Tropical Spastic Para Paresis Management-A New Hope
Avinash Shankar1*, Amresh Shankar2 and Anuradha Shankar3
1Postgraduate in Endocrinology & Metabolism, National Institute of Health & Research, India
2Aarogyam Punarjeevan, India
3Centre for Indigenous Medicine & Research, Regional Institute of Ayurveda, India
Submission:April 26, 2019; Published:May 20, 2019
*Corresponding author:Avinash Shankar, Postgraduate in Endocrinology & Metabolism, (AIIMS Delhi) Chairman, National Institute of Health & Research, Warisaliganj (Nawada), Bihar, India
How to cite this article: Avinash Shankar, Amresh Shankar, Anuradha Shankar. Tropical Spastic Para Paresis Management-A New Hope. J Complement Med Alt Healthcare. 2019; 9(3): 555765. DOI:10.19080/JCMAH.2019.09.555765
Abstract
Tropical spastic para paresis a disease of nervous system is caused by Human T lymphotrophic virus type I thus also known as HTLV-I associated myelopathy and common among female of age group 30-50 years in approximately 2-3% of HTLV-1 affected person. In spite advancement in diagnostic procedure i.e.-CTscan, MRI its treatment with α- interferon, steroid, antiviral drugs, Neurovitamin supplementation, physiotherapy fails to ensure cure or improve quality of life except transient pain relief with analgesics and muscle relaxants, thus a therapeutic regime composite consisting a proven herbal neurogenic been evaluated. Objective of study: To assess the herbal neurogenic and immune boosting composite in ensuring clinical relief and improving quality of life in patients deterred from various medi centres without any relief. Material and method: 63 diagnosed and already treated cases of Tropical spastic para paresis attending at Centre for Critical Care National Institute of Health & Research Warisaliganj (Nawada)Bihar been selected, interrogated, examined clinically, assessed and analyzed their previous investigation reports, therapeutics taken and their effect. Irrespective of their clinical severity all patients were advocated the prescribed regime and were followed for post therapy 2 years for which patients been given a follow up card to record the changes. Result: 88.9% patients had grade I clinical response while rest 11.1% grade II without any untoward effect or any withdrawal during post therapy 2 years follow up.
Keywords: Tropical spastic para paresis; Human T lymphotrophic virus-type I; CT; MRI; Herbal neurogenic; Quality of life
Introduction
Tropical spastic para paresis, a chronic and progressive clinical condition affecting Nervous system remained of obscure etiopathogenesis for long but now a days an important association of this condition been established between Human retrovirus (Human T cell lymphotropic virus type I) thus this condition is also termed as HTLV1 associated myelopathy (HAM). As per WHO estimate worldwide 10-20 million peoples are carrying HTLV1 and 5% of it are affected with TSP of age group 30-50 years [1-10]. TSP is very common in Latin America, the Caribbean Basin, sub-Saharan Africa and Japan but these days incidence of this clinical state is increasing even in India. Common presentation of the clinical condition is [11-14] 1. Gradual weakening and stiffening of lower extremity 2. Raditing back pain down to legs 3. Burning and pricking sensation (paraesthesia) 4. Urinary and bowel function disturbances 5. In male erectile dysfunction 6. Inflammatory skin condition like dermatitis or psoriasis 7. Rarely may present with eye inflammation, arthritis, and muscle inflammation The common mode of transmission of this virus is through [15-16] 1. Breastfeeding 2. Sharing infected needles during intravenous drug use 3. Sexual activity
Blood transfusions
In spite of advancement in diagnostics (CT scan and MRI) and its established etiopathogenesis till date no established therapeutic regime ensured its reversal but only symptomatic relief, i.e. α- interferon, intravenous immunoglobulin, antiviral drugs and muscle relaxants Tizanidine signs and symptoms vary but may include slowly progressive weakness and spasticity of one or both legs, exaggerated reflexes, muscle contractions in the ankle, and lower back pain. Other features may include urinary incontinence and minor sensory changes, especially burning or prickling sensations and loss of vibration sense. Considering the poor quality of life with present therapeutics a clinical study was planned to evaluate the clinical efficacy of proved neurogenic herbal composite with neuro modulator at National Institute of Health & Research and Centre For Research in Indigenous Medicine.
Objective of the study
To evaluate he clinical efficacy and safety profile of herbal neurogenic with neuromodulator in TSP
Duration of study
Jan 2014 to December 2018
Material and Methods
Material
Patients of proved and treated cases of Tropical spastic Para paresis without any clinical response, attending at Centre For Critical Care, National Institute of Health & Research were considered for evaluation of the herbal neurogenic constituting therapeutic regime.
Methods
Patients of spastic para paresis diagnosed by myelogram, computerized tomography(CT) and magnetic resonance imaging (MRI) been interrogated thoroughly for the onset, duration and evolution of the disease, Family history of neurological illness, history of extramarital sexual exposure, abortion, blood transfusions, dietary with emphasis on strict vegetarianism, Lathyrus sativus, Socio-economic status, housing, sanitary conditions, treatment taken and their response. A detailed general examination and a meticulous neurological assessment were done (Table 1).

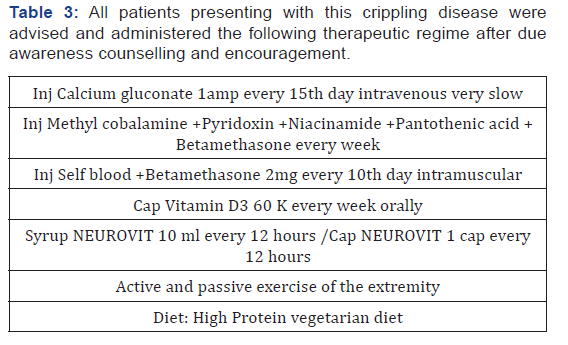
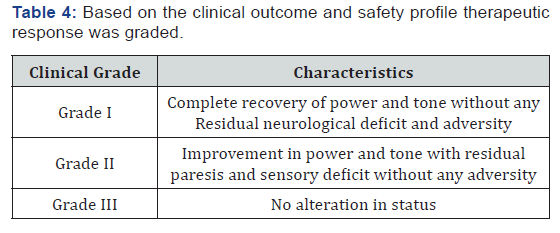
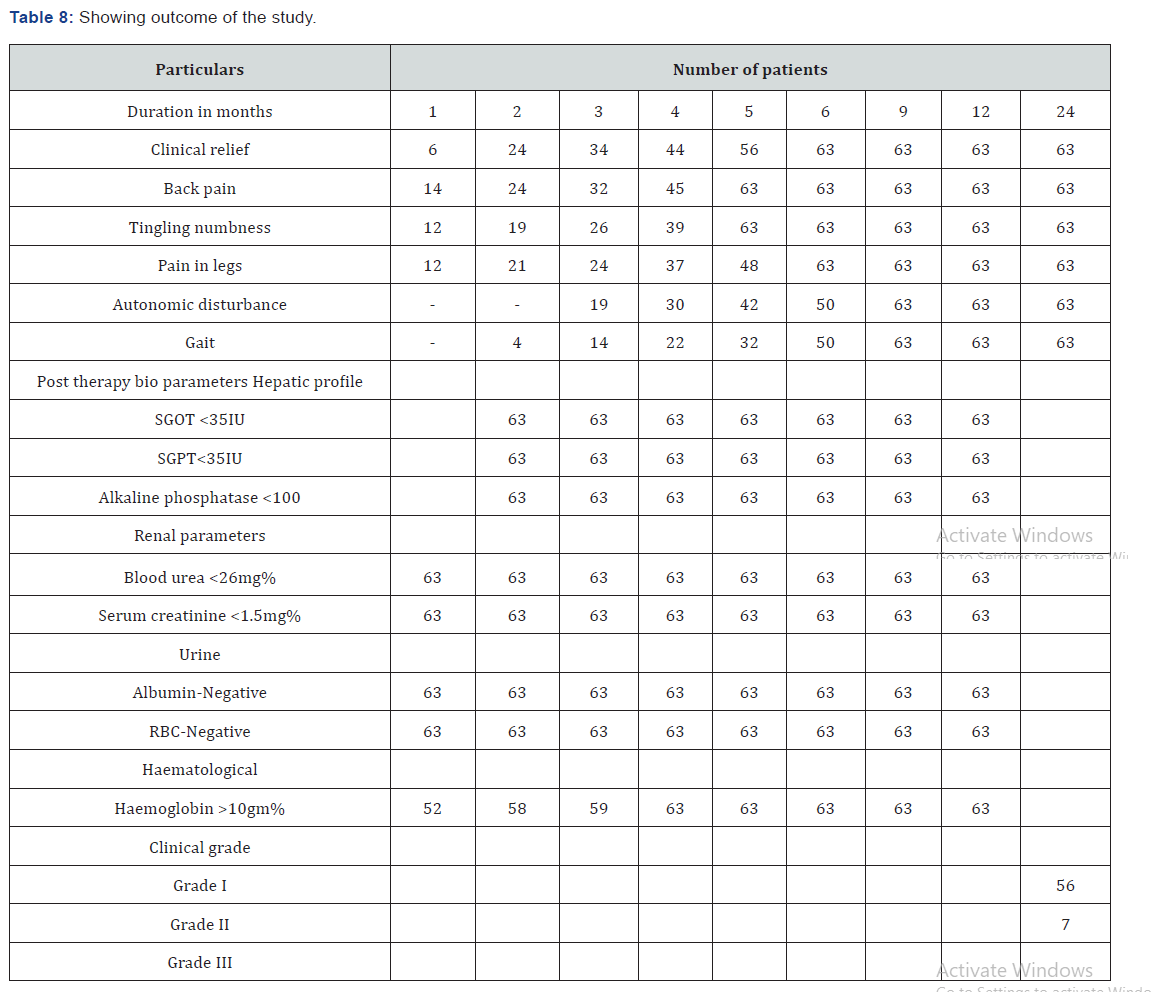
Patients were investigated for hemoglobin concentration, total and differential leucocyte count, erythrocyte sedimentation rate (ESR), peripheral smear, fasting and postprandial blood sugar, renal and liver function tests, and serological test for syphilis (Table 2). All patients underwent conventional myelography CT and MRI scans. The serum samples of all the patients were tested for HTLV-1 antibodies by the serodia technique. (Table 3). Herbal composite NEUROVIT Syr Or Capsule constitutes: Cap 500mg Or Syr. 5ml constitutes 100mg each of Acorus calamua (rhizome), Nardostachys jatamansi (Flower), Herpestis monnieri (leaf), Convolvulus pluricaulis (flower), Cassia acutifolia (seed). Patients were assessed for improvement in tone and power of the muscle, tingling and numbness, gait and autonomic function (passage of stool and urine ) for which patients were given a follow up card to mention date of achievement and any untoward manifestation experienced. Patients were advised to visit the center on any unusual manifestation or contact on helpline for needful redresses. To adjudge the safety profile of the regime practiced basic bio parameters were repeated every month for first three month and then every 3 months (Table 4).

Results
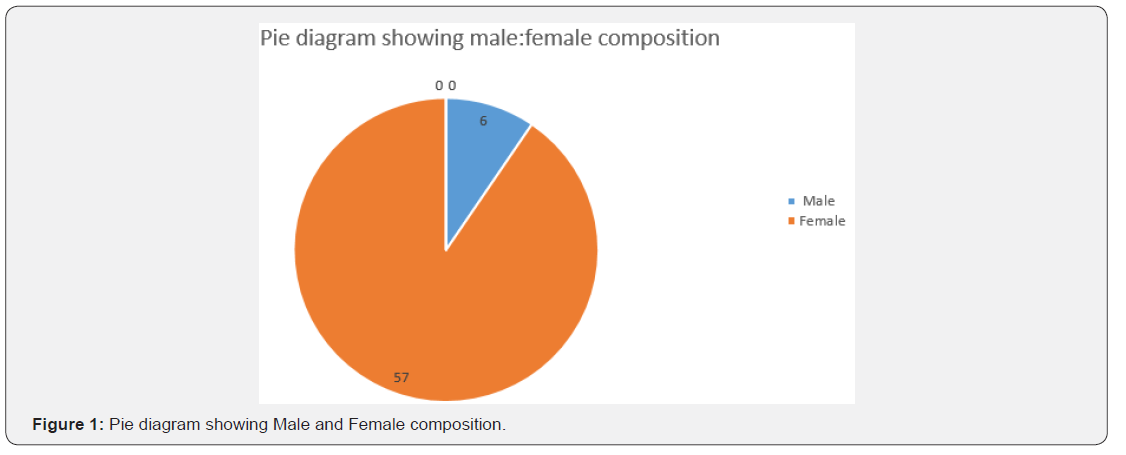
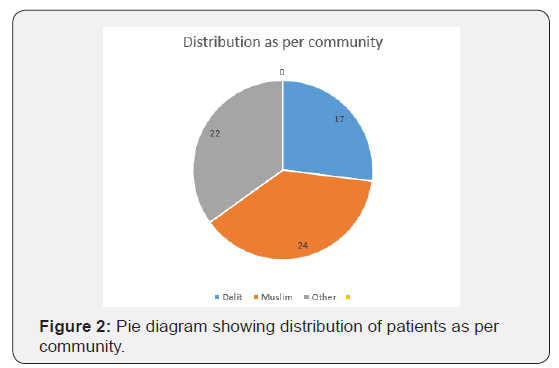
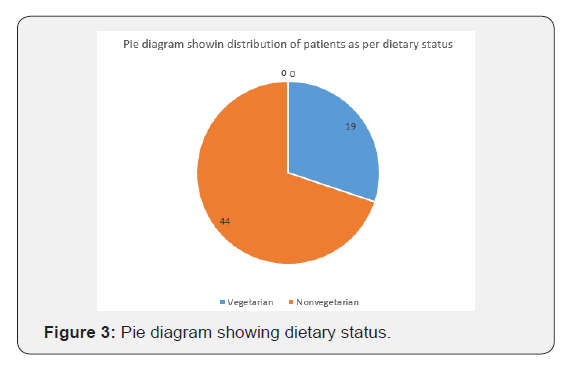
63 identified, diagnosed and treated Patients of Tropical spastic para paresis considered for study were of age group 30- 50 years and out of them majority (30/63) were of age group 30- 35 years with female dominance (Table 5 and Figure 1) and all were from rural background and community representation was (Figure 2) Out of all majority were non-vegetarian and non-had any history of taking Lathyrus sativus (Figure 3).
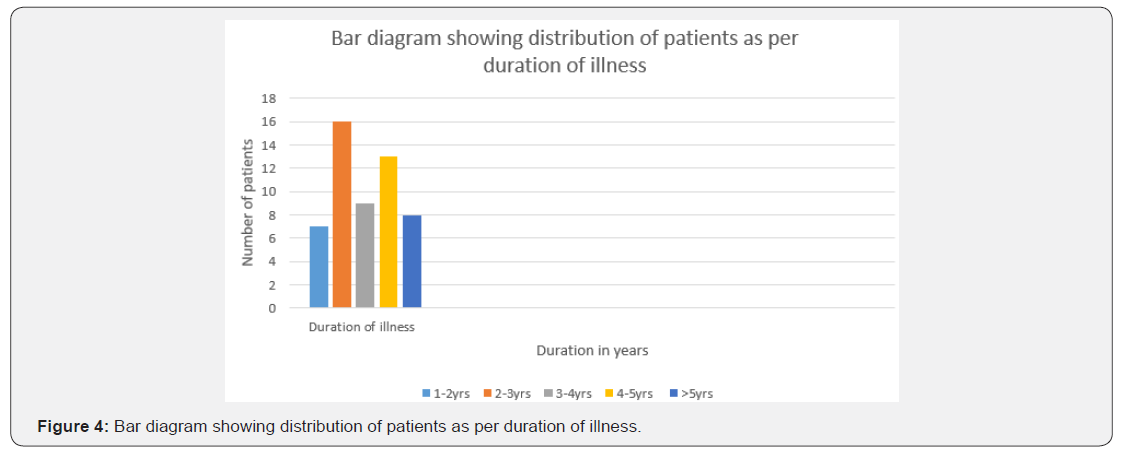
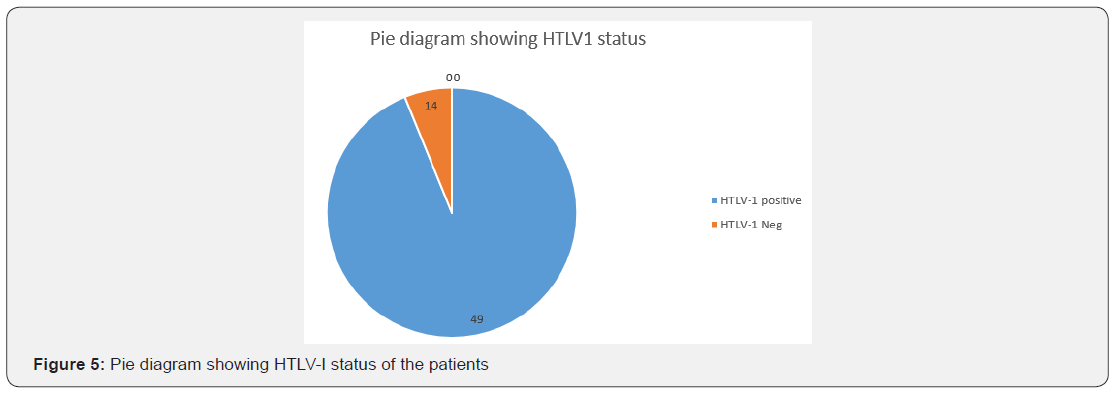
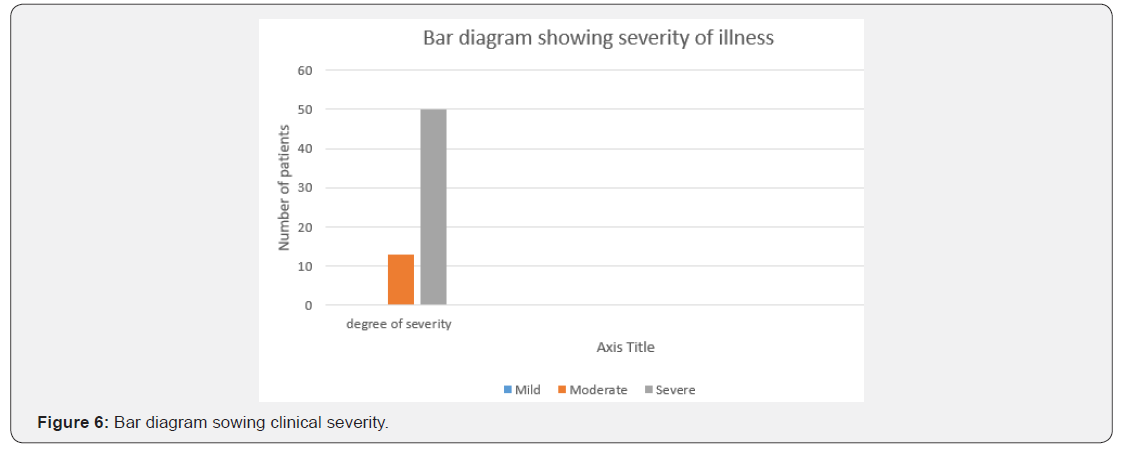
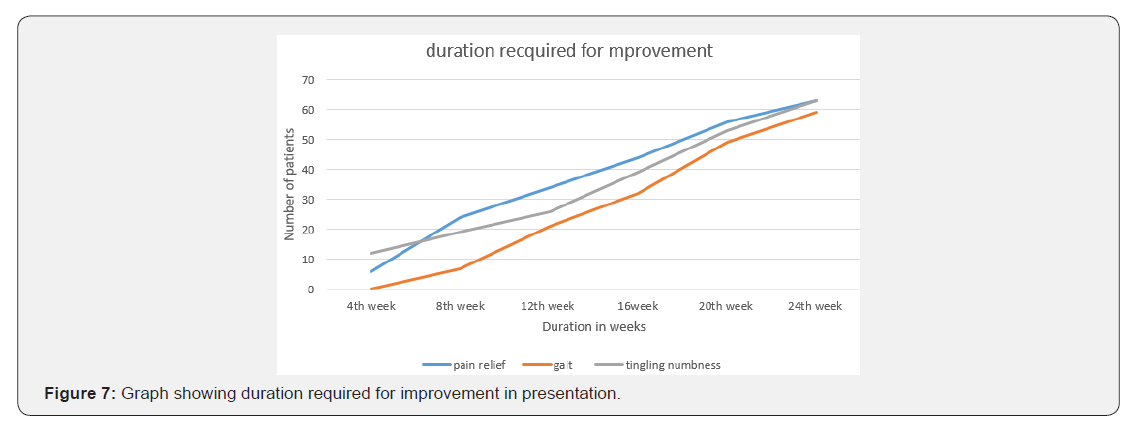
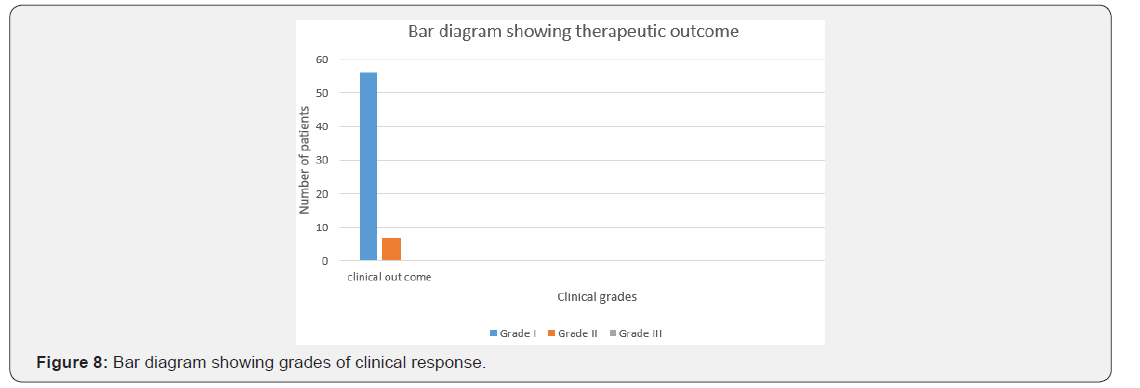
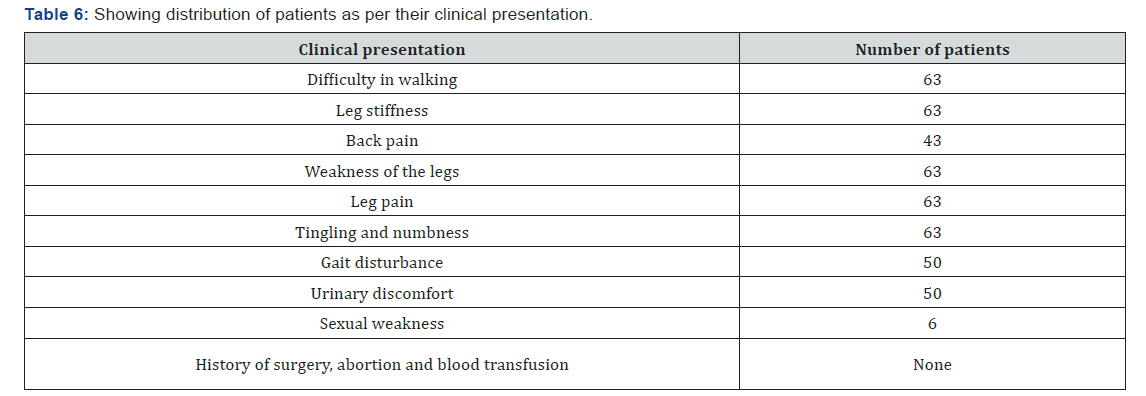
The age of onset of clinical presentation varied from 20-40 years and duration of illness from 1 year to 12 years (Figure 4) Symptoms at the onset were difficulty in walking, stiffness of legs, back pain ,weakness of legs, leg pain and urinary discomfort while presenting presentation at our center were disturbed gait, leg stiffness, back pain, leg pain urinary discomfort, urinary retention, tingling and numbness, erectile deficiency in male cases (Table 6). No history of blood transfusion, abortion, delivery or surgery prior to onset of the disease but serum samples revealed positive for HTLV-1 in 49 cases out of 63. In addition, all the bio parameters (Hepatic, hematological and renal profile remain normal) (Figure 5). No patients were positive for Tuberculosis, any sexually transmitted disease, CT and MRI also shows normal in all the cases. Out of 63 patients 13 were of moderate and 50 were of severe status (Figure 6). Patients had taken treatment with α interferon, muscle relaxants, neuro vitamin supplementation at various medicare centers without any positive therapeutic outcome (Table 7). Symptomatic relief started from 4th week of therapy and by 24th week all had symptomatic relief (Figure 7). The minimum and maximum duration of therapy required for complete reversal of clinical presentation (both symptom & sign) is 9 months and 2 years respectively. Out of all 56 patients achieved Grade I clinical improvement and 7 Grade II (Figure 8). No patients shown any adversity, recurrence of presentation or any alteration in bio parameters in 2 years of post-therapy follow up (Table 8).
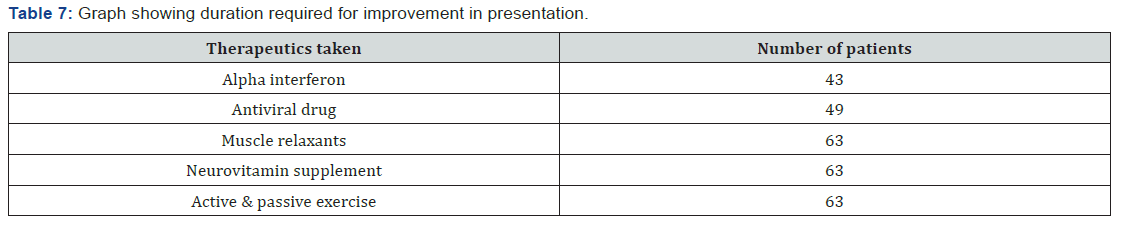
Discussion
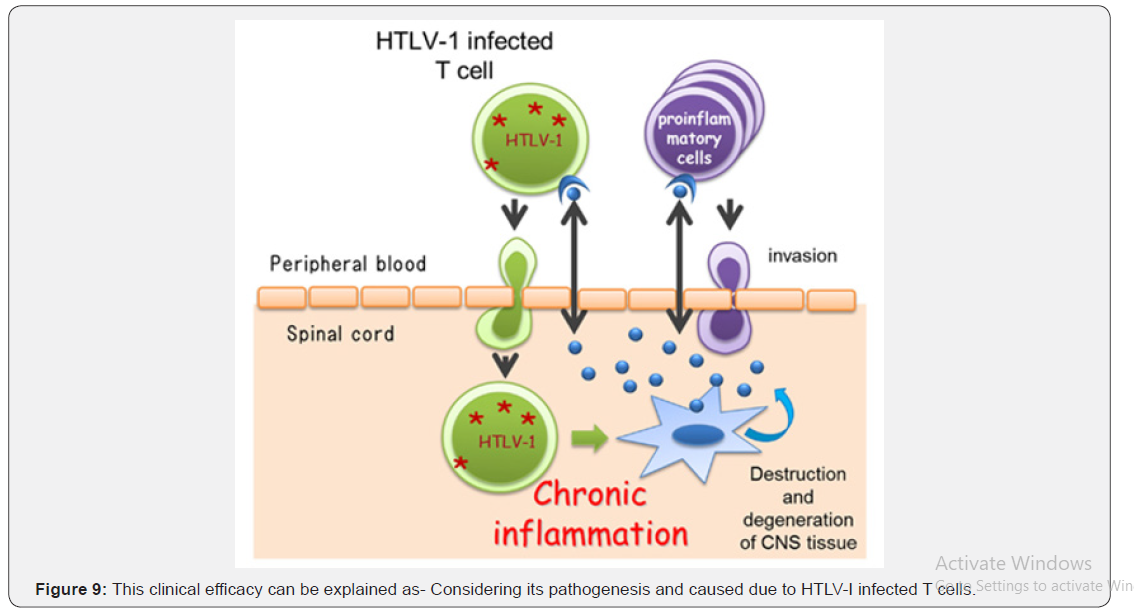
Tropical spastic para paresis is also common neurological disorder in India though it’s a common in different parts of the world i.e. including Jamaica, Martinique, Seychelles, Colombia and Japan. Though it was considered as a neurological disorder of obscure etiology but these days it is proved to be caused by Human T cell lymphotropic virus type I (HTLV-I). In spite of advancement in diagnostics like CT, MRI, CSF and Serum for HTLV-I antigen [17-19] the therapeutics used i.e. alpha interferon, muscles relaxant and neuro vitamin supplement [20- 22] fails to ensure cure or improve quality of life except transient symptomatic relief. Clinical supermacy in term of marked improvement in pain, sensation and gait of the already treated patients with other regime and achieving Grade I clinical response in 88.9% patients and Grade II in rest 11.1%. No patients had any withdrawal or drug adversity in 2 years post therapy follow up. This clinical efficacy can be explained as- Considering its pathogenesis and caused due to HTLV-I infected T cells Self blood with Betamethasone intramuscular induces antibody formation against the released toxin and ensure their neutralization while betamethasone acting as anti-inflammatory reduces neural edema synergized by Intravenous Calcium administration whose inclusion of one mole exit 2 mole of Sodium acting on Sodium potassium ATPase pump and facilitate decrease in neural edema and calcium ion improves neural conduction. Methyl cobalamine, pyridoxine, Niacin and pantothenic acid support neural cells in its normal neural conduction and Neurovit a herbal composite by its neurogenic activity helps in restoration of neural viability and vitality which combinedly ensure relief in pain, neuropathic manifestation, gait and autonomic function and provide better quality of life to all.
Conclusion
Present regime constituting Calcium gluconate intravenous, Methyl cobalamine + Pyridoxin + Niacin intravenous, Selfblood( 2ml) and Betamethasone 2mg intramuscular, cap Cholecalciferol 60K, Syrup Herbal neurotonic (Neurovit) proves worth in management of Tropical spastic para paresis even in chronic and long term treated cases (Figure 9).
References
- World Health Organization (WHO) (1989) Human T lymphotropic virus type 1, HTLV-1, Wkly. Epidemiol 64: 382-383.
- Orland JR, Engstrom J, Fridey J, Sacher RA, Smith JW, et al. (2003) Prevalence and clinical features of HTLV neurologic disease in the HTLV outcomes study. Neurology 61(11): 1588-1594.
- Blattner WA, Gallo RC (1985) Epidemiology of human retroviruses. Leuk Res 9(6): 697-698.
- Oomman A, Madhusoodanan M (2003) Tropical spastic paraparesis in Kerala. Neurol India 51(4): 493-496.
- Roman GC (1988) The neuroepidemiology of tropical spastic paraparesis. Ann Neurol 23: S113-S120
- Arango C, Concho M, Zaninovic V, Biojor, Borrero I, et al. (1988) Epidemiology of tropical spastic paraparesis in Colombia and associated HTLV-1 infection. Ann Neurol 23: S161-S165.
- Richardson JH, Newell AL, Newman PK, Mani KS, Rangan G, et al. (1989) HTLV-1 and neurological disease in South India. Lancet 1(8646): 1079
- Gessain A, Barin F, Vernant JC, Gout O, Calendar A, et al. (1985) Antibodies to human T lymphotropic virus type I in patients with tropical spastic paraparesis. Lancet 2(8452): 407-410.
- Rubin M (2016) Tropical Spastic Paraparesis/HTLV-1-Associated Myelopathy (TSP/HAM). Merck Manual.
- Tropical Spastic Paraparesis Information Page. National Institute of Neurological Disorders and Stroke.
- Iwasaki Y (1990) Pathology of Chronic myelopathy associated with HTLV-Infection (HAM/TSP) J Neurolo Sci 96(1): 103-123.
- Izumo S, Umehara F, Osame M (2000) HTLV-1 associated myelopathy, Neuropathology 20: 565-568.
- Osame M (2002) Pathological mechanisms of human T cell lymphotropic virus type I-associated myelopathy (HAM/TSP). J Neurovirol 8(5): 359-364.
- Lezin A, Olindo S, Oliere S, Varrin Doyer M, Marlin R, et al. (2005) Human T lymphotropic virus type I (HTLV-I) proviral load in cerebrospinal fluid: a new criterion for the diagnosis of HTLV-I-associated myelopathy/tropical spastic paraparesis?. J Infect Dis 191: 1830-1834.
- Matsuzaki T, Nakagawa M, Nagai M, Usuku K, Higuchi I, et al. (2001) HTLV-I proviral load correlates with progression of motor disability in HAM/TSP: analysis of 239 HAM/TSP patients including 64 patients followed up for 10 years. J Neurovirol 7(3): 228-234.
- De Castro Costa CM, Araújo AQ, Barreto MM, Takayanagui OM, Sohler MP, et al. (2006) Proposal for diagnostic criteria of tropical spastic paraparesis/HTLV-I-associated myelopathy (TSP/HAM). AIDS Res Hum Retroviruses 22: 931-935.
- Bagnato F, Butman JA, Mora CA, Gupta S, Yamano Y, et al. (2005) Conventional magnetic resonance imaging features in patients with tropical spastic paraparesis. J Neurovirol 11(6): 525-534.
- Scadden DT, Freedman AR, Robertson P (2018) Human T-lymphotropic virus type I: Disease associations, diagnosis, and treatment.
- Sandbrink F (2015) Tropical Myeloneuropathies Treatment & Management. Medscape Reference.
- Arimura K, Nakagawa M, Izumo S, Usuku K, Itoyama Y, et al. (2007) Safety and efficacy of interferon-α in 167 patients with human T-cell lymphotropic virus type 1-associated myelopathy. J Neurovirol 13(4): 364-372.
- Croda MG, De Oliveira AC, Vergara MP, Bonasser F, Smid J, et al. (2008) Corticosteroid therapy in TSP/HAM patients: the results from a 10 years open cohort. J Neurol Sci 269(1-2): 133-137.
- Taylor GP, Goon P, Furukawa Y, Green H, Barfield A, et al. (2006) Zidovudine plus lamivudine in human T-lymphotropic virus type-l-associated myelopathy: a randomised trial. Retrovirology 3: 63.






























