Exploration of Antibacterial and Anti-inflammatory Activities of Premna integrifolia Plant Extracts in Bubaline Mastitis
Anand Kumar P1, Lakshminarasaiah S1, Rao GS2, Azad R3, Sharma P4 and Reddanna P3*
1 Department of Veterinary Microbiology, NTR College of Veterinary Science, Sri Venkateswara Veterinary University, Gannavaram – 521 102 Andhra Pradesh, India
2 Department of Veterinary Pharmacology & Toxicology, NTR College of Veterinary Science, Sri Venkateswara Veterinary University, Gannavaram – 521 102 Andhra Pradesh, India
3Department of Animal Biology, School of Life Sciences, University of Hyderabad, Hyderabad, India
4 National Institute of Animal Biotechnology, Hyderabad, India
Submission: August 16, 2018; Published: August 31, 2018
*Corresponding author: Dr. P. Reddanna, Professor, Department of Animal Biology, School of Life Sciences, University of Hyderabad, Gachibowli, Hyderabad- 500 046 Telangana India, Tel: +91 40 23134542; Email: prsl@uohyd.acernet.in;predanna@gmail.com
How to cite this article: Anand Kumar P, Lakshminarasaiah S, Rao GS, Azad R, Sharma P, Reddanna P. Exploration of Antibacterial and Antiinflammatory Activities of Premna integrifolia Plant Extracts in Bubaline Mastitis. J Complement Med Alt Healthcare. 2018; 7(5): 555724. DOI: 10.19080/JCMAH.2018.07.555724
Abstract
Mastitis, which affects the milk production of dairy animals, is usually due to mammary gland invasion by bacterial pathogens. Emergence of antimicrobial resistance in bacteria and side effects associated with the use of anti-inflammatory cortisones in mastitis prompted for use of alternate/complementary therapeutics. As the plant Premna integrifolia was reported to exhibit antibacterial, anti-inflammatory/immunomodulatory properties, its leaf and root aqueous extracts were tested for their antibacterial activity against Staphylococcus aureus and Escherichia coli, either individually or in combination with the antibiotics. The anti-inflammatory properties of the extracts were also tested against the bubaline mammary epithelial cells (MEC) infected with S. aureus and E. coli. In microbroth dilution assays for assessing minimum inhibitory concentration (MIC) in vitro, the leaf and root extracts of Premna integrifolia didn’t exhibit any antimicrobial activity against S. aureus but showed significant antimicrobial activity on E. coli. In combination with the plant extract, the sensitivity of S. aureus to amoxicillin is not only increased but also the S. aureus isolates that were resistant to amoxicillin also became sensitive. The Premna integrifolia leaf and root extracts, however, showed antagonism on antimicrobial activity of enrofloxacin in combination. In addition the aqueous root extract of Premna integrifolia exhibited anti-inflammatory activity through down regulation of cytokines IL-6, IL-8 and TNF-α in S. aureus and IL-6 and IL-8 in E. coli infected MEC. These studies reveal antimicrobial activity of leaf and root extracts of Premna integrifolia on E. coli. In combination with amoxicillin these plant extracts increased the sensitivity of S. aureus to amoxicillin. The anti-inflammatory activity of root extract of Premna integrifolia on MEC infected with S. aureus and E. coli is also demonstrated in these studies.
Keywords:Mastitis; S. aureus; E. coli; Premna integrifolia; Mammary epithelial cells; cytokines; Amoxicillin; Enrofloxacin
Introduction
Mastitis in dairy animals is inflammatory reaction of the udder tissue against the invading microbial pathogens. Bacterial pathogens are majorly implicated in the mastitis of cows and buffaloes leading to major production losses in dairy animals resulting in huge economic losses to dairy farmers and industry [1]. Staphylococcus aureus and Escherichia coli are the major bacterial pathogens of bovine/bubaline mastitis [2]. However, the emergence of antimicrobial resistance in bacterial pathogens that cause mastitis in dairy animals is a cause of grave concern [3-5]. Also controlling the inflammation in mastitis is very essential as the persistent inflammation of mammary gland tissue may result in permanent unproductivity in dairy animals [6-7]. Mastitis is the most frequent reason for the use of antimicrobial drugs in dairy herds, which eventually has resulted in antimicrobial resistance [8].
Development of new antibiotics will take long time and there is chance of further developing antimicrobial resistance against these molecules in due course. In this context exploration of natural compounds from medicinal plants that exhibit both antibacterial and anti-inflammatory/immunomodulatory properties may offer promising solution for therapeutic approach to mastitis in dairy animals. Medicinal uses of the plant Premna integrifolia that has prominent value in Indian system of medicine Ayurveda was reviewed by different researchers [9-11]. Reports on increased sensitivity of bacterial pathogens to antibiotics, when used in combination with anti-inflammatory compounds like Non-Steroidal Anti-inflammatory Drugs (NSAIDs) [12], also encourages us to take up research work on the natural compounds with anti-inflammatory activity. As the development of resistance to natural products of plant origin is highly remote and the issue of antibiotic residues in milk doesn’t arise with the natural compounds, the present investigation was taken up to study the antibacterial and anti-inflammatory/immunomodulatory activities of aqueous young leaf and root extracts of the plant Premna integrifolia. The study is aimed to test the anti-bacterial activity of the plant extracts on S. aureus and E. coli, either individually or in combination with the antibiotics. It is also aimed to test the anti-inflammatory/ immunomodulatory activity of the plant extracts on Mammary epithelial cells (MEC) cultured from fresh milk of buffaloes and further infected with the selected bacterial pathogens of mastitis.
Materials and Methods
Plant material
Plant materials were collected from Maharastra region of India. The plant was identified as Premna integrifolia L. belonging to Verbenaceae by Dr. S. K. Srivastava, Scientist-E, BSI, Dehradun with accession no. 116123. Sample herbarium sheets deposited with Northern Regional Centre, Botanical Survey of India, Dehradun.
Preparation of Premna integrifolia extracts
The young roots and leaves of Premna integrifolia were sun dried for 15 days, powdered and successively extracted with soxhlet apparatus with petroleum ether, ethyl acetate, methanol and water in the increasing polarity index. These extracts were dried using a rotatory evaporator followed by lyophilization. Similarly, leaves were dried in shade for 10 days and extracted as above. In the present study the aqueous extracts were evaluated for their anti-microbial and anti-inflammatory effects./p>
Bacterial isolates
The bacterial pathogens Staphylococcus aureus and Escherichia coli were isolated from the mastitic milk samples of buffaloes and the bacteria were subjected to characterization by culturing on selective bacteriological media. Mannitol salt agar (MSA) and Eosin methylene blue (EMB) agar (Oxoid, UK) were used for culture of S. aureus and E. coli, respectively. These bacteria were further characterized in polymerase chain reaction (PCR) test by reactivity with species-specific oligonucleotide primers [2].
Microbroth dilution method for measuring the minimum inhibitory concentration (MIC) of antibiotic/ minimum inhibitory concentration (MIC) of antibiotic/
The antimicrobial activity of the plant extracts was evaluated by microbroth dilution method in serial wells of microtitre plate (Axygen, USA) [13], with suitable modifications. Briefly, two-fold dilution of antibiotic/plant extract (10mg/ml) is made with cation adjusted Mulleur Hinton broth, in their respective wells of 96-well microtiter plate. The antimicrobial activity of the plant extracts was tested individually, also in combination with antibiotic. In the combination studies a fixed volume of 50μl of plant extract (10mg/ml) was added to the wells with serial dilution of respective antibiotic. Separate row(s) of wells with serial dilution of antibiotic alone were also maintained to compare the MIC values of antibiotic with the MIC values of plant extract or antibiotic & plant extract combination. Appropriate controls were also maintained. Amoxicillin and enrofloxacin (SRL, India) antibiotics in powder form were used for S. aureus and E. coli, respectively. To all the wells constant volume of 300μl of 0.5 McFarlands standard bacterial culture (S. aureus/E. coli) was added. The culture plates were incubated for 18hrs. and the absorbance readings were taken at 660 nm (Multiskan plate reader, Thermo). The MIC values of the antibiotic/plant extract or combination of antibiotic & plant extract corresponding to the absorbance readings of respective wells were noted. Then indicator dye p-iodonitrotetrazolium violet (INT) (SRL, India) was added to all the wells to visually appreciate the extent of antimicrobial activity of the compounds tested. The breakpoints of amoxicillin and enrofloxacin/ciprofloxacin in MIC assays were taken as per Clinical and Laboratory Standard Institute (CLSI) guidelines 2012.
In Microbroth dilution method for measuring the MIC a loopful of inoculum was picked up from the wells in microtiter plates where there is inhibition of bacterial growth and streaked on bacteriological medium, further incubated to confirm the absence of live bacteria/bacterial growth in those wells.
Isolation and culture of mammary epithelial cells (MEC) from milch buffaloes
Mammary epithelial cells were isolated form the fresh milk of apparently healthy milch buffaloes maintained at Livestock Farm Complex, NTR College of Veterinary Science, Gannavaram as per the established procedure [14] with suitable modifications. Briefly, the fresh milk samples were centrifuged at 500 x g and the cell pellet was washed with phosphate buffer saline (pH 7.2). Then the cell pellet was cultured in DMEM/F12 (Sigma, USA) medium with 10% Foetal Bovine Serum (Thermo Fisher) for 48 hrs. in 5% CO2 atmosphere. Four groups of the cultured mammary epithelial cells (MEC) were maintained. First group was maintained normal untreated. Second group was maintained as normal & treated (plant extract treated), third group was maintained as infected by infecting with 300μl of 0.5 Mcfarlands standard bacterial culture. The fourth group was maintained as plant extract treated & infected, where in MEC were treated with 300μl of plant extract (10mg/ml). After 6 hrs. of incubation with plant extract the MEC were infected with 300μl of 0.5 Mcfarlands standard bacterial culture and further incubated for 6 hrs. The S. aureus broth culture was used to infect MEC, whereas heat inactivated (65 °C/30 minutes) E. coli was used to treat the MEC.
Detection of cytokines expression in bubaline MEC by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
Two step qRT-PCR was carried out in this study. In the first step the total RNA from MEC of different groups of cells was extracted, separately, by using Trizol reagent (Invitrogen, USA) as per the manufacturer’s instructions. The quality of RNA was checked in Nanodrop (Thermo, USA). The cDNA from RNA of different groups of cells was synthesized by standard protocol using reagents/chemical/enzymes from Thermo Fisher Scientific, USA. Briefly, the 200 ng of RNA extracted was incubated with Random Hexamers, then treated with RNAase inhibitor RiboLock. The RNA was reverse transcribed to cDNA using M-MuLV Reverse Transcriptase RNaseH+ at 37 °C in a thermal cycler (Eppendorf Master cycler, Germany). Any contamination of genomic DNA was removed by using DNA free TM DNA removal kit. The resultant cDNA was quantified in Nanodrop.
In the second step the qRT-PCR tests were performed in 25μl of reaction volume in Quant Sudio3 Real Time PCR instrument (Applied Biosystems, USA). The levels of gene expression of cytokines Interleukin-6 (IL-6), Interleukin-8 (IL-8), Tumour Necrosis Factor - α (TNF-α) in MEC after 6 hrs. of infection with bacterial pathogens in normal and plant extracts treated MEC were studied. The house keeping β-actin gene was kept as endogenous control. The sequence of oligonucleotide primers used in this study (Bioserve Biotechnologies, India) were adopted from the earlier research reports (15). In the qRT-PCR tests KAPA SYBR Fast qPCR master mix based on SYBR Green technology was used under the test conditions of initial denaturation 95 °C/ 3 minutes; then 94 °C / 3 sec, 60 °C / 3 sec & 70 °C / 10 sec for 50 cycles, followed by standard melt curve conditions.
results
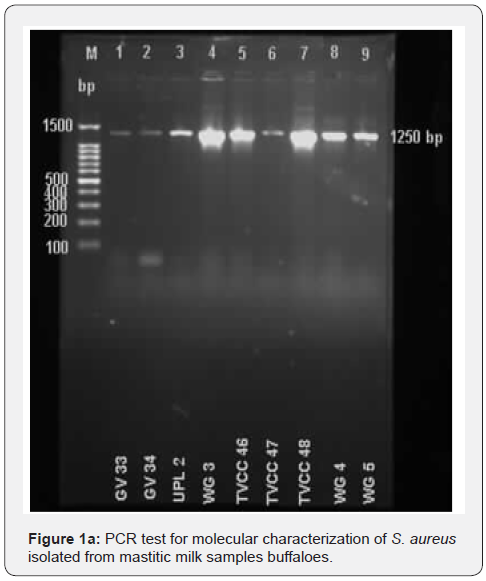
A total of 42 isolates of S. aureus and 11 isolates of E. coli were isolated from mastitic milk samples of buffaloes in and around Gannavaram, Krishna District, Andhra Pradesh. Certain mastitic milk samples were positive for mixed infections of S. aureus and E. coli. The S. aureus produced typical mannitol fermentation on MSA and the E. coli produced greenish metallic sheen on EMB agar, during the culture. In PCR test the S. aureus produced a specific PCR product of 1250 bp (Figure 1a) and E. coli produced a specific PCR product of 662 bp (Figure 1b).

In MIC assays, 31% isolates (n=13) of S. aureus were found to be resistant to amoxicillin. The isolates were GV28, GV40, GV42, GV43, GV45, TVCC41, TVCC47, TVCC49, TVCC53, PMNR1, KSP35, KSP36 and KSP39. Both the plant extracts (each extract separately) didn’t exhibit any significant antimicrobial activity against all the isolates (n=42) of S. aureus. However, for 45.2% of isolates (n=19) amoxicillin exhibited antimicrobial activity even at a lower concentration when combined with the plant leaf extract. The isolates were GV29, GV30, GV35, GV38, GV39, GV40, GV41, GV42, GV43, GV44, GV45, TVCC42, TVCC43, TVCC47, TVCC49, TVCC53, KSP35, KSP36 and KSP39. The MIC values of amoxicillin in antibiotic & leaf extract combination wells are found to be lower (to the extent of 0.00006μg/ml of concentration) than the MIC value of amoxicillin alone. Out of 13 isolates of S. aureus that were found to be resistant for amoxicillin, 10 isolates showed sensitivity to amoxicillin, when it is used in combination with the leaf extracts. For 23.8% isolates (n=10) of S. aureus there is no significant variation in MIC values of amoxicillin, when it is used alone or in combination with leaf extract. For 40.5% of isolates (n=17) amoxicillin exhibited antimicrobial activity at a lower concentration when combined with the plant root extract. The isolates were GV30, GV40, GV41, GV42, GV43, GV44, GV45, TVCC46, TVCC48, TVCC49, WG3, WG4, WG5, KSP35, KSP36, KSP39 and KSP43. The MIC values of amoxicillin in antibiotic & root extract combination wells are found to be lower than the MIC values of amoxicillin alone. Out of 13 isolates of S. aureus that were found to be resistant for amoxicillin, 9 isolates showed sensitivity to amoxicillin when it is used in combination with the root extract.
In MIC assays, all the E. coli isolates (n=11) were found to be sensitive to enrofloxacin. The isolates were GV26, GV27, GV28, GV29, WG1, KSP35, KSP38, GV46, GV47, KSP44 and KSP45. The leaf extract exhibited significant antimicrobial activity against 81.81% isolates (n=9) of E. coli. For these 9 isolates of E. coli the MIC values of leaf extract were significantly lower than the MIC values of enrofloxacin. The root aqueous extract exhibited antimicrobial activity against all the 11 isolates of E. coli. The MIC values of plant extracts was in the range of 31.25 to 0.98 μg/ml for different isolates of E. coli, whereas the MIC values of enrofloxacin are in the range of 500 - 62.5 μg/ml. In MIC assays with combination of enrofloxacin & plant extract (each extract separately), the enrofloxacin didn’t exhibit antimicrobial activity at its higher concentration but showed antimicrobial activity at its lower concentration.
After 48 hr. culture the MEC attained full confluence in tissue culture flaks and they were used for infection studies. The cDNA obtained from different groups of MEC was quantified by Nanodrop (Thermo) and same concentration cDNA from all the groups was used in qRT-PCR assays.
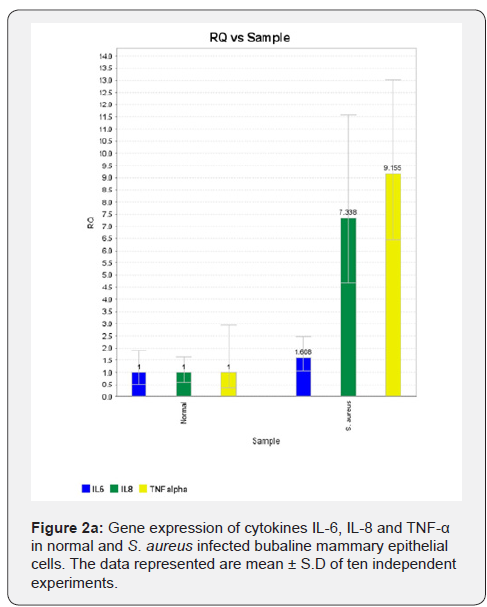
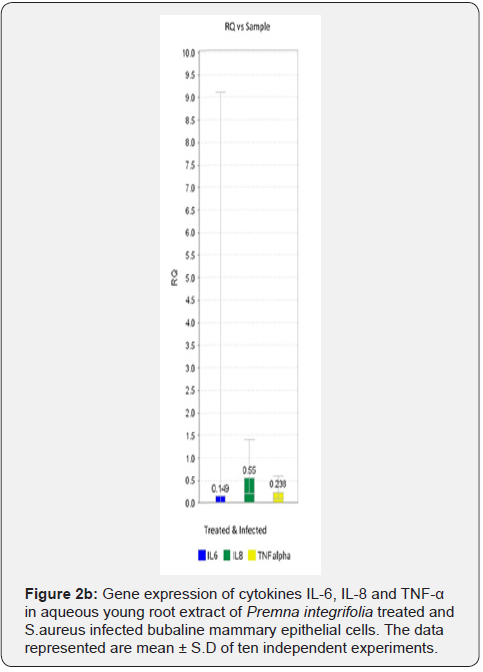
In MEC infection studies with S. aureus, the expression of cytokines IL-6, IL-8 and TNF-α genes were upregulated in S. aureus infected MEC (Figure 2a). In plant (young root) extract treated & infected MEC the gene expression of these cytokines was significantly downregulated compared to infected MEC (Figure 2b).
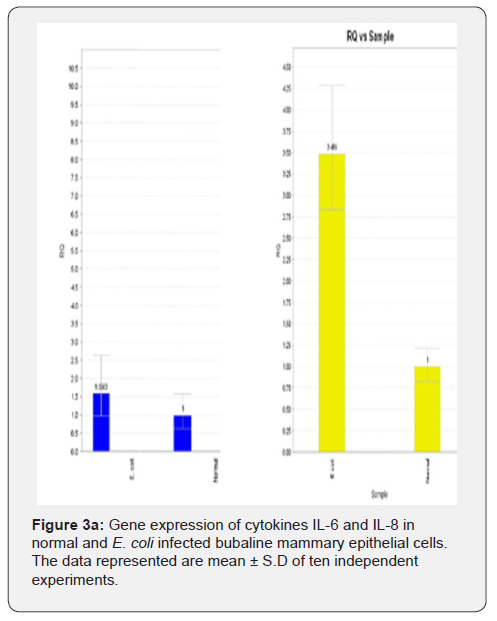
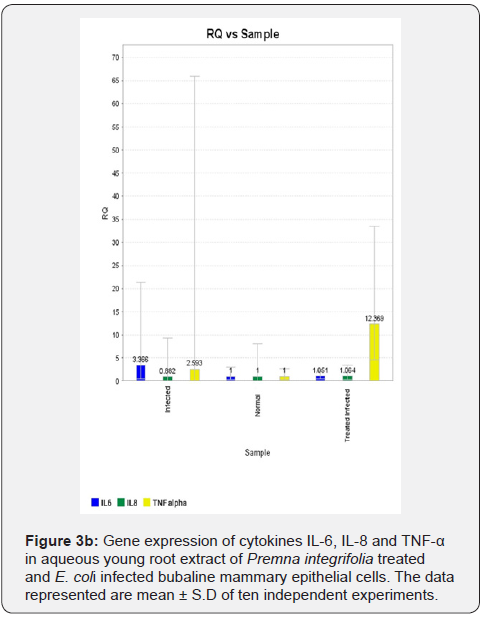
In MEC infection studies with E. coli, the gene expression of cytokines IL-6, IL-8 and TNF-α was upregulated in infected MEC compared to normal MEC (Figure 3a). Figure depicting upregulation of TNF-α gene expression was not shown. The gene expression of the cytokines IL-6 and IL-8 was downregulated in plant (young root) extract treated & infected MEC compared to infected MEC (Figure 3b). However, the gene expression of cytokine TNF-α was found to be upregulated in plant (young root) extract treated & infected MEC compared to infected MEC (Figure 3b).
Discussion
Mastitis in dairy bovines is usually caused by bacterial pathogens leading to inflammation of udder tissue and its further damage [1,2]. As the use of conventional antibiotics and antiinflammatory agents have certain disadvantages like development of antimicrobial resistance in bacteria, presence of antibiotic residues in milk during treatment, immunosuppression associated with cortisone administration etc., it is proposed to explore the antibacterial and anti-inflammatory/immunomodulatory activity of leaf and root aqueous extracts of the plant Premna integrifolia. The antibacterial activity of the plant extracts was tested on clinical isolates of S. aureus and E. coli isolated from mastitic milk samples of buffaloes. The isolated S. aureus and E. coli from different samples in this study were further characterized and the results were in accordance with the earlier reports [2].
Out of 42 characterized isolates of S. aureus 31% showed resistance to amoxicillin. The MIC values for indicating the resistance to amoxicillin in S. aureus were as per the CLSI guidelines, 2012. Due to the emergence of anti-microbial resistance, it is not surprising to find resistance to amoxicillin in S. aureus isolates from mastitic milk samples of dairy bovines [4,5]. Though antibacterial activity was reported with different extracts of Premna integrifolia [11,15-17], in the present study both the leaf and root aqueous extracts of the plant didn’t show any antimicrobial activity against all the isolates of S. aureus. This may be due to use of different solvent in the process of extraction. Also, in the previous studies the antimicrobial activity of the leaf extract was investigated by disc diffusion method [11], whereas in the present study the antimicrobial activity of plant extracts was tested by micro broth dilution method. In addition, all the isolates used in the present study were clinical isolates.
For 45.2% of isolates of S. aureus, amoxicillin exhibited antimicrobial activity at a lower concentration when combined with the plant leaf extract. It was reported that anti-inflammatory drug celecoxib sensitizes S. aureus to antibiotics [12] and the combinatorial effect of celecoxib and ampicillin was further demonstrated [18,19]. Anti-inflammatory activity of Premna integrifolia root was also reported [9]. Therefore, the antimicrobial activity exhibited by amoxicillin at lower concentrations may be due to combinatorial effect of plant extract (with anti-inflammatory activity) and amoxicillin. This may be correlated to the finding that out of 13 isolates of S. aureus that were found to be resistant to amoxicillin, 10 isolates showed sensitivity to amoxicillin when used in combination with the leaf extracts and 9 isolates showed sensitivity to amoxicillin when used in combination with the root extract. However, further research is to be carried out to find out the precise mechanism of this combinatorial effect.
In MIC assays the antibiotic enrofloxacin exhibited antimicrobial activity against all the isolates of E. coli. The sensitivity of E. coli to enrofloxacin in antimicrobial assays was already established [5,20]. Although the leaf extract didn’t exhibit any significant antimicrobial activity against S. aureus isolates, it exhibited significant antimicrobial activity against 9 isolates of E. coli. In fact MIC values of leaf extract were significantly lower than the MIC values of enrofloxacin for these E. coli isolates. The antimicrobial activity exhibited by the leaf extracts against E. coli is in accordance with the earlier reports on antibacterial activity of Premna integrifolia [11,16,17]. The aqueous root extract of the plant also exhibited significant antimicrobial activity against all the 11 isolates of E. coli. Specific research reports on the antimicrobial activity of the root extract are not available. Though the enrofloxacin has an established antimicrobial activity against E. coli when used alone, it is very interesting to observe in the present study that the enrofloxacin in combination with the plant extract (each extract separately) didn’t exhibit antimicrobial activity at higher concentration but exhibited its antimicrobial activity at lower concentrations. So, in two-fold serial dilution wells of enrofloxacin with combination of constant concentration of plant extract (each extract separately), bacterial growth was not inhibited at higher concentrations of enrofloxacin, whereas at lower concentrations of enrofloxacin the bacterial growth was inhibited. However, usually in MIC assays as the dilution of antibiotic progresses in the series of wells its concentration decreases and the bacterial growth is not inhibited in wells of microtiter plates with lower concentration of antibiotic. These findings are also in contrary to the reports on synergism of natural products and antibiotics [21].
From the studies on antimicrobial activity of fluoroquinolone antibiotic ciprofloxacin in combination with antioxidants it was reported that antioxidants exhibited antagonistic activity on ciprofloxacin [22,23]. It was observed that as the fluoroquinolones kill the bacteria by increasing the oxidative stress in bacterial cells, the concurrent/combinatorial use of antioxidants inhibit the oxidative stress induced by the ciprofloxacin. The antioxidant properties of Premna integrifolia were already reported [9- 11]. Therefore, it may be summrised that in the present study the antioxidant properties of the plant extracts antagonized the antimicrobial activity of enrofloxacin, which belongs to fluoroquinolones. This is supported by the observation that with plant extract combination E. coli growth was not inhibited in the microtiter plate wells with higher concentration of antibiotic, whereas in the wells with lower concentration of enrofloxacin the E. coli growth was inhibited. Perhaps there might be optimum levels of enrofloxacin and antioxidant plant extract combination in the microtiter plate wells with higher concentrations of enrofloxacin, leading to antagonistic action of plant extract on enrofloxacin. However, further studies are required for conclusive evidence on this aspect.
The aqueous leaf extract of the plant Premna integrifolia didn’t have any activity on downregulation in the expression of cytokines, IL-6, IL-8 and TNF-α genes in S. aureus and E. coli infection studies in MEC. However, in MEC infection studies with S. aureus the aqueous root extract of the plant Premna integrifolia showed antiinflammatory activity by downregulating the expression of genes of cytokines IL-6, IL-8 and TNF-α. But in MEC infection studies with E. coli the aqueous root extract showed anti-inflammatory activity by downregulating the expression of cytokines IL-6 and IL-8 genes only but not TNF-α. This may be due to the potent action of endotoxin of E. coli on MEC even after heat inactivation. This study thus forms the first report on the pattern of expression of cytokines IL-6, IL-8 and TNF-α genes in Premna integrifolia plant extract treated and infected cells of any system.
Conclusion
In conclusion, although the young leaf and root extracts of the plant Premna integrifolia didn’t exhibit any antimicrobial activity on S. aureus, significant antimicrobial activity was exhibited by these extracts on E. coli in microbroth dilution assays for MIC in vitro. However, in combination with the plant extract, the sensitivity of S. aureus to amoxicillin is not only increased but also the S. aureus isolates that were resistant to amoxicillin also showed sensitivity to the same antibiotic in this combination. The effect of plant extracts on E. coli, however, were in contrast with the findings of S. aureus as the antioxidant natural products showed antagonism on antimicrobial activity of enrofloxacin in its combination with the plant extracts. The aqueous root extract of Premna integrifolia exhibited anti-inflammatory activity through down regulation of genes of cytokines IL-6, IL-8 and TNF-α in S. aureus infected MEC. However, the down regulation of genes of cytokines was limited to only IL-6 and IL-8 only in E. coli infected MEC. Therefore, the plant extracts of Premna integrifolia offer promising solution for therapeutic approach to mastitis in dairy animals with a caution on its antioxidant property as it antagonizes the action of fluoroquinolone antibiotics.
Acknowledgment
The authors acknowledge the funding by National Medicinal Plants Board (NMPB), Ministry of AYUSH, Government of India, New Delhi to carry out this research project (Z. 18017/187/CSS/ R&D/AP-01/2014-15).
References
- Constable PD, Hinchcliff KW, Done SH, Grunberg W (2017) Veterinary Medicine: A text book of the diseases of Cattle, Horses, Sheep, Pigs and Goats (11th Edn). Elsevier, Missouri, USA, pp.1904-1996.
- Anand Kumar P (2009) Evaluation of PCR test for detecting major pathogens of bubaline Mastitis directly from mastitic milk samples of buffaloes. Tropical Animal Health and Production 41(8): 1643-1651.
- Kumar R, Yadav BR, Singh R S (2010) Genetic determinants of antibiotic resistance in Staphylococcus aureus isolates from milk of mastitic crossbred cattle. Current Microbiology 60(5): 379-386.
- Kumar R, Yadav BR, Anand SK and Singh RS (2011) Molecular surveillance of putative virulence factors and antibiotic resistance in Staphylococcus aureus isolates recovered from intra-mammary infections of river buffaloes. Microbial Pathogenesis 51(1-2): 31-38.
- Chandrasekaran D, Venkatesan P, Tirumurugaan KG, Nambi AP, Tirunavukkarasu PS, et al. (2014) Pattern of antibiotic resistant mastitis in dairy cows. Veterinary World 7(6): 389-394
- Alluwaimi AM (2004) The cytokines of bovine mammary gland: prospects for diagnosis and therapy. Research in Veterinary Science 77(3): 211-222.
- Bannerman DD (2009) Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intramammary infection of dairy cows. Journal of Animal Science 87 (Suppl. 1):10-25.
- Li X, Mehrotra M, Ghimire S, Adewoye L (2007) β-Lactam resistance and β-lactamases in bacteria of animal origin. Veterinary Microbiology 121(3-4): 197-214.
- Gokani RH, Lahiri SK, Santani DD, Shah MB (2011) Evaluation of antiinflammatory and antioxidant activity of Premna integrifolia root. J Complement Integr Med 8(1).
- Mali PY (2015) Premna integrifolia L: A review of its biodiversity, traditional uses and phytochemistry. Anc Sci Life 35(1): 4-11.
- Mali PY (2016) Pharmacological potentials of Premna integrifolia L. Ancient Science of Life 35(3):132-142.
- Kalle AM, Rizvi A (2011) Inhibition of bacterial multidrug resistance by celecoxib, a cycloxigenase-2 inhibitor. Antimicrob Agents Chemother 55(1): 439-442.
- Eloff JN (1998) A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica 64(8): 711-713.
- Krishnakanth G, Selokar NL, Saini M, Chauhan MS, Manik RS, et al. (2012) Production of nuclear transfer embryos by using somatic cells isolated from milk in buffalo (Bubalus bubalis). Reprod Domest Anim 47(5): 842-848.
- Leutenegger CM, Alluwaimi AM, Smith WL, Perani L, Cullor JS (2000) Quantitation of bovine cytokine mRNA in milk cells of healthy cattle by real-time TaqMan ® polymerase chain reaction. Vet Immunol Immunopathol 77(3-4): 275-287.
- Kurup KK, Kurup PA (1964) Antibiotics substances from the root bark of P. integrifolia. Die Naturwissenschaften 20: 484.
- Kumar KU, Soma P, Kumar SS, Chandra SM, Kumar BS (2011) Assessment of analgesic and antibacterial activity of Premna integrifolia Linn (Family: Verbenaceae) leaves. International Journal of Pharmaceutical Sciences and Research 2: 1430-1435.
- Madhavi A, Arunasree MK (2014) Celecoxib sensitizes Staphylococcus aureus to antibiotics in macrophages by modulating SIRT1. PLOS ONE 9(6): e99285.
- Madhavi A, Varma GYN, Anuradha K, Arunasree MK (2017) Celcoxib enhances the efficiency of Low-dose antibiotic treatment against polymicrobial sepsis in mice and clinical isolates of ESKAPE pathogens. Front Microbiol 8: 805.
- Chauhan PM, Thumar HK, Bhagat A, Sharma VK, Chauhan HC, Patel MR (2016) Comparitive efficacy of antibiotic sensitivity tests for management of acute clinical Escherichia coli mastitis in crossbred cow. Journal of Livestock Science 7: 41-45.
- Hemaiswarya S, Kruthiventi AK, Doble M (2008) Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 15(8): 639-652.
- Goswami M, Sharma D, Khan NM, Checker R, Sandur SK, et al. (2014) Antioxidant supplementation enhances bacterial peritonitis in mice by inhibiting phagocytosis. Journal of Medical Microbiology 63: 355-366.
- Masadeh MM, Alzoubi KH, Al-azzam SI, Khabour OF, Al-buhairan AM (2016) Ciprofloxacin induced antibacterial activity is attenuated by pretreatment with antioxidant agents. Pathogens 5(1): 28.






























