In Vitro Studies on Alpha Glucosidase Inhibitory Activity of Some Indigenous Plants
Mangesh A Bhutkar*, Somnath D Bhinge, Dheeraj S Randive, Ganesh H Wadkar and Sachin S Todkar
Department of Pharmaceutics, Rajarambapu College of Pharmacy, India
Submission: April 17, 2018; Published: August 22, 2018
*Corresponding author: Mangesh A Bhutkar, Department of Pharmaceutics, Rajarambapu College of Pharmacy, Kasegaon, Tal-Walwa, Dist-Sangli, Maharashtra, India, Tel: +91 2342 238200;+91 9423263907; Email: mangesh_bhutkar@rediffmail.com
How to cite this article: Mangesh A B, Somnath D B, Dheeraj S R, Ganesh H W, Sachin S T. In Vitro Studies on Alpha Glucosidase Inhibitory Activity of Some Indigenous Plants. J Complement Med Alt Healthcare. 2018; 7(2): 555706. DOI: 10.19080/JCMAH.2018.07.555706
Abstract
CDiabetes mellitus is a common and very prevalent disease affecting the citizens of both developed and developing countries. The deficiency or insensitivity of insulin in diabetes mellitus causes glucose to accumulate in the blood, leading to various complications. Currently available pharmacotherapies for the treatment of diabetes mellitus include oral hypoglycemic agents and insulin. One of therapeutic approach to treat diabetes is to decrease postprandial hyperglycemia in patients with type II diabetes that can be achieved by the inhibition of carbohydrate hydrolyzing enzymes like α-amylase and α-glucosidase. These enzyme inhibitors which are currently used in the clinical practice for management of diabetes also exhibit certain gastrointestinal side effects. Thus, there is an urgent need to identify and explore such enzyme inhibitors, especially from the natural sources having fewer side effects. In the present study, aqueous extracts of selected plants namely Catharanthus roseus, Caesalpinia bonducella and Mucuna pruriens which are used in the Ayurvedic traditional system of medicine to treat diabetes were tested for their inhibitory effect on α-glucosidase. The results of the study revealed that the extract of C.roseus was found to be more effective in inhibition of the enzyme α-glucosidase as compared to C.bonducella and M.pruriens, whereas Acarbose exhibited maximum α-glucosidase inhibitory activity with 4.5mm as the diameter of the inhibitory ring. Further studies are necessary for use of the extracts of C.roseus, C. bonducella and M.pruriens as chemotherapeutic agents for clinic use in the treatment of diabetes and obesity. .
Keywords: Antidiabetic; α-glucosidase; Inhibitory effects; Indigenous plants
Introduction
Diabetes mellitus is a common and very prevalent disease affecting the citizens of both developed and developing countries and has become a major source of ill health worldwide [1,2]. It has been predicted that by 2030, India, China and the United States will have the largest number of people with diabetes [3]. Diabetes mellitus is characterized by hyperglycaemia, lipidaemia and oxidative stress and predisposes affected individuals to long-term complications afflicting the eyes, skin, kidneys, nerves and blood vessels [4]. One of the therapeutic approaches to treat diabetes is to decrease postprandial hyperglycemia in patients with type II diabetes. It can be effectively achieved by the inhibition of carbohydrate hydrolyzing enzymes like α-amylase and α-glucosidase. However, such inhibitors which are currently used in the clinical practice for management of diabetes are known to exhibit various gastrointestinal side effects. Thus, there is an urgent need to identify and explore inhibitors of carbohydrate hydrolyzing enzymes from natural sources having fewer side effects. Plant and plant products are being used as a source of medicine since long. Traditional herbal medicines form an important part of healthcare system in India [5]. Ayurveda and other ancient Indian literature have mentioned several herbal plants and their preparations useful in the treatment of various diseases and disorders [6,7]. Ethanopharmacological surveys have shown that more than 1200 plants have been mentioned in traditional medicine for their alleged hypoglycemic activity [8]. Numerous plants and their products have been widely prescribed and used for diabetic treatment all around the world with less known mechanistic basis of their functioning. The present study was therefore undertaken to make a comparative study for the ability of the selected plants to inhibit α-glucosidase activity.
Catharanthus roseus a traditionally known medicinal plant, which belongs to the family Apocynaceae, is an erected procumbent herb or under shrub containing latex. It has been known to possess antibacterial, antimicrobial, antifungal, antioxidant, anticancer and antiviral activities. The organic extracts of C. roseus has been used in the folklore for the treatment of diabetes, malaria, leukemia wasp stings, sore throat, eye irritation, infections and to stop bleeding. It is also used a as an astringent, diuretic and expectorant [9,10].
Caesalpinia bonducella (Family: Caesalpiniaceae) is prickly shrub claimed to have multiple therapeutic properties. All parts of the plant have medicinal properties, so it is a very valuable medicinal plant which is utilized in traditional system of medicine. The plant has been reported to possess anxiolytic, antinociceptive, antidiarrhoeal, antidiabetic, adaptogenic, anthelmintic, anti inflammatory, antimalarial, antimicrobial, antipyretic, analgesic, antibacterial, antispasmodic, antioxidant, antiproliferative, antipsoriatic, antitumor, larvacidal, muscle contractile, hepatoprotective, anticonvulsant and antifilarial activities [11,12].
Mucuna pruriens (Fabaceae) is one of the popular drugs in the Ayurvedic system of medicine. Various preparations from the seeds of this plant are used for the management of several free radical mediated diseases such as ageing, rheumatoid arthritis, diabetes, atherosclerosis, male infertility and nervous disorders [13,14].
The present study thus aimed to make a comparative study for the ability of the extracts of selected plants to inhibit in-vitro α-glucosidase activity.
Materials and methods
Chemicals and reagents
All the chemicals used during the experimental work were of analytical grade obtained from S.D. Fine Chemicals Pvt. Ltd., Mumbai, Sigma chemical company, USA and Loba chemicals, Mumbai.
Plant material
The seeds of M.pruriens, and C. bonducella were collected from local areas of Kasegaon, District Sangli, (MS), India, whereas the roots of C.roseus were collected from local areas of Karad, District Satara, (MS), India. The plant material was further identified and authenticated by the Department of Botany, YC College of Science, Karad. The plant material was cleaned thoroughly, dried in a hot air oven (50 °C) separately powdered, passed through 60 mesh sieve (BS) and thereafter stored in an airtight container at 4 °C till further use.
Preparation of plant extracts
Aqueous extracts were prepared by extracting the powders of the plant materials with hot water (70 °C) in a mechanical shaker (24 h), filtered and freeze dried.
Alpha glucosidase inhibition [15]
The plant extracts were spotted on the plate (diameters≤ 2mm). Agar solution containing 1.6% starch was poured on the plate. Enzyme (2mg/ml) in 0.1 mol/l acetate buffer (pH 4.5) was impregnated on to the paper. After the substrate solution gelled, the paper was overlaid on the substrate agar layer. The plate was incubated for 1.5h at 55 0C, 0.05 mmol/l iodine solution was poured on the surface after the paper was peeled off. Excess iodine was removed by rinsing the sheet with water. A purple ring would appear around a sample spot that contained inhibitors.
Statistical analysis
All the analyses were carried out in triplicate and the results were expressed in mean ± SD.
Results and Discussion
One of the therapeutic approaches to treat diabetes is to decrease postprandial hyperglycemia in patients with type II diabetes that can be achieved by the inhibition of carbohydrate hydrolyzing enzymes like α-amylase and α-glucosidase. Pancreatic α -amylase is a key enzyme in the digestive system and catalyses the initial step in hydrolysis of starch to a mixture of smaller oligosaccharides consisting of maltose, maltotriose, and a number of α-(l-6) and α-(1-4) oligoglucans. These are then acted on by α -glucosidases and further degraded to glucose which on absorption enters the blood-stream. Degradation of this dietary starch proceeds rapidly and leads to elevated PPHG (postprandial hyperglycemia). It has been shown that activity of HPA (human pancreatic α -amylase) in the small intestine correlates to an increase in post-prandial glucose levels, the control of which is therefore an important aspect in treatment of type 2 diabetes [16,17].
Inhibitors of pancreatic α -amylase delay carbohydrate digestion causing a reduction in the rate of glucose absorption and lowering the post-prandial serum glucose levels. Some inhibitors currently in clinical use are Acarbose, Miglitol and Voglibose have their own limitations. They are non-specific, produce serious side effects and fail to elevate diabetic complications. Acarbose which has strong α-amylase inhibitory activity produces digestive tract disorder such as abdominal distention, bloating, flatulence, and possibly diarrhea [18].
Therefore, natural inhibitors from dietary plants are useful as they have lower inhibitory activity against α-amylase and a stronger inhibitory activity against α- glucosidase and can be used as effective therapy for postprandial hyperglycemia with minimal side effects. Many phytoconstituents have been reported to possess α-amylase and α-glucosidase inhibitors. Some phenolic compounds in sweet potato, strawberry, Raspbery, Olive oil, pears, coca and Lentils are reported to be effective human α-amylase inhibitors. Flavonoids and anthocyacin are also reported to have inhibitory activity against α-amylase [19]. Natural polyphenols have been described to have potential to hinder the activity of carbohydrate hydrolyzing enzymes like α-amylase and α-glucosidase [20].
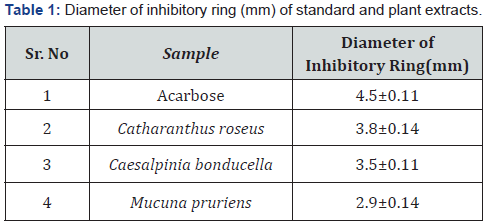
In the present study α-glucosidase inhibitory activity of the plant extracts was studied by TLC plate method. It is a rapid and possesses high capacity; about 25 samples can be performed in one TLC plate of 36cm2 area with results in just 2 hours. The doses of plant extracts were spotted on the plate and the inhibitory ring of Acarbose and the plant extracts was determined after its treatment with starch. The diameter of the inhibitory ring for the selected plant extracts and Acarbose are given in Table 1.
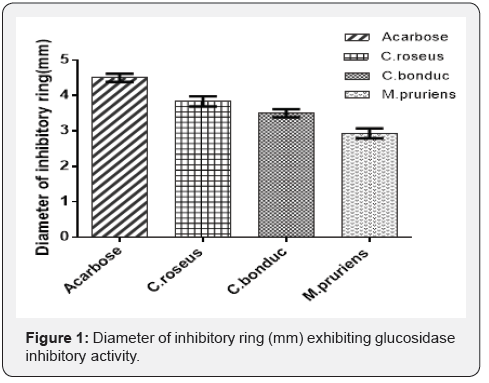
Acarbose, the standard used exhibited maximum α-glucosidase inhibitory activity with 4.5mm as the diameter of the inhibitory ring whereas it was observed as 3.8mm, 3.5mm and 2.9mm for C.roseus, C.bonducella and M.pruriens respectively. Diameter of inhibitory ring (mm) obtained with different plant extracts is shown in Figure 1.
The results revealed that C.roseus, C.bonducella and M.pruriens showed α-glucosidase inhibitory activity and can thus reduce the rate of digestion and absorption of carbohydrates. The α-glucosidase inhibitory activity exhibited by the selected plant extracts may be attributed to the presence of various phytoconstituents in these extracts.
Conclusion
The results of the present study indicate that the selected plant extracts exhibited maximum α-glucosidase inhibitory activity and thus, will prove to be beneficial to reduce the rate of digestion and absorption of carbohydrates. The plant extracts may essentially contain herbal bioactive compounds inhibiting α-glucosidase enzyme activity. Further studies have to be carried out in order to identify the bioactive constituents for use of the extracts of C.roseus, C.bonducella and M.pruriens as chemotherapeutic agents in clinic use for the treatment of diabetes and obesity
References
- Bhutkar MA, Bhise SB (2011) Comparison of antioxidant activity of some antidiabetic plants. Int J Res Pharm Biomed Sci 2(3): 982-987.
- Bhutkar MA, Bhinge SD, Randive DS, Wadkar GH (2017) Hypoglycemic effects of Berberis aristata and Tamarindus indica extracts in vitro. Bull Fac Pharm Cairo Univ 55(1):91-94.
- Bhutkar MA, Bhise SB (2011) Anti-oxidative effect of Tamarindus indica in alloxan induced diabetic rats. Int J Res Pharm Biomed Sci 2(3):1006-1009.
- Bhutkar MA, Bhise SB (2013) Studies on antiglycation potential of some traditional antidiabetic plants. Asian J Plant Sci Res 3(6): 60-63.
- Bhutkar MA, Bhise SB (2011) Antioxidant properties of Mentha piperita and Mentha spicata: A comparative study. J Herbal Med Toxicol 5(2): 109-113.
- Randive DS, Bhinge SD, Sayyad SF, Wadkar GH (2016) Comparative standardization of marketed formulations of fermented biomedicine - Arjunaristha. Indonesian J Pharmacy 27: 220-225.
- Randive DS, Sayyad SF, Bhinge SD, Bhutkar MA (2016) Preparation of Arjunarishta using microbes isolated from Woodfordia fruticosa flowers (Dhayati). Ancient Sci Life 36(1): 42-47.
- Pranav K, Doble P, Doble M (2008) A target based therapeutic approach towards Diabetes mellitus using medicinal plants. Current Diabetes Reviews 4(4): 291-308.
- Jayanthi M, Sowbala N, Rajalakshmi G, Kanagavalli U, Sivakumar V (2010) Study of anti- hyperglycemic effect of Catharanthus roseus in alloxan induced diabetic rats. Int J Pharm Pharm Sci 2(4): 114-116.
- Bhutkar MA, Bhise SB (2011) Comparative studies on antioxidant properties of Catharanthus rosea and Catharanthus alba. Int J Pharm Tech Res 3(3):1551-1556.
- Wadkar GH, Sayyad FJ (2017) Pharmacognostic, physicochemical and phytochemical investigation of root bark of Caesalpinia bonducella. Int J Pharmacogn Phytochem Res 9(1): 26-30.
- Bhutkar MA, Bhinge SD, Randive DS, Wadkar GH, Todkar SS (2017) Studies on in-vitro antiglycation potential of some indigenous antidiabetic plants. Global J Pharm Pharm Sci 3(5): 1-5.
- Sharma BK, Shamim A, Singh R (2012) A review on M. pruriens its phytoconstituents and therapeutic uses. Novel Sci Int J Pharm Sci 1(6): 308-312.
- Bhutkar MA, Bhise SB (2013) In vitro hypoglycemic effects of Albizzia lebbeck and Mucuna pruriens. Asian Pac J of Trop Biomed 3(11): 866- 870.
- Haimin C, Xiaojun Y, Lin W, Zheng LI, Zhang W (2004) A new method for screening α-glycosidase inhibitors and application to marine microorganisms. Pharm Biol (42): 416-421.
- Eichler HG, Korn A, Gasic S, Prison W, Businger J (1984) The effect of new specific α-amylase inhibitor on post-prandial glucose and insulin excursions in normal subjects and Type 2 (non-insulin dependent) diabetic patients. Diabetologia 26(4): 278-281.
- Bhandari MR, Nilubon JA, Gao H, Kawabata J (2008) α-glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliate). Food Chem 106(1): 247-252.
- Vichayanrat A, Ploybutr S, Tunlakit M, Watanakejorn P (2002) Efficacy and safety of Voglibose in comparison with acarbose in type 2 diabetic patients. Diabetes Res Clin Pract 55(2): 99-103.
- Bischoff H (1994) Pharmacology of glucosidase inhibitor. Eur J Clin Invest 24(3): 3-10.
- Mai TT, Thu NN, Tien PG, Van CN (2007) α-glucosidase inhibitory and antioxidant activities of Vietnamese edible plants and their relationships with polyphenol contents. J Nutr Sci Vitaminol 53(3): 267-276.






























