Cytotoxicity Effects of LPR-1®, CCE100 and ONCOBLOC® on a Panel of Cancer Cell Lines
Wong Teck Yew2, Patrick Loh Heng Meng2,3, Zhang Peng Fei3 and Lim Yang Mooi1,2*
1Department of Pre-clinical Sciences, Malaysia
2Centre for Cancer Research, Faculty of Medicine and Health Sciences, University Tunku Abdul Rahman, Malaysia
3Link (THM) Sciences Pte Ltd (Singapore), 8 Chang Charn Road, Link (THM) Building, Singapore
Submission: March 13, 2018; Published: April 02, 2018
*Corresponding author: Lim Yang Mooi, Department of Pre-clinical Sciences, Faculty of Medicine and Health Sciences, University Tunku Abdul Rahman, Malaysia, Email: ymlim@utar.edu.my
How to cite this article: Wong T Y, Patrick L H M, Zhang P F, Lim Yang M. Cytotoxicity Effects of LPR-1®, CCE100 and ONCOBLOC® on a Panel of Cancer Cell Lines. J Complement Med Alt Healthcare. 2018; 6(1): 555676. DOI: 10.19080/JCMAH.2018.06.555676.
Abstract
LPR-1®, CCE100 and ONCOBLOC® are LPR-1® Standardized C. militaris (L.) Link Extract (LSc 1501) Conventional Cordyceps extract (100X concentration) and ONCOBLOC® standardized novel C. militaris (L.) Link extract (SLS-161), respectively. All these three extracts are obtained from C. militaris through conventional and proprietary extraction processes. These extracts possess a broad spectrum of chemoprotective properties against free radicals and oxidative stress. In this study, the cytotoxicity ONCOBLOC® were assessed against 24 human cancer cell lines using MTT of LPR-1®, CCE100 and cytotoxicity assay. In addition, the cytotoxic effects of these extracts were benchmarked to chemotherapy drug, doxorubicin on a panel of cancer cell lines. From the results obtained, LPR-1® showed cytotoxicity to HONE-1, NCI-H23 and Fa Du cells; CCE100 caused cytotoxicity to HONE-1, Fa Du, T-47D, MCF-7 and U-2 OS cells; whereas ONCOBLOC® exhibited cytotoxicity to HONE-1, FaDu, T-47D, MDA- MB-231 and K-562. Among the 24 cancer cells, ONCOBLOC® showed the strongest cytotoxic effects against nasopharyngeal carcinoma epithelial cell line (HONE-1), with the IC50 value of 15.34^g/mL. These preliminary results suggest that ONCOBLOC® possesses promising anti-cancer activities and could be developed as a novel anticancer agent. Hence, more efficacy studies will be carried to confirm its anti cancer properties.
Keywords: Cytotoxicity; C. militaris; LPR-1®, CCE100, ONCOBLOC®
Introduction
LPR-1® and ONCOBLOC® were extracted by using SINGAPORE LINK SCIENCES PTE LTD’s proprietary protocol LSc 1501 and SLS-161, respectively. Proprietary extraction refers to the combination use of formulated extraction solvent, proprietary extraction process protocol and customized fabricated extraction equipment. LPR-1® is currently available in the market and is being marketed for the application on organ-ageing associated conditions, it is also prescribed by endocrinologists for kidney and diabetic related conditions. CCE100 is Conventional Cordyceps Extracts with 100X concentration. Conventional extraction refers to the use of 100% water as the sole extraction solvent, followed by air-drying of the extracts. This is done repeatedly to achieve a 1:100 weight/weight ratio of the initial versus final extract. CCE100 is prepared as a benchmark for comparison of conventional extract against targeted extracts LPR-1® and ONCOBLOC® is developed and formulated for oncology-related (adjuvant) applications. C. militaris has been reported to have the medicinal beneficial on improving male reproduction [1], anti-aging [2], immunomodulatory [3], anti-microbial [4], antibacterial [4], anti-viral [5], anti-fungal [5], insecticidal [5], larvicidal [6], anti-fibrotic [7], anti-diabetic [8], anti-HIV [9], anti-malarial [10], anti-fatigue [11], anti-platelets aggregation [12], neuroprotective [13], liverprotective [14], reno-protective [15,16], as well as pneumo-protective [17]. C. militaris contains many active compounds, such as cordycepin, polysaccharides, ergosterol and mannitol [18]. The major bioactive compound cordycepin (3'-deoxyadenosine) might contribute to anticancer activities. The anticancer activities of cordycepin have been reported by other researchers, such as, anti-angiogenetic [19,20], anti-inflammatory [21], anti- oxidant [22], growth inhibition on U937 leukemia cells [23], anti-leukemic [24], anti-tumour [25], anti- proliferative [26], anti-metastatic [27,28], inhibit purine biosysnthesis, DNA/RNA biosynthesis, shut down mTOR signalling pathway [29], induce apoptosis [30] and regulate cell cycle [31].
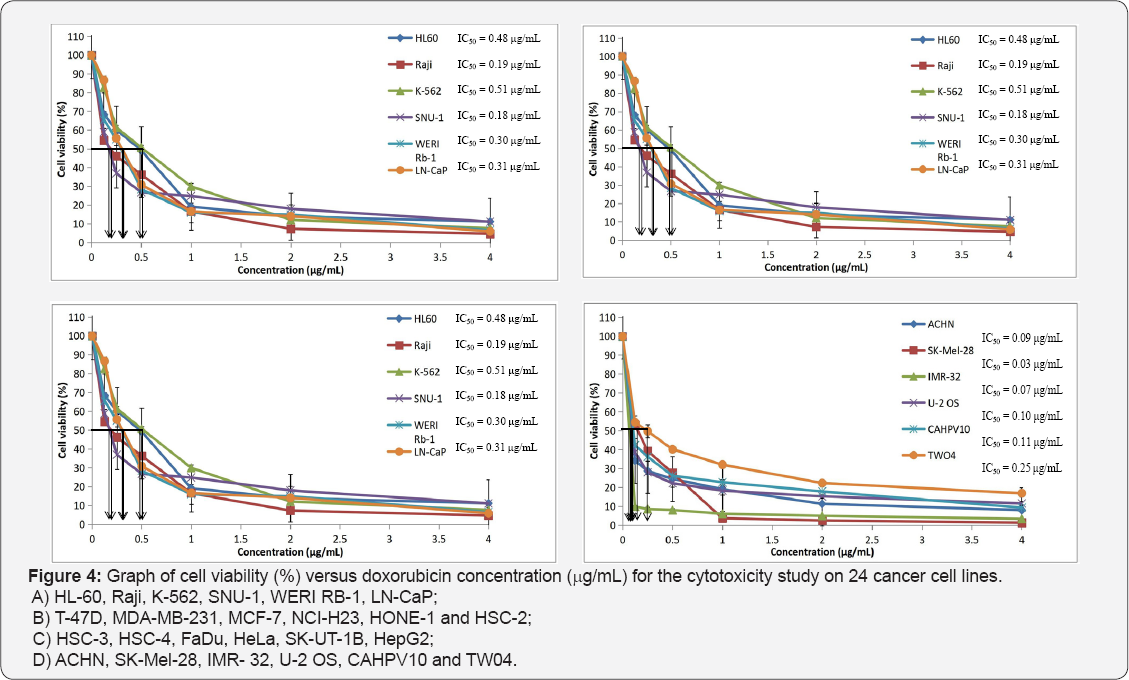
The cytotoxic effects of LPR-1®, CCE100 and ONCOBLOC® on various cancer cells are yet to be studied. Thus, this study aims to evaluate the cytotoxicity of LPR-1®, CCE100 and ONCOBLOC® against 24 cancer cell lines using MTT assay. The 24 cancer cell lines tested were acute promyelocytic leukaemia cell line (HL60), chronic myeloid leukaemia cell line (K-562), lymphoma cell line (Raji), gastric carcinoma cell line (SNU-1), retinoblastoma cell line (WER1-Rb-1), two prostate adenocarcinoma cell lines (LN-CaP and CAHPV 10), three breast cancer adenocarcinoma cell lines (T-47D, MDA-MB-231, and MCF-7), non- small cell lung carcinoma cell line (NCl-H23), nasopharyngeal carcinoma epithelial cell line (HONE-1), upper aerodigestive tract carcinoma cell line (HSC-2), oral squamous cell carcinoma cell line (HSC-3), tongue squamous cell carcinoma cell line (HSC-4), hypopharyngeal squamous carcinoma cell line (FaDu), cervical adenocarcinoma cell line (HeLa), Uterus/Leiomyosarcoma cell line (SK-UT-1B), hepatocellular carcinoma cell line (HepG2), renal cell adenocarcinoma cell line (ACHN), melanoma cell line (SK-Mel-28), neuroblastoma cell line (lMR-32), osteosarcoma cell line (U-2 OS) and nasopharyngeal carcinoma cell line (TW04). The cytotoxicity effects of LPR-1®, CCE100 and ONCOBLOC® were benchmarked with doxorubicin, a standard chemotherapeutics drug on 24 cancer cell lines. The most active extract will be further studied on its anti-cancer, anti-tumour- promoting and anti-inflammatory effects before developed it as a potential cancer preventive agent.
Materials and Methods
Preparation of LPR-1®, CCE100, ONCOBLOC® and Doxorubicin
LPR-1®, CCE100 and ONCOBLOC® were provided by LINK (THM) SCIENCES PTE LTD (Singapore). LPR-1® is in pellet form whereas CCE100 and ONCOBLOC® are in powder form. All three samples were soaked in water, sonicated and filtered through PTFE 0.45μm membrane filter to obtain the supernatant of LPR- 1®, CCE100 and ONCOBLOC®. The supernatant collected was then freeze-dried. The dried extracts were then dissolved in water for preparing the concentrations needed in this experiment. The concentrations of LPR-1®, CCE100 and ONCOBLOC® were prepared at 3.125, 6.25, 12.5, 25, 50 and 100μg/mL. The cytotoxic effects of these extracts were benchmarked with doxorubicin that was purchased from Sigma-aldrich (USA). The concentrations of doxorubicin were prepared at 0.125, 0.25, 0.5, 1, 2 and 4μg/mL.
Determination of Optimal Cell Seeding Concentration
Optimum cell seeding density of each cell line was determined prior to MTT assay. A total of 1x106, 5 x105, 1 x105, 1 x104 and 1 x103 cells/ml of cells were seeded in each well accordingly in a 96-well plate. The cells were incubated for 72 hours prior subjected to cytotoxicity evaluation by MTT assay. Absorbance of each well was read at 570 nm. Absorbance versus cell density graph was plotted to determine the optimum cell concentration of each cancer cell. The optimum cell concentration was obtained from the middle point of exponential phase of each graph plotted for each cell line. The optimum cell concentration for each cell line obtained was served as the cell seeding concentration for cytotoxicity evaluation.
MTT Assay
LPR-1®, CCE100, ONCOBLOC® and doxorubicin were tested for cytotoxicity against 24 cell lines. A total of 100μL of cells at the optimum cell concentration were seeded into each well of 96-well plate. Three wells containing medium only were included as a negative control. After 24 hours of incubation, each well was added with 100μL mixture of fresh medium containing respective extracts (LPR-1®, CCE100, ONCOBLOC® or doxorubicin). The concentrations of LPR-1®, CCE100 and ONCOBLOC® tested were at 3.125, 6.25, 12.5, 25, 50 and 100μg/ mL, whereas, the concentrations of doxorubicin tested were at 0.125, 0.25, 0.5, 1, 2 and 4μg/mL. The plate containing culture was then incubated for 72 hours. After 72 hours of incubation, 20μL of MTT was added into each well and incubated for another 4 hours. After 4 hours of incubation, the plate was centrifuged at 3000 rpm for 5 minutes. A total of 140 μL medium was carefully removed from each well. A total of 100μL iso-propanol was then added into each well and swirled gently at room temperature in the dark for 15 minutes. The absorbance of each well was read at 570 nm. The assays were conducted in triplicate and repeated in three independent experiments. The average absorbance against number of cells/mL graph was plotted and 1C50 value was calculated.
Results and Discussion
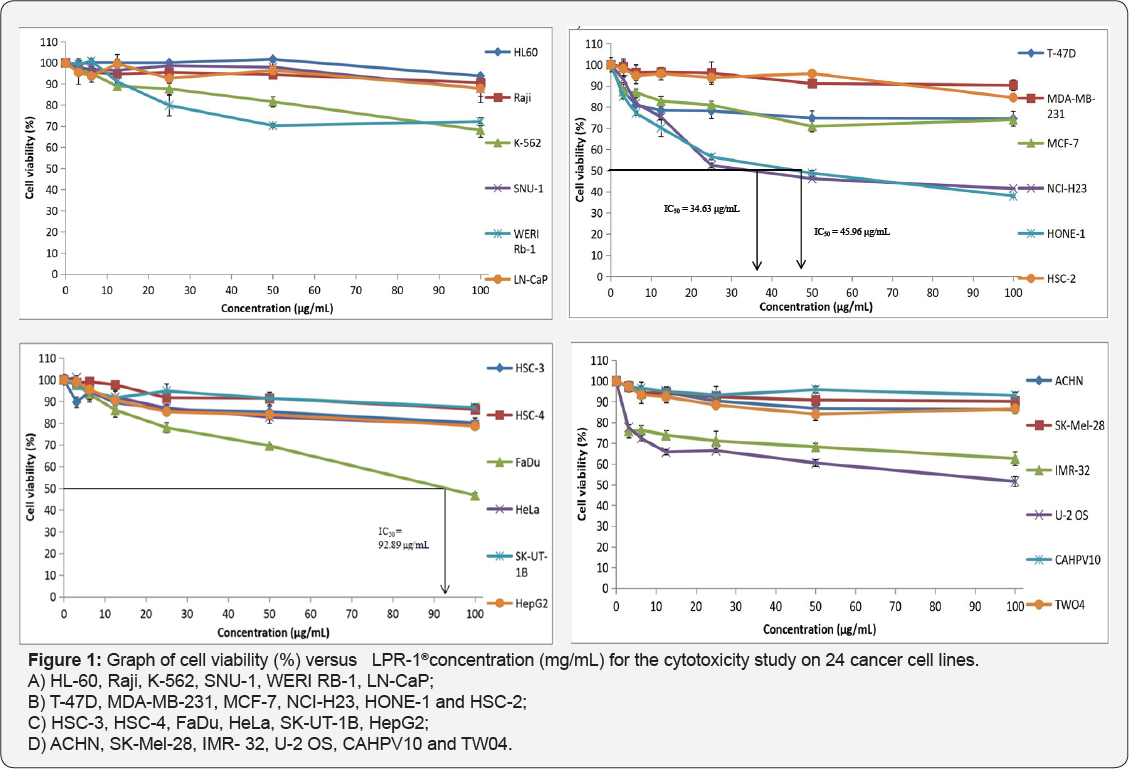
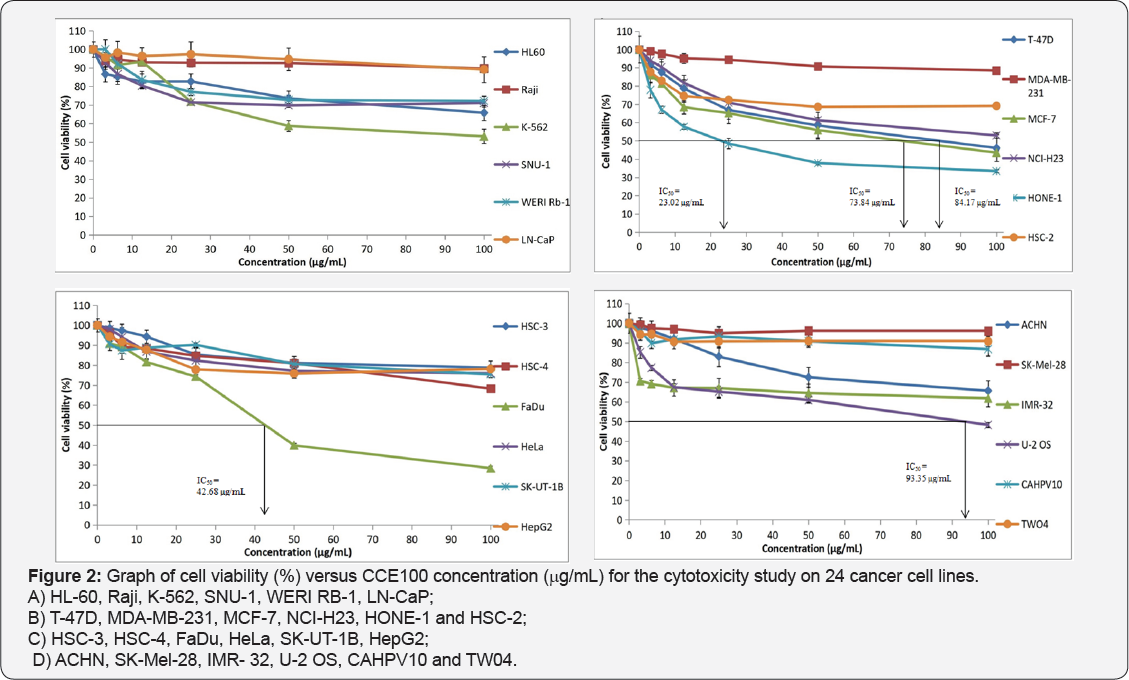
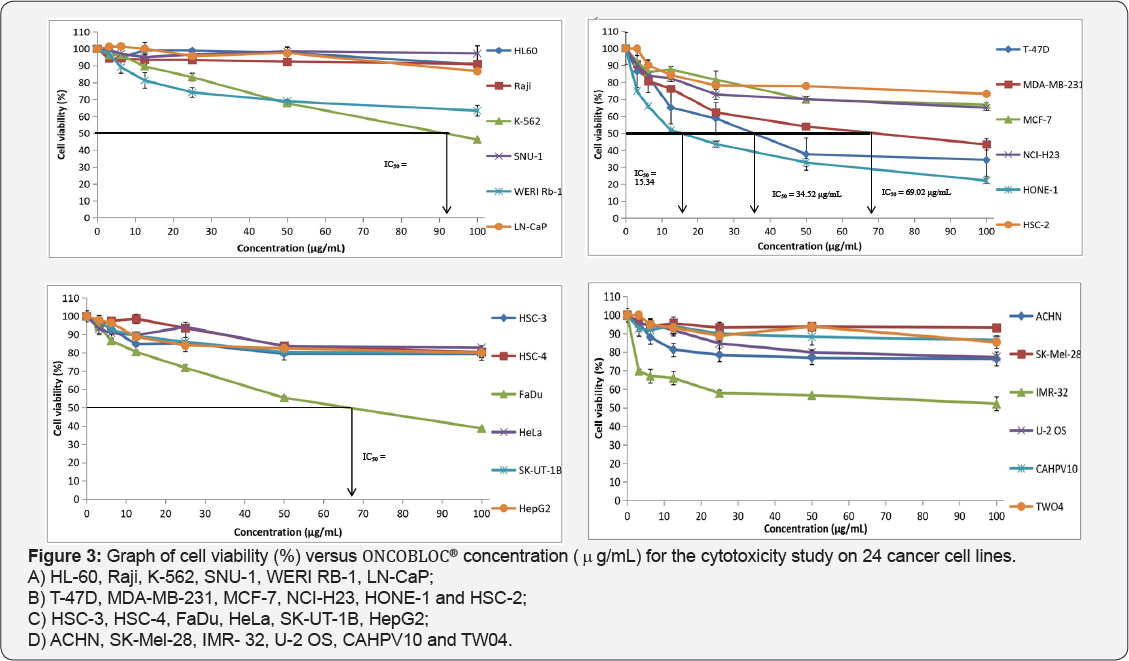
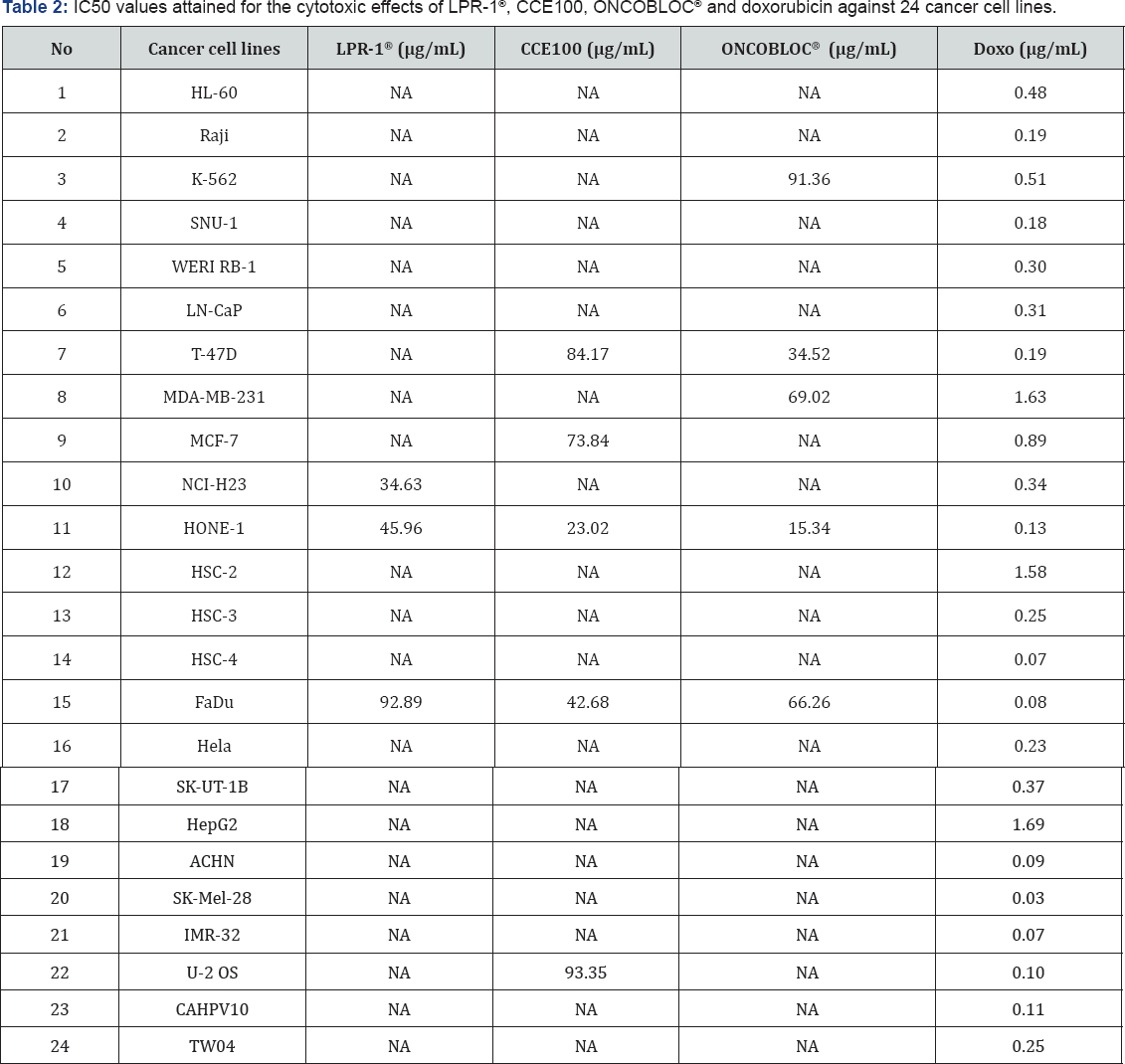
Table 1 displays the results obtained for the optimum cell seeding density of each cell line. The optimum cell seeding density of each cell line was ranging from 0.4 X 104 to 2 X 104 cells. The 1C50 values for the cytotoxic effects of LPR-1®, CCE100, ONCOBLOC® and doxorubicin evaluated are indicated in Figures 1-4, respectively and summarised in Table 2. The results obtained indicate that the three extracts of LPR-1®, CCE100 and ONCOBLOC® exhibited different cytotoxic effects towards different kinds of cancer cells, which is categorized as weak, moderate, and strong cytotoxic effects (Table 3). From the results obtained, ONCOBLOC® showed the strongest cytotoxic effects towards HONE-1 with the 1C50 value of 15.34μg/mL, followed by CCE100 with the 1C50 value of 2 3.02μg/mL. LPR-1® exhibited moderate cytotoxic effect towards HONE-1 and the 1C50 value obtained was at 45.96 μg/mL. Figure 5 shows the images of HONE-1 cells treated with LPR-1®, CCE100, ONCOBLOC® and doxorubicin. Meanwhile, both CCE100 and ONCOBLOC® showed moderate cytotoxic effects towards FaDu with the 1C50 values of 42.68 and 66.26 μg/mL, respectively. LPR-1® exhibited weak cytotoxic effect towards FaDu and the 1C50 value attained was at 92.89 μg/mL. Figure 6 shows the images of FaDu cells treated with LPR-1® CCE100, ONCOBLOC® and doxorubicin.
Note: NA = Nonachievable, the IC50 values cannot be obtained due to the cytotoxic effects are above 50% cell death
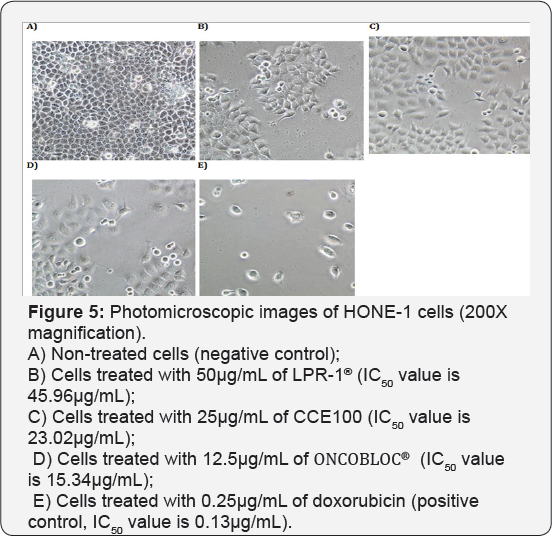

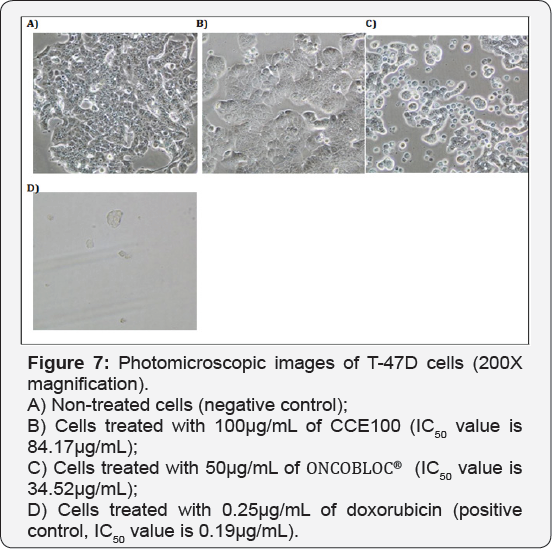
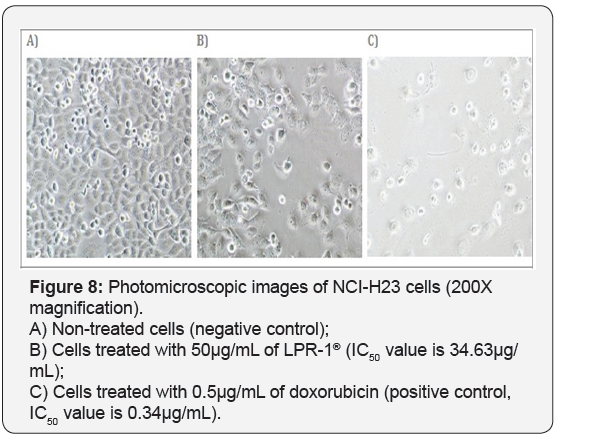
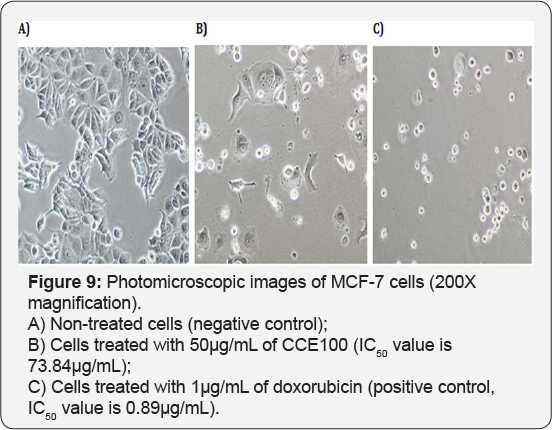
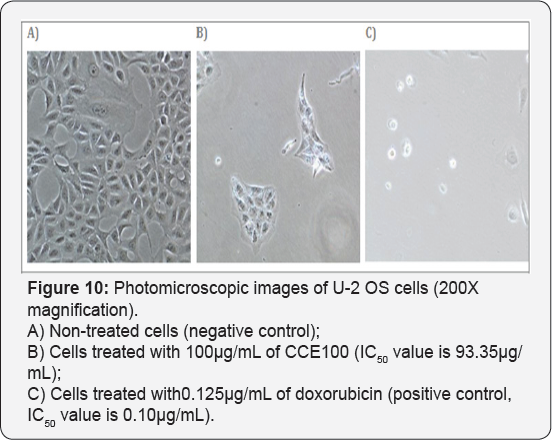
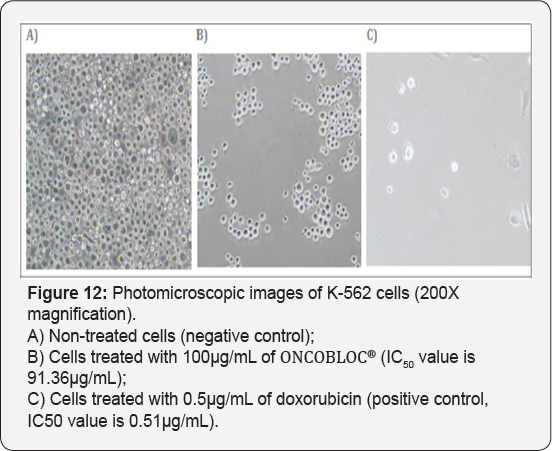
For T-47D cell line, CCE100 showed weak cytotoxic effects with the IC50 value of 84.17 μg/mL, whereas ONCOBLOC® exhibited moderate cytotoxic effect with the IC50 value of 34.52 μg/mL. Figure 7 shows the images of T-47D cells treated with CCE100, ONCOBLOC® and doxorubicin. LPR-1® exhibited moderate cytotoxic effect towards NCI-H23 and the IC50 value obtained was at 34.63 μg/mL. Figure 8 shows the images of NCI-H23 cells treated with LPR-1® and doxorubicin. CCE100 exhibited weak cytotoxic effects towards MCF-7 and U-2 OS cells with the IC50 values of 73.84 and 93.35 μg/mL respectively Figures 9,10 show the images of MCF-7 and U-2 OS cells treated with CCE100 and doxorubicin, respectively. ONCOBLOC® showed moderate cytotoxic effect towards MDA-MB-231 with the IC50 value of 69.02 μg/mL. Figure 11 shows the images of MDA-MB-231 cells treated with ONCOBLOC® and doxorubicin. Meanwhile, ONCOBLOC® showed weak cytotoxic effect towards K-562 with the IC50 value of 91.36 μg/mL. Figure 12 shows the images of K-562 cells treated with ONCOBLOC® and doxorubicin.
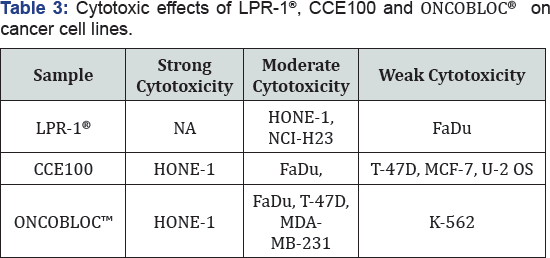
Note: Strong cytotoxicity = IC50 value < 30 μg/mL; Moderate cytotoxicity = 30 μg/mL < IC50 value ≤ 70 μg/mL; Weak cytotoxicity = IC50 value > 70 μg/mL; NA = Nonachievable
The findings obtained from this study demonstrate that ONCOBLOC® has the greatest cytotoxic effects towards cancer cells compared to LPR-1® and CCE100. Photomicroscopic images of cell with IC50 values only (Figures 5-12) are displayed in this report. There was no cytotoxic effect observed for LPR-1®, CCE100 and ONCOBLOC® against HL-60, Raji, SNU-1, WERI RB-1, LN-CaP, HSC-2, HSC-3, HSC-4, HeLa, SK-UT-1B, HepG2, ACHN, SK- Mel-28, IMR-32, CAHPV10 and TWO4 cancer cells. The cytotoxic effects of these three extract may contributed by the major active compounds present in C. militaris, such as cordycepin and water- soluble polysacharides. Cordycepin has been reported to induce apoptosis in neuroblastoma SK-N-BE (2)-C and melanoma SK- MEL-2 cells [30]. This preliminary study reported for the first time that LPR-1®, CCE100 and ONCOBLOC® exhibit various level of cytotoxicity towards different cancer cell lines.

Conclusion
In conclusion, these extracts exhibit cytotoxic effects on nasopharyngeal carcinoma epithelial cells (HONE-1), hypopharyngeal squamous carcinoma cells (FaDu), breast cancer cells (T-47D, MCF-7 & MDA-MB-231), non-small cell lung carcinoma cells (NCI-H23), osteosarcoma (U-2 OS) and chronic myeloid leukaemia cells (K-562). ONCOBLOC® showed the most cytotoxicity effects compared to LPR-1® and CCE100. The preliminary findings of this study suggest that ONCOBLOC® could be potentially developed as a novel therapeutic candidate for cancer treatment. Nevertheless, more efficacy studies should be conducted to confirm its anticancer activities.
Acknowledgement
The authors would like to express their deepest gratitude to Link (THM) Sciences Pte Ltd (Singapore) for sponsoring this research (Vote no. 4472/001).
References
- Chen YC, Chen YH, Pan BS, Chang MM, Huang BM (2017) Functional study of Cordyceps sinesis and cordycepin in male reproduction: A review. Journal in Food and Drug Analysis 25(1): 197-205.
- Hekimi S, Lapointe J, Wen Y (2011) Taking a "good” look at free radicals in the aging process. Trends in Cell Biology 21(10): 569-576.
- Wang M, Meng XY, Yang RL, Qin T, Wang XY, et al. (2012) Cordyceps militaris polysaccharides can enhance the immunity and antioxidation activity in immunosuppressed mice. Carbohydrate Polymers 89(2): 461-466.
- Ahn YJ, Park SJ, Lee SG, Shin SC, Choi DH (2000) Cordycepin: selective growth inhibitor derived from liquid culture of Cordycceps militaris against Clostridium spp. J Agric Food Chem 48(7): 2744-2748.
- Mao XB, Zhong JJ (2006) Significant effect of NH4+ on cordycepin production by submerged cultivation of medicinal mushroom Cordyceps militaris. Enzyme Microb Technol 38(3-4): 343-350.
- Kim JR, Yeon SH, Kim HS, Ahn YJ (2002) Larvicidal activity against Plutella xylostella of cordycpin from the fruiting body of Cordycceps militaris. Pest Manage Sci 58(7): 713-717.
- Hung YP, Lee CL (2017) Higher Anti-Liver Fibrosis Effect of Cordyceps militaris-Fermented Product Cultured with Deep Ocean Water via Inhibiting Proinflammatory Factors and Fibrosis-Related Factors Expressions. Marine Drugs 15(6): 168.
- Choi SB, Park CH, Choi MK, Jun DW, Park S (2004) Improvement of insulin resistance and insulin secretion by water extracts of Cordyceps militaris, Phellinus linteus, and Paecilomyces tenuipes in 90% pancreatectomized rats. Biosci Biotechnol Biochem 68(11): 22572264.
- Mueller WEG, Weiler BE, Charubala R, P fleiderer W, Leserman L, et al. (1991) Cordycepin analogues of 2'5'-oligoadenylate inhibit human immunodeficiency virus infection via inhibition of reverse transcriptase. Biochemistry 30(8): 2027-2033.
- Sugar AM, Mc Caffrey RP (1998) Antifungal activity of3'-deoxyadenosine (cordycepin). Antimicrob Agent. Chemother 42(6): 1424-1427.
- Jung K, Kim IH, Han D (2004) Effect of medicinal plant extracts on forced swimming capacity in mice. J Ethmopharmacol 93: 75-81.
- Yoshikawa N, Kunitomo M, Kagota S, Shinozuka K, Nakamura K (2009) Inhibitory effect of cordycepin on hematogenic metastasis of B16-F1 mouse melanoma cells accelerated by adenosine- 5'-diphosphate. Anticancer Res 29(10): 3857-3860.
- 13. Gu YX, Want ZS, Li SX, Yuan QS (2007) Effects of multiple factors on accumulation of nucleosides and bases in Corydyceps militaris. Food Chem 102(4): 1304-1309.
- Wang B, Lee CP, Chen Z, Yu HM, Duh P (2012) Comparison of the hepatoprotective activity between cultured Cordyceps militaris and natural Cordyceps sinensis. Journal of Functional Foods 4(2): 489-495.
- Wu ZL, Wang XX, Cheng WY (2000) Inhibitory effect of Cordycceps sinensis and Cordycceps militaris on human glomerular mesangial cell proliferation induced by native LDL. Cell Biochem Funct 18(2): 93-97.
- Wang Y, Yin H, Lv X, Wang Y, Gao H (2010) Protection of chronic renal failure by a polysaccharide from Cordyceps sinensis. Fitoterapia 81(5): 397-402.
- Yu RM, Yang W, Song LY, Yan CY, Zhang Z, et al. (2007) Structural characterization and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordycceps militaris. Charbohydrate Polym 70: 430-436.
- Nag TB, Wang HX (2005) Pharmacological actions of Cordyceps, a prized folk medicine. J Pharm Pharmacol 57(12): 1509-1519.
- Yoo HS, Shin JW, Cho JH, Son CG, Lee YW, Park SY, Cho CK (2004) Effects of Cordyceps militaris extract on angiogenesis and tumor growth. Acta Pharmacol Sin 25(5): 657-665.
- Won SY, Park EH (2005) Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. J Ethnopharmacol 96(3): 555-561.
- Kim H, Naura AS, Errami Y, Ju J, Boulares AH (2011) Cordycepin blocks lung injury-associated inflammation and promotes BRCA1-deficient breast cancer cell killing by effectively inhibiting PARP. Mol Med 17(9- 10): 893-897.
- Chena XL, Wub GH, Huang ZL (2013) Structural analysis and antioxidant activities of polysaccharides from cultured Cordyceps militaris. International Journal of Biological Macromolecules 58: 18- 22.
- Park C, Hong SH, Lee JY, Kim GY, Choi BT, et al. (2005) Growth inhibition of U937 leukemia cells by aqueous extract of Cordyceps militaris through induction of apoptosis. Oncol Rep 13: 1211-1216.
- Kodama EN, McCaffrey RP, Yusa K, Mitsuya H (2000) Antileukemic activity and mechanism of action of cordycepin against terminal deoxynucleotidyl transferase-positive (TdT+) leukemic cells. Biochem Pharm 59(3): 273-281.
- Lin YW, Chiang BH (2008) Anti-tumor activity of the fermentation broth of Cordyceps militaris cultured in the medium of Radix astragali. Proc Biochim 43: 244-250.
- Liu J, Yang S, Yang X, Chen Z, and Li J (1997) Anticarcinogenic effect and hormonal effect of Cordycceps militaris. Zhongguo Zhong Yao Za Zhi 22(2): 111-113.
- Shih IL, Tsai KL, Hsieh C (2007) Effects of culture conditions on the mycelial growth and bioactive metabolite production in submerged culture of Cordyceps militaris. Biochem Eng J 33: 193-201.
- Noh EM, Youn HJ, Jung SH, Han JH, Jeong YJ, et al. (2010) Cordycepin inhibits TPA-induced matrix metalloproteinase-9 expression by suppressing the MAPK/AP-1 pathway in MCF-7 human breast cancer cells. Int J Mol Med 25(2): 255-260.
- Hardeep ST, Anil KS, Sardul SS, Dharambir K (2013) Cordycepin: A bioactive metabolite with therapeutic potential. Life Sciences 93(23): 863-869.
- Baik JS, Kwon HY, Kim KS, Jeong YK, Cho YS, Lee YC (2012) Cordycepin induces apoptosis in human neuroblastoma SK-N-BE(2)-C and melanoma SK-MEL-2 cells. Indian J Biochem Biophys 49(2): 86-91.
- Jung SM, Park SS, Kim WJ, Moon SK (2012) Ras/ERK1 pathway regulation of p27KIP1-mediated G1- phase cell-cycle arrest in cordycepin-induced inhibition of the proliferation of vascular smooth muscle cells. Eur J Pharmacol 681(1-3): 1S-22.






























