjuvant (CFA) induced arthritis has strong correlation with rheumatoid arthritis in humans which elicits its activity primarily in the joints of the hind limbs with reduction of motor activity, increased itching and scratching behaviors. The initial inflammatory response is developed within hours but more severity is observed during 28 days [43]. AV11-03 brought about reduction in the development of arthritis, behavioral parameters, vascular permeability, histamine release as also in the various serum and liver enzymes.
There was reduced oedema in the AV11-03 treated animals. This is also supported by the reduction in severe redness, swelling, erythema at the ankle joints thereby suggesting that the AV11-03 inhibited the release of localized inhibitory mediators. The improvement observed with AV11-03 treatment might be due to reduction in pannus formation and cartilage damage caused by proliferation of synovium.
Reduced locomotor activity and increased scratching and itching behaviors in CFA induced arthritic rats are spontaneous behaviors towards an attempt to protect the affected paw. Soft tissue swelling around the ankle joint and diffused demineralization are characteristic of this arthritic model. Improvement in all these parameters by AV11-03 suggests its preventive role in the progress of the disease.
CFA administration results in swelling of joint knees and leads to blood vascular permeability within four weeks of administration. The decrease in extravasation of dye in the treated animals is due to reduced endothelial gap in vascular component at joints of rats. Evans blue has high binding affinity to plasma proteins. Normally large plasma proteins and bound Evans blue dye cannot pass through the endothelial gaps and therefore get restricted in the vascular component. When the endothelial gaps get enlarged in the diseased state, the plasma protein and Evans blue dye complex can escape to the interstitial tissues. The measurement of the amount of Evans blue in the synovial capsule can provide an indication of relative vascular permeability. The decreased extravasation in AV11-03 treated groups might be due to decreased endothelial gaps.
The enhanced histamine concentration in the blood of CFA arthritis rats was significantly brought down by treatment with AV11-03. Pre-formed histamine is present in the mast cell granules and gets released by mast cell degranulation in diseased state. It triggers a cascade of immune reactions and release of pro-inflammatory cytokines like IL-a, IL-18 etc. which play key role in rheumatoid arthritis [44]. The radiographic analysis of the joints showed the reversal/prevention of soft tissue swelling, bone erosion and bone resorption by AV11-03. This is supportive of the above pharmacological, behavioral and biochemical observations.
ALP, AST, ALT, LDH and creatinine are indicators and suggestive for disturbances in the cellular integrity induced by pathological conditions like arthritis and persistent inflammation. Loss of semi-permeability of the synovial membrane has also been correlated with significant elevation of these enzyme levels. Reduction in these enzymes on treatment with AV11-03 is suggestive of its protective role in arthritis.
LPO is the oxidative deterioration of poly-saturated lipids to form radical intermediates that bring about cellular damage. Melondialdehyde (MDA), major product of this reaction, is an index lipid peroxidation and has been estimated as TBARS [45]. Free radicals and reactive oxygen species are generated by the infiltrating cells and lead to destruction of the inflamed joints. As a result SOD is utilized, and its activity reduced in arthritis. The level of glutathione, a reducing agent that traps free radicals also decreases in arthritis. Treatment with AV11-03 potentially maintained the oxidative homeostasis. This indicates strong antioxidant activity of the formulation against inflammation mediated oxidative stress.
Histopathological studies showed that AV11-03 treatment significantly reduced infiltration of leukocytes and disruption and loss of articular damage in the arthritis rats.
The three ingredients in AV11-03 (i.e. T. chebula, B. serrata and Glucosamine) have been proved to be highly effective in various rheumatoid and osteo-arthritis conditions in different animal models and human clinical trials. The main chemical constituent in T. chebula and boswellic acids in B.serrata showed suppression of generation of a number of pro-inflammatory mediators especially TNF-α, INF-γ, IL-1β, IL-6, NF-κB etc. T.chebula and B. serrata inhibit both cyclooxygenase and lipooxigenase enzymes. Further, the phenolic components of T.chebula have strong antioxidant activities. The physical and radiological observation recorded in different animal experiments and human clinical trials unequivocally established the potential of these ingredients in improving the overall patho-physiological and anatomical condition of arthritis. The concentrations of these ingredients in AV11-03 are rationally selected by going through various reported studies. There is a strong synergistic activity of these ingredients and as a whole the formulation acts through diverse mechanisms to ultimately bring about improvement in the arthritic condition.
Conclusion
AV11-03, a poly- herbal tablet formulation incorporating three very potent anti-arthritic plant drugs exhibited strong anti-arthritis activity in carrageenan and CFA induced acute and chronic arthritis. At 200 mg/Kg and 400 mg/Kg dose (actives), there was very significant improvement in swelling, behavioral parameters, locomotor parameters, histamine release, biochemical, histological, and radiographic parameters.
References
- Lee DM, Weinblatt ME (2001) Rheumatoid arthritis. Lancet 15(358): 903-911.
- Matsumoto I, Maccioni M, Lee DM, Maurice M, Simmons B, et al. (2002) How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nat Immunol 3(4): 360-365.
- Feldmann M, Brennan FM, Maini RN (1996) Rheumatoid arthritis. Cell 85(3): 307-310.
- Smolen JS, Steiner G (2003) Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov 2(6): 473-488.
- Schaffer D, Florin T, Eagle C, Marschner I, Singh G, Grobler M, et al. (2006) Risk of serious NSAID-related gastrointestinal events during long-term exposure: A systematic review. Med J Aust 185(9): 501-506.
- Ravindran V, Rachapalli S, Choy EH (2009) Safety of medium- to long-term glucocorticoid therapy in rheumatoid arthritis: A meta-analysis. Rheumatology (Oxford) 48(7): 807-811.
- Said M (1997) Hamdard Pharmacopoeia of Eastern Medicine. Indian Medical Science Series 55. Delhi: Sri Satguru Publications.
- Khare CP (2007) Indian Medicinal Plants-An Illustrated Dictionary.
- Seo JB, Jeong JY, Park JY, Jun EM, Lee SI, et al. (2012) Anti-arthritic and analgesic effect of NDI10218, a standardized extract of Terminalia chebula, on arthritis and pain model. Biomol Ther (Seoul) 20(1): 104-112.
- Shin TY, Jeong HJ, Kim DK, Kim SH, Lee JK, et al. (2001) Inhibitory action of water-soluble fraction of Terminalia chebula on systemic and local anaphylaxis. J Ethnopharmacol 74(2): 133-140.
- Reddy DB, Reddanna P (2009) Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-kappa B and MAPK activation in RAW 264.7 macrophages. Biochem Biophys Res Commun 381(1): 112-117.
- Murdock M, Gupta CR, Vega N, Kotora K, Miller J, et al. (2016) Evaluation of Terminalia chebula extract for anti-arthritic efficacy and safety in osteoarthritic dogs. J Veterinar Sci Technol 7:1.
- Reddy DB, Reddy TCM, Jyotsna G, Sharan S, Priya N, et al. (2009) Chebulagic acid, a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line. J Ethnopharmacol 124(3): 506-512.
- Das ND, Jung KH, Park JH, Mondol MA, Shin HJ, et al. (2011) Terminalia chebula extract acts as a potential NF-κB inhibitor in human lymphoblastic T cells. Phytother Res 25(6): 927-934.
- Nair V, Singh S, Gupta YK (2010) Anti-arthritic and disease modifying activity of Terminalia chebula Retz. in experimental models. J Pharma Pharmacol 62(12): 1801-1806.
- Kumar CU, Pokuri VK, Pingali U (2015) Evaluation of the analgesic activity of standardized aqueous extract of Terminalia chebula in healthy human participants using hot air pain model. J Clin Diagn Res 9(5): FC01-FC04.
- Fox BA, Stephens MM (2007) Glucosamine hydrochloride for the treatment of osteoarthritis symptoms. Clini Interv Aging. 2(4): 599-604.
- Gouze JN, Bianchi A, Becuwe P, Netter P, Magdalou J, et al. (2002) Glucosamine modulates IL-1-induced activation of rat chondrocytes at a receptor level, and by inhibiting the NF- Kappa B pathway. FEBS Lett 510(3): 166-170.
- Gouze JN, Gouze E, Popp MP, Bush ML, Dacanay EA, et al. (2006) Exogenous glucosamine globally protects chondrocytes from the arthritogenic effects of IL-1beta. Arthritis Res Ther 8(6): R173.
- Nakamura H, Shibakawa A, Tanaka M, Kato T, Nishioka K (2004) Effects of glucosamine hydrochloride on the production of prostaglandin E2, nitric oxide and metalloproteases by chondrocytes and synoviocytes in osteoarthritis. Clin Exp Rheumatol 22(3): 293-299.
- Henderson CJ, Panush RS (1999) Diets, dietary supplements, and nutritional therapies in rheumatic diseases. Rheum Dis Clin North Am 25(4): 937-968.
- Ammon HPT (2010) Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine 17(11): 862-867.
- Kumar R, Singh S, Saksena AK, Pal R, Jaiswal R, et al. (2019) Effect of Boswellia Serrata extract on acute inflammatory parameters and tumor necrosis factor-a in Complete Freund's Adjuvant-induced animal model of rheumatoid arthritis. Int J Appl Basic Med Res 9(2): 100-106.
- Alluri VK, Kundimi S, Sengupta K, Golakoti T, Kilari EK (2020) An Anti-Inflammatory composition of Boswellia serrata resin extracts alleviates pain and protects cartilage in mono iodoacetate-induced osteoarthritis in rats. Evidence-Based Comple Alter Med 21: 12.
- Sengupta K, Alluri VK, Satish AR, Mishra S, Golakoti T, et al. (2008) A double blind, randomized, placebo-controlled study of the efficacy and safety of 5-Loxin®for treatment of osteoarthritis of the knee. Arthritis Res Ther 10(4): 1-11.
- Ganpeng Yu , Xiang W, Zhang T, Zeng L, Yang K, et al. (2020) Effectiveness of Boswellia and Boswellia extract for osteoarthritis patients: a systematic review and meta-analysis. BMC Complementary Med Ther 20: 225.
- Singh H, Ghosh MN (1968) Modified plethysmometer for measuring foot volume of unanesthetized rats. J Pharm Pharmacol 20(4): 316-
- Newbould BB (1963) Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Br J Pharmacol Chemother 21(1): 127-
- Costa MD, Sutter PD, Gybel J, Hees JV (1981) Adjuvant induced arthritis in rats: A possible animal model of chronic pain. Pain 10(2): 173-185.
- Tomita T, Yoshimi K, Philip ST (2006) THR-0921, a novel peroxisome proliferation-activated receptor gamma agonist, reduces the severity of collagen-induced arthritis. Arthritis Res Ther 8(1): R7.
- Parent AJ, Beaudet N, Beaudry H, Bergeron J, Bérubé P, et al. (2012) Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behav Brain Res 229(1): 160-167.
- Franchis FY, Hilda HLW, Ethel SKN (2004) The time course and substance P effects on the vascular and morphological changes in adjuvant induced monoarthritis rats. Int Immunol 4: 299-310.
- Tiligada E, Kakolyri M, Ennis M (2017) Histamine quantification in human blood samples. In: Tiligada E, Ennis M (Eds.), Histamine Receptors as Drug Targets (Methods in Pharmacology and Toxicology Book Series). Humana Press, New York, USA, pp. 489-508.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem. 193 :265-275.
- Chandrasekhar VM, Ranapriya VL, Parashar A, Muchandi AA (2010) Ganapathy S. Neuroprotective activity of Matricaria recutita against the global model of ischemia in rats. J Ethnopharmacol 127: 645-651.
- Mishra H P, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a sample assay for superoxide dismutase. J Bio Chem 247: 3170-3175.
- Claliborne A (1985) Catalase activity. In: Greenwald RA (Ed.), CRC Handbook of Methods for Oxygen Radical Research. CRC Press, Boca Raton, Florida, USA, pp. 283-284.
- Sedlak, Lindsay H (1968) Estimation of protein-bound and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Ana Biochem 267: 4904-4911.
- Shankaranarayan J, Christina AJM, Kalyan SB, Jagan A, Sundara SK, et al, (2009) Evaluation of Glycine max Merill. seeds for anti-arthritic activity in male Wistar rats. Int J Pharm Sci. Nonotech 4: 363-366.
- Jain AK (2021) Manual of Practical Physiology for M.B.B.S. 1st Ed, Arya Publication, Sirmour, India, p. 42-43.
- Kalpesh RP, Chandragouda RP, Ramchandra BJ, Vallabh KM, Prabhakar RP, et al. (2009) Anti-arthritic activity of bartogenic acid isolated the fruits of Barringtonia racaemosa Roxb. Evidence based Complementary and Alternative Medicine 1-7.
- Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, et al. (1996) Selective inhibition of cyclooxygenase (COX)‑2 reverses inflammation and expression of COX‑2 and interleukin- 6 in rat adjuvant arthritis. J Clin Invest 97: 2672‑
- Dimitrijevic M, Laban O, Djuric VJ, Stanojevic S, Miletic T, et al. (2001) Behavior and severity of adjuvant arthritis in four rat strains. Brain, Behavior, and Immunity 15: 255-265.
- Chime HR, Mallikarjun M, Chandrasekhar VM, Prashant PM (2010) Anti allergic activity of Aristolochia bracteolate Lnk in animal model. Indian J Exp Biol 48: 46-52.
- Kamanli A, Naziroglu M, Aydilek N, Hacievliyagil C (2004) Plasma lipid peroxidation and antioxidant levels in patients with rheumatoid arthritis. Cell Biochem Funct 22: 53-57.






























