Different Treatment Schemes with Canova Medication® Modify Differently the Course of Mice Infection by Trypanosoma cruzi
Gabriel M1, Ciupa L2*, Aleixo DL3, Amado CAB4, Dalálio MMO5, Ferreira EC6, Moreira NM7, Pupulim ART8, Kaneshima EN9, de Araújo SM10
1Departamento de Ciências Básicas da Saúde, Universidade Estadual de Maringá, Maringá -Paraná, Brazil
2Programa de Pós Graduaçào em Biociencias e Fisiopatologia, Universidade Estadual de Maringá, Brazil
3Centro de Ciências Biológicas e da Saúde-Curso de Medicina, Centro Universitário UNICESUMAR, Maringá-Paraná, Brazil
4Departamento de Farmacologia e Terapeutica, Universidade Estadual de Maringá, Maringá-Paraná, Brazil
5Departamento de Ciências Básicas da Saúde, Universidade Estadual de Maringá, Maringá-Paraná, Brazil
6Departamento de Ci234;ncias Básicas da Saúde, Universidade Estadual de Maringá, Maringá-Paraná, Brazil
7Departamento de Enfermagem, Universidade Estadual do Oeste do Paraná, Foz do Iguafu, Paraná, Brazil
8Departamento de Ciências Básicas da Saúde, Universidade Estadual de Maringá, Maringá-Paraná, Brazil
9Departamento de Medicina Universidade Estadual de Maringá, Maringá-Paraná, Brazil
10Departamento de Ciências Básicas da Saúde, Universidade Estadual de Maringá, Maringá-Paraná, Brazil
Submission: October 30, 2017; Published: December 01, 2017
*Corresponding author: Larissa C, Programa de Pós Graduaçào em Biociencias e Fisiopatologia, Universidade Estadual de Maringá, Brazil, Tel: +55 (44] 99-877-2949; Email: ciupalarissa@gmail.com
How to cite this article: Gabriel M, Ciupa L, Aleixo D, Amado C, Dalálio M, et al. Different Treatment Schemes with Canova Medication® Modify Differently the Course of Mice Infection by Trypanosoma cruzi. J Complement Med Alt Healthcare. 2017; 4(2): 555632. 009 DOI: 10.19080/JCMAH.2017.04.555632
Abstract
Objective: Purpose of this study was to evaluate the action of the Canova Medication (CM) in experimental infection of mice with Colombian strain belonging to genetic group T. cruzi I in different treatment regimens.
Methods: In a blind, controlled, and randomized trial, using 60 Swiss mice males, 28 days old. Swiss mice were inoculated with 104tripomastigotes/Colombian strain and treated with CM following different schemes: DI-20CM (treated with CM from the infection for 20 days; CI-9CM: treated with CM from the confirmation of infection for 9 days; AI-24CM: treated for 24 days beginning the treatment three days before infection; CONTROL: infected and untreated; NI: healthy uninfected. We evaluated: parasitaemia curve; mortality rate; histopathology; cytokines levels.
Results: The TNFα showed more concentration in all schemes of treatments. IL10 in AI-24CM group were statistically lower compared to the CONTROL (2.91pg/mL, 26.38pg/mL; p=0.046) can be related to the higher mortality rate, thus explaining the presence of diffuse inflammation in the heart of the animals of this group. The group CI-9 CM showed the best performance diminishing parasitological parameters and longer survival.
Conclusion: Administration of CM after infection confirmation was beneficial, however, it is still not still the ideal. When administered before the infection it promoted early death of animals. CM modulates the production of cytokines in experimental infection of mice by Trypanosoma cruzi. The modulation is depentent on the treatment scheme. The results alert to the indiscriminated use of ultra diluted medication, considering the animal species versus doses and treatment schemes.
Keywords: Trypanosoma cruzi; Chagas disease; Canova medication; Citokyne; Mice infection; Highly diluted medication
Abbreviations: NI: Non-Infected and Non-Treated Animals; CD: Chagas Disease; BZ: Benznidazole (N-Benzyl-2-Nitro-1-Imidazole Acetamide); CM: Canova Medication®; PPP: Prepatent Period; PP: Patent Period; MPD: Maximum Peak Day; MPP: Maximum Parasitemia Peak; TP: Total Parasitemia; Control: Infected Animals, Treated For 20 Days with a 1% Hydro Alcoholic Solution from Parasite Inoculation; G-16 CM: Infected Animals, Treated with CM for 16 Days from the 5 th Day of Parasite Inoculation; AI-24 CM: Infected Animals, Treated with CM for 24 Days, Initiating the Treatment Three Days Before Parasite Inoculation; DI-20 CM: Infected Animals, Treated for 20 Days with CM from Parasite Inoculation; CI-9 CM: Infected Animals, Treated with CM for 9 Days After the 12th Day of Infection from the Infection Confirmation
Introduction
Chagas disease (CD), caused by Trypanosoma cruzi [1], is an anthroponosis vastly spread in most part of the American continent. It is considered neglected by the World Health Organization, being one of the most severe tropical diseases, in addition to being a great public health problem in Central and South America [2,3]. Currently the disease has been detected in other continents [4]. The host-parasite relationship in CD is complex, involving profound immune system reactions. It is characterized by intense and widespread inflammatory process, with severe immune suppression component, expressed, among others, by changes in the levels of several cytokines and their receptors [5]. Currently in Brazil, there is a single antiparasitic drug the benznidazole (N-benzyl-2-nitro-1-imidazole acetamide) (BZ), which is partially effective in the acute phase of the disease. However, it has low efficacy in the chronic phase and can cause significant side effects [6,7]. For this reason, searching for a more effective treatment that covers all the stages of the disease is the goal of many studies and investigations [8-11].
In recent years, there has been an increased interest from health professionals and the general population, in both Brazil and other countries, in the "Alternative or Complementary Practices” [12-14]. Several studies indicate that some natural products can be used to aid in the cure of diseases, as well as to maintain the resistance of the body against infections [15,16]. The modulation of the immunological system can be suggested as one of the way these agents exert their effects [15,17,18]. The new forms of immune modulatory therapy can be directed to specific cells or their products. Particularly, the use of immune modulatory medicinal products could be an alternative in CD treatment. The Canova Medication® (CM) is a complex composed of Aconitum napellus, Arsenicum album, Bryonia alba, Lachesis muta and Thuya occidentalis in dilution from 10-11 to 10-19 [19]. It is used as an immune modulator, and the literature describes it as having stimulating effect on the immune system by activating the macrophages [20-22]. The immune modulatory action of CM was observed in experimental infection, induced by Leishmania (L.) amazonensis [23], and also in the reduction of lesions caused by the Paracoccidioides braziliensis, controlling the progression of the infection [24].
The CM has been clinically utilized, with benefits, in association with other medicines in the treatment of diseases wherein the immune system is impaired [25-27]. Considering that the infection caused by the T. cruzi presents immunosuppressive characteristic [5], and just like others highly diluted medications [28,29] the CM presents effect on the course of the experimental infection by this protozoan [30], studies evaluating the effect of different treatment schemes using Canova are welcome. In recent studies, Ferraz et al. [29], and Aleixo et al. [28] showed that different administration schemes of ultra diluted solutions cause different effects on the course of murine infection by T. cruzi. Parasitemia curve, the mortality rate, the tissue distribution of T. cruzi and its consequences are all-important tools to study the relationship parasite/host in this infection, being also used to evaluate the action of the medications tested [8,9,30-34]. Taking into account all the previous information, the objective of this study was to investigate whether different treatment schemes interfere in the development of the experimental infection by T. cruzi. In order to achieve the objective proposed here, it was used parasitological, histopathological and immunological parameters of infected mice with BZ-resistant strain and treated with CM.
Methods
Parasite and inoculum
It was used the Colombian strain, belonging to T. cruzi I. The inoculum was determined according to Brener [35] for 10,000 blood trypomastigotes at 0.2mL/animal, intraperitoneal (i,p,) route. The parasites were obtained from mice previously inoculated, at the 14th day of the infection, with blood collected from the orbital plexus with heparinized Pasteur pipette.
Animals
Swiss male mice (23.7±1.2g) with 28 days of living, coming from the central animal house of UEM. The animals were acclimated for three days in the animal house of the sector, maintained in a controlled temperature environment (22.7±2.01 °C), a light/dark cycle of 12h, water and food offered ad libitum. All cages contained wood shavings. All behavioral experiments were conducted in the light phase, and under daily surveillance. Sixty Swiss male mice were divided in to standard cages so that the averages of the initial weights of mice in each group were statistically equal, with ten animals per cage [36]. The cages were numbered 1-6, and were randomized into treatment groups by picking numbers out of a hat.
Six treatment groups of 10 Swiss male mice each were studied:
i. NI: non-infected and non-treated animals.
ii. Control: infected animals, treated for 20 days with a 1% hydro alcoholic solution from parasite inoculation.
iii. G-16 CM: infected animals, treated with CM for 16 days from the 5th day of parasite inoculation.
iv. AI-24 CM: infected animals, treated with CM for 24 days, initiating the treatment three days before parasite inoculation.
v. DI-20 CM: infected animals, treated for 20 days with CM from parasite inoculation.
vi. CI-9 CM: infected animals, treated with CM for 9 days after the 12 th day of infection from the infection confirmation.
Canova medication®
Canova Medication® (CM) [19] is a homeopathic complex, offered in the form of pills or solutions, for oral, intravenous or inhalant administration. It is produced in Brazil only by authorized laboratories or pharmacies. The CM is registered as a magistral formula, according to the Law 5991/73. Its preparation is according to Hahnemannian homeopathy method. It is a colorless and odorless solution, formulated from natural substances known in the Pharmacopoeia World. This experiment was done with the commercial medicament produced by Canova do Brazil® (Canova of Brazil®).
Treatment schemes
For the G-16 CM group, the medicine was administered in daily doses of 0.2mL per animal, orally, with a steel cannula with a ball at the end to relieve stress. The treatment started on the 5th day from the induction of the infection, considering that most researchers to evaluate medicines in experimental infection with T. cruzi [37-40] use this procedure. One person who did not take part in assessment of the parasitological parameters gave the treatment. The treatment was randomized. For the treatment of the AI-24 CM, DI-20 CM, CI-9 CM groups, the CM was diluted in water (10μL/mL water), offered ad libitum in an amber bottle, and hygienized once every three days. According to Aleixo et al. [28], in veterinary medicine this is the most frequent form of administering highly diluted drugs. The treatment schemes were as follows:
i. AI-24 CM group: The treatment started three days before the induction of the infection. Considering that the CM is a modulator of the immunological response [20-22] and will promote the change in the host response after contact with the parasite.
ii. DI-20 CM Group: The treatment started on the day of the inoculation of the parasites - based on the idea that the host response will be different considering that the organism has contact with the medicine and with the parasite at the same time [28].
iii. CI-9 CM Group: The treatment initiated on the 12th day after the induction of the infection and after the confirmation of infection. The premise was that the mice would be already with the immune system activated by the presence of the parasite, and according to Aleixo et al. [28], the host would be more benefited.
Parasitemia curve
The parasitemia curve was evaluated after the 4th day of infection and for 30 days. The blood was obtained from the mouse-tails and the number of parasites was determined by the method of Brener [41]. The parasitemia of the mice of all groups was obtained blind: those who assessed the parasitemia were not aware of the treatment the animals had received. Based on the parasitemia curve, it was obtained information related to pre-patent period (PPP), patent period (PP), maximum peak of parasitemia (MP), day of maximum parasitemia (DMP) and total parasitemia (TP). Results were expressed as mean± standard deviation for the studied groups.
Mortality rate
The mortality rate was evaluated along the course of the experiment, resulting in the following parameters: days of survival and percentage of mortality expressed by the number of animals died within a group. The results were expressed as percentage of cumulated deaths during the period evaluated. The animals that survived to the patent period of infection remained under observation for a period of 120 days after inoculation. The animals used in the survival study died as a direct result of infection.
Histopathology
For histopathological analysis, three animals from each experimental group were euthanized on the 21st day of infection. They were selected randomly by picking numbers out of a hat. The day of euthanasia was determined in previous studies, being the day of peak parasitemia since the tissue distribution and the cytokine levels are a direct reflection of levels of parasite in the blood [34-37,42].
The method of euthanasia was exsanguination by cardiac puncture after general anesthesia. All efforts were made to minimize suffering. Fragments of the heart, skeletal muscle, liver, spleen and large intestine were collected, fixed in 10% buffered formalin, and paraffin-embedded. For each animal, four sections of each organ were made, with 5μm each, and separated by of 20μm intervals. The sections were stained with hematoxylin-eosin (H&E). In the cuts, tissues were evaluated for the presence of parasites and inflammation, and this assessment is expressed in numbers of amastigotes nests per field, number of amastigotes per nest, number of inflammatory foci per field in the case of focal inflammation, and number of the fields with the presence of diffuse inflammation. Ten random fields from each cut were researched, with a 40X objective. The values shown for each organ in each group are the mean ± standard deviation of four slides for each animal, being three animals per group.
Cytokines dosing
The same animals used in histopathological studies was used for cytokine analysis, the serumwas collected. The assay was performed using the CBA KIT for the B&D flow cytometer. The cytokines 1L-2, 1L-4, 1L-6, 1L-10, 1L-17A, IFNγ, TNFα were dosed. The technique was developed in accordance to the instructions of the manufacturer. 1t was used the FACS Calibur flow cytometer (Becton-Dickinson, Rutherford, NJ, USA), equipped with Cell Quest software (Joseph Trotter, Scripps Research Institute, La Jolla, CA, USA). The result was expressed in mean ± standard deviation of the values achieved from each animal.
Statistical analysis
It was used the Kolmogorov-Sminorv Test to assess the normality of the data. For comparing parasitism tissue and parasitological parameters among the groups, the Kruskal-Wallis ANOVA, the Median, and the LSD tests were employed. The analysis considered 5% significance level for all comparisons.
Ethics
All procedures involving mice followed the Ethical Principles of Animal Experimentation established by the National Council for the Control of Animal Experimentation (CONCEA) [43], Brazilian College of Animal Experimentation (COBEA) and Brazilian Society of Laboratory Animal Science (SBCAL). Were performed according to protocols approved by the Committee for Ethical Conduct in Animal Experimentation (CEUA) of the State University of Maringa (report 032/2005 on 21/Jun/2005).
Results
Parasitological evaluation
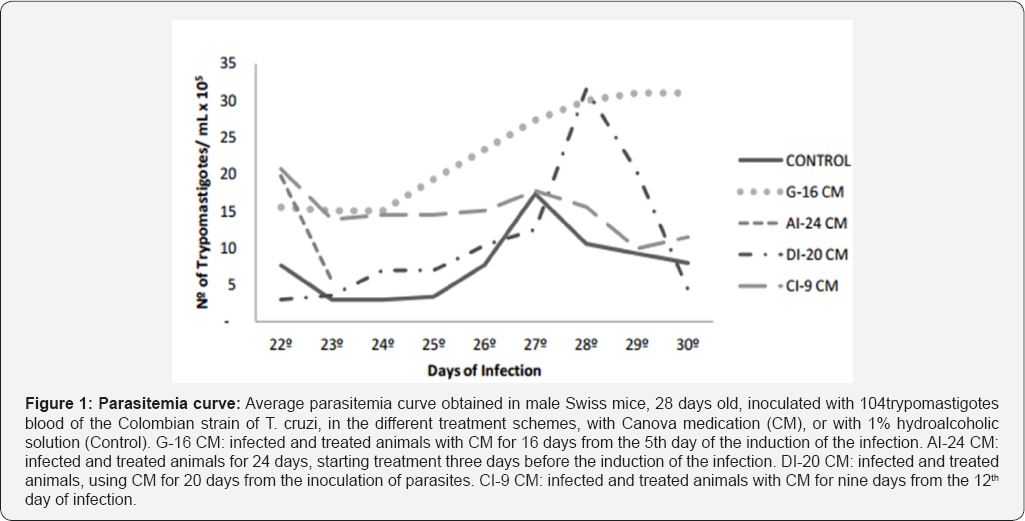
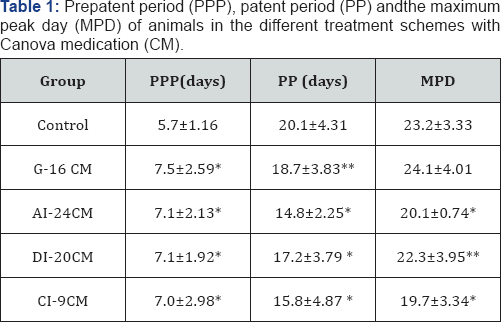
Figures (1&2), and Tables (1&2) present the parasitological data in detail. Parasitemia curve observed for the control group followed the graphic standards described in the literature for the Colombian strain [35-39]. Parasitemia curves were distinct for each treatment scheme. After the 22 nd day of infection, the differentiation between the curves became clearer (Figure 1); the effect of each treatment scheme can be easily individualized. In the G-16 CM group, the parasitemia increases until the death ofthe animals, and it is higher than in the Control group (p=0.0248). In the DI-20 CM group, the curve follows the same pattern observed for the Colombian strain, however with delayed parasitemia peak, and parasitemia levels higher than those observed in the Control group. In the AI-24 CM group, the parasitemia observed (Figure 1) is only from two days, considering that all the animals died by the 23rd day of infection; the parasitemias observed are higher (p=0.0014) than in the Control group. Parasitemia curve of the CI-9CM group is higher than in the Control group, but more stable and with downward trend.
Average parasitemia curve obtained in male Swiss mice, 28 days old, inoculated with 104 trypomastigotes blood of the Colombian strain of T. cruzi, in the different treatment schemes, with Canova medication (CM), or with 1% hydroalcoholic solution (Control). G-16CM: infected and treated animals with CM for 16 days from the 5th day of the induction of the infection. AI-24CM: infected and treated animals for 24 days, starting treatment three days before the induction of the infection. DI- 20CM: infected and treated animals, using CM for 20 days from the inoculation of parasites. CI-9CM: infected and treated animals with CM for nine days from the 12th day of infection. Prepatent period (PPP) was significantly higher (p =0.0000) for the treated groups compared to the Control group (Table 1). Likewise, all treated groups showed significantly lower patent period (PP) (G-16 CM = 18.7 days/p=0.0232; AI-24 CM = 14 days/p =0.0000; CM-20 DI=17 days/p =0.0000 and CI-9CM=15 days/p =0.0000) compared to that observed for the Control group (20 days) (Table 1). Excepting the G-16CM group, all the other groups significantly advanced the maximum peak day (MPD) of parasitemia, as it can be seen in Table 1.
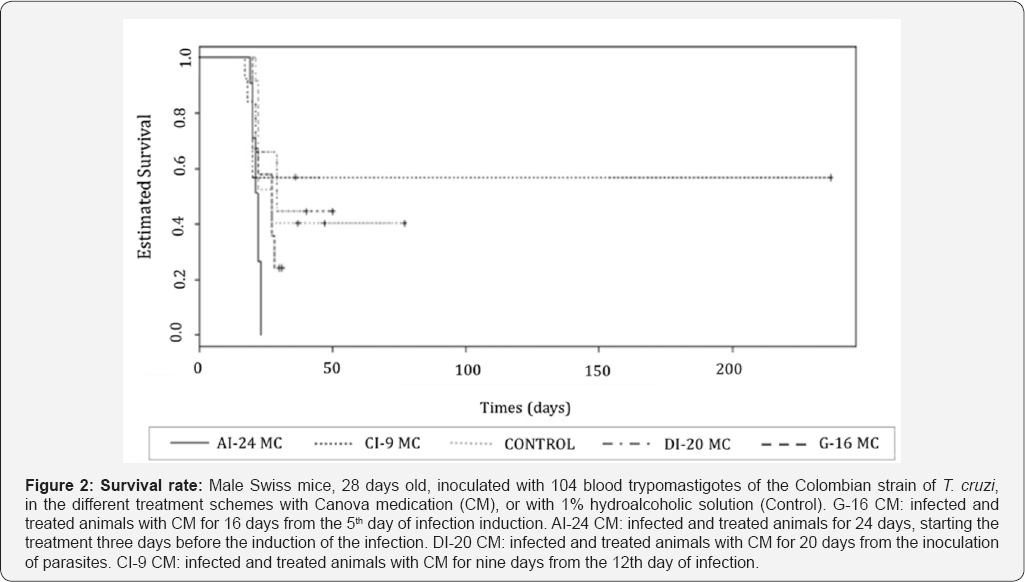
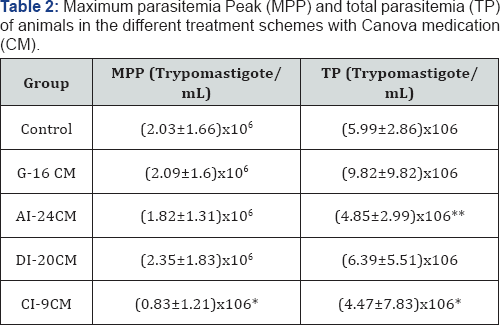
Statistical significance (p<0.000* and p<0.05**) compared to control group. Control: infected animals treated with 1% hydroalcoholic solution. G-16CM: infected animals treated using CM for 16 days from the 5th day of the induction of infection. AI- 24CM: infected animals and treated for 24 days starting treatment three days before the induction of the infection. DI-20CM: infected animals and treated, using CM for 20 days from the inoculation of parasites. CI-9CM: infected animals and treated using CM for nine days from the 12th day of infection. Maximum parasitemia peak (MPP) and the total parasitemia (TP) were significantly lower in the CI-9CM group (8.33x105 trypomastigotes/mL and 4.47 x106 trypomastigotes/mL, respectively) than in the Control group (2.03x106 trypomastigotes/mL and 5.99x106 trypomastigotes/ mL) (p<0.0000 and p<0.0000) (Table 2). Statistical significance (p<0.000* and p<0.05**) compared to control group. Control: infected animals treated with 1% hydroalcoholic solution. G-16 CM: infected animals treated using CM for 16 days from the 5th day of the induction of infection. AI-24CM: infected animals and treated for 24 days starting treatment three days before the induction of the infection. DI-20 CM: infected animals and treated, using CM for 20 days from the inoculation of parasites. CI-9 CM: infected animals and treated using CM for nine days from the 12th day of infection. Survival time was significantly different between groups (p=0.0001) (Figure 2). The CI-9CM group presented longer survival time, with an animal surviving indefinitely.
Male Swiss mice, 28 days old, inoculated with 104 blood trypomastigotes of the Colombian strain of T. cruzi, in the different treatment schemes with Canova medication (CM), or with 1% hydroalcoholic solution (Control). G-16 CM: infected and treated animals with CM for 16 days from the 5th day of infection induction. AI-24 CM: infected and treated animals for 24 days, starting the treatment three days before the induction of the infection. DI-20 CM: infected and treated animals with CM for 20 days from the inoculation of parasites. CI-9 CM: infected and treated animals with CM for nine days from the 12th day of infection. Mortality rate of animals from the Control group, G-16CM, AI-24CM and DI-20CM was 100%. In the group CI-9CM, mortality rate was 90%. All the animals in the AI-24CM group died faster (between 20th and 24th dpi) than the ones in the Control group (between 21st and 78th dpi).
Histopathological evaluation
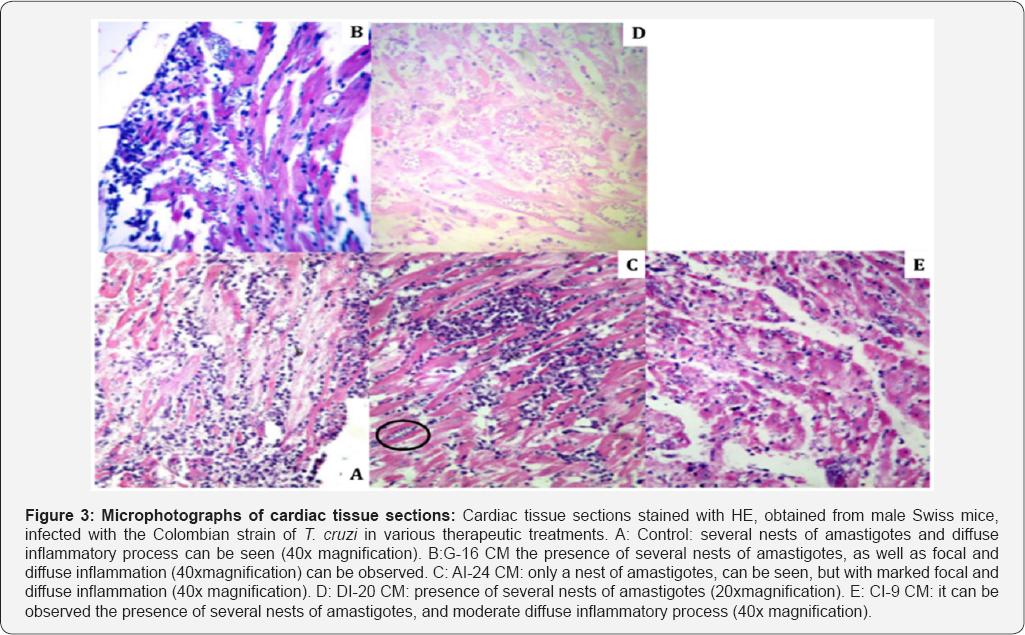
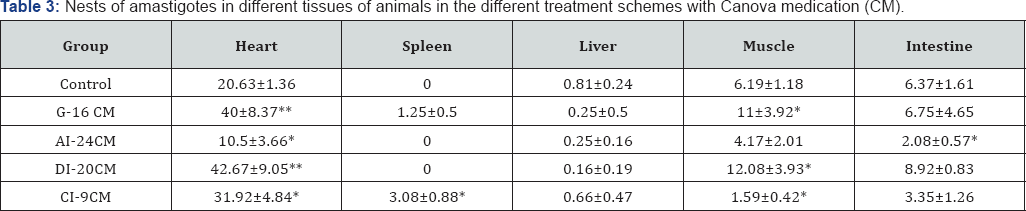
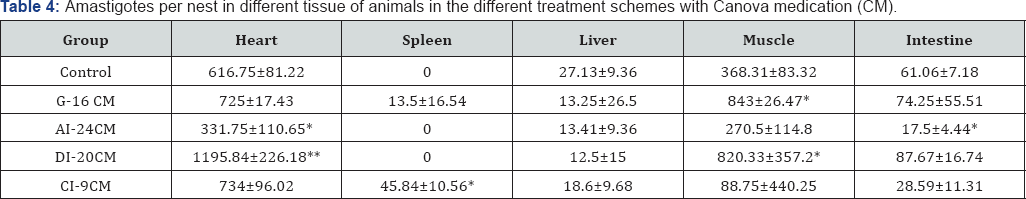
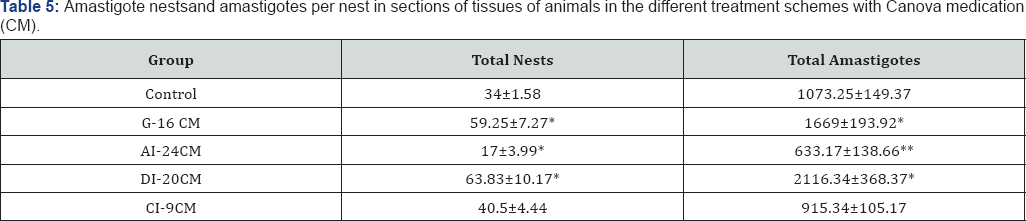
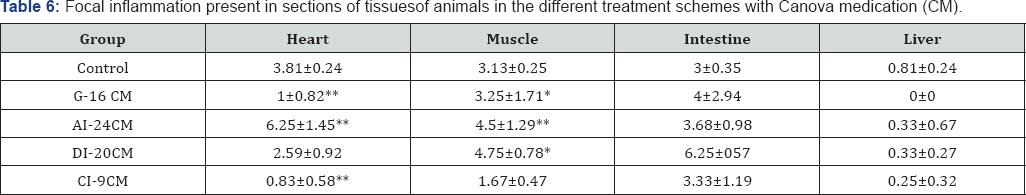
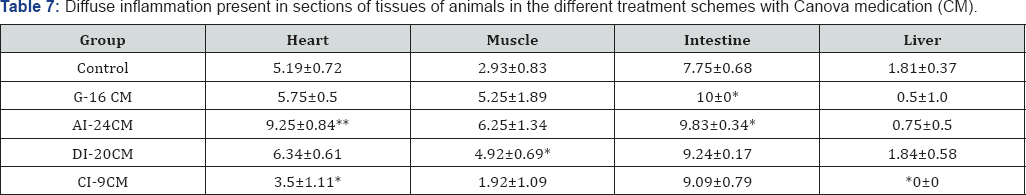
Table (3-7) & Figure 3 show details of the histopathological evaluation for the different experimental groups. In Table 3 & Figure 3, it can be observed that in the heart, excepting the A1-24CM group (p = 0.0349), all the treated groups showed significantly larger number of "amastigotes nests” compared to the Control group (G1-16CM-p=0.0005; D1 20CM-p=0.0001; C1-9CM-p=0.0206). Average number of nests of amastigotes in different tissue. Statistical significance (p <0.05* and p <0.005**) compared to control group. Control: infected animals treated with 1% hydroalcoholic solution. G-16CM: infected animals treated using CM for 16 days from the 5th day of the induction of infection. A1-24CM: infected animals and treated for 24 days starting treatment three days before the induction of the infection. D1-20 CM: infected animals and treated, using CM for 20 days from the inoculation of parasites. C1-9CM: infected animals and treated using CM for nine days from the 12th day of infection.
Cardiac tissue sections stained with HE, obtained from male Swiss mice, infected with the Colombian strain of T cruzi in various therapeutic treatments. A: Control: several nests of amastigotes and diffuse inflammatory process can be seen (40x magnification). B: G-16CM the presence of several nests of amastigotes, as well as focal and diffuse inflammation (40xmagnification) can be observed. C: A1-24CM: only a nest of amastigotes, can be seen, but with marked focal and diffuse inflammation (40x magnification). D: D1-20CM: presence of several nests of amastigotes (20xmagnification). E: C1-9CM: it can be observed the presence of several nests of amastigotes, and moderate diffuse inflammatory process (40x magnification).
Considering the parameter "number of amastigotes per nest” in the heart (Table 4), while the AI-24 CM group presented less than the Control group (p = 0.0153), the DI-20 CM group had the greatest "number of amastigotes per nest” compared to the Control group (p=0.0001). In the skeletal muscle, the "number of amastigotes nests” are significantly greater in the G-16 CM and DI-20 CM groups (p=0.0235 and p=0.0075, respectively), and lower in the C1-9 CM group (p=0.0291) compared to the Control group. The G-16 CM and D1-20 CM groups had higher (p=0.0059 and p=0.0081) "number of amastigotes per nest” than the Control group. In the intestine, the AI-24 CM group showed lower parasitism tissue than the Control group (p=0.0192) for both the "number of nests” and the "number of amastigotes per nest” (p=0.0356).
In the spleen, the C1-9 CM group showed significantly more "nests of amastigotes” and "amastigotes per nest” than the Control group (p=0.0412 and p=0.0497). In the liver, there was no statistical difference between the different groups. Average number of amastigotes per nest in different tissue.Statistical significance (p <0.05* and p <0.005**) compared to control group. Control: infected animals treated with 1% hydroalcoholic solution. G-16 CM: infected animals treated using CM for 16 days from the 5th day of the induction of infection. A1-24CM: infected animals and treated for 24 days starting treatment three days before the induction of the infection. D1-20 CM: infected animals and treated, using CM for 20 days from the inoculation of parasites. C1-9 CM: infected animals and treated using CM for nine days from the 12th day of infection.
Table 6 & 7 shows, in detail, the inflammatory process in the heart, the skeletal muscle, the intestine and the liver in the different treatment schemes studied. 1n the heart, it shows the presence of focal inflammation in G-16 CM (p=0.0005) and CI-9 CM (p=0.0003) groups, and diffuse inflammation in CI-9 CM (p=0.0099) group, being lower than in the Control group. 1n the intestine, it shows that the presence of diffuse inflammation was statistically lower in the C1-9 CM group (p=0.0453) than in the Control group.
Average per group of the total number of amastigote nests and amastigotes per nest. Statistical significance (p <0.001* and p <0.05**) compared to control group. Control: infected animals treated with 1% hydroalcoholic solution. G-16 CM: infected animals treated using CM for 16 days from the 5th the induction of infection. AI-24CM: and treated for 24 days starting treatment three days before the induction of the infection. DI-20 CM: infected animals and treated, using CM for 20 days from the inoculation of parasites. CI-9 CM: infected animals and treated using CM for nine days from the 12th day of infection. Average of total number of diffuse inflammation present in sections of tissue. Statistical significance (p <0.05* and p <0.005**) compared to control group. Control: infected animals treated with 1% hydroalcoholic solution. G-16 CM: infected animals treated using CM for 16 days from the 5th day of the induction of infection. AI-24CM: infected animals and treated for 24 days starting treatment three days before the induction of the infection. DI-20 CM: infected animals and treated, using CM for 20 days from the inoculation of parasites. CI-9 CM: infected animals and treated using CM for nine days from the 12th day of infection.
Cytokines dosage
Cytokines values for IL-2, 1L-4, IL-6, 1L-10, 1L-17A, IFNγ, and TNFα evaluated for different treatment regimens are shown in Table 8. The IL-2 was only detected in the NI group. It was observed lower production of IL-17A in the CI-9 CM group (0.02 pg/mL, p=0.0463) compared to the Control group (0.85 pg/mL). In the same way, the serum levels of IL-17A were significantly higher in the Control group (0.85 pg/mL) compared to the other groups treated with CM water diluted (DI-20 CM-0,003 pg/mL, p=0.049, AI-24 CM - 0.02 pg/ml, p=0.049).
The IL-10 levels were significantly higher in the Control group compared to the AI-24-CM group (p=0.046). The TNFα and IFNγ serum levels were significantly higher in the treated groups (p=0.0495) compared to the NI group, however without significant difference compared to the Control group.
Mean and standard cytokines levels (pg/mL) in the serum of mice. Statistical significance: p <0.05* compared to NI and p <0.005** compared to control group. Control: infected animals treated with 1% hydroalcoholic solution. G-16CM: infected animals treated using CM for 16 days from the 5th day of the induction of infection. AI-24CM: and treated for 24 days starting treatment three days before the induction of the infection. DI- 20CM: infected animals and treated, using CM for 20 days from the inoculation of parasites. CI-9CM: infected animals and treated using CM for nine days from the 12th day of infection.
Discussion
In the present study, the different treatment schemes exerted different effects in the course of the experimental mice infection, expressed by changes in the parasitological, histopathological and immunological parameters evaluated, compared to the Control animals infected with the Colombian strain of T cruzi that showed the classic pattern for this strain infection [35-39]. While for some parameters, it was not possible to identify differentiation between the treatment schemes, for others it was clear that the different schemes of the CM utilization could be beneficial, or harmful, to the animals infected and treated.
The treatment scheme of the CI-9CM group, with the animals being treated from the 12 th day of inoculation and confirmation of the infection showed better results considering the parasitological parameters. In this group, the PPP was significantly higher; both the parasitemia peak and the total parasitemia were lower than that observed in the Control group. All these alterations indicate a more balanced host-parasite relationship [28,29]. In the same way, the CI-9CM group resulted in longer survival, with an animal surviving indefinitely, reinforcing the parasitological data that showed a better performance for this group. This change in the infection course, in this studied model, reflects a trend of lower mortality rate that, indisputably, is an observed benefit for such treatment scheme.
This result is supported by the findings of Aleixo et al. [28], showing that the administration of medication highly diluted in water and four days after the detection of the infection, in mice inoculated with the strain Y of T. cruzi, promoted better clinical and parasitological evolution, with lower parasitemia and mortality, tending to higher animal survival. Aleixo et al. [28] also demonstrated that the administration of highly diluted medication offered by gavage produced harmful changes in the course of the infection in mice infected by the Y strain of T. cruzi. Pupulin et al. [30] also showed that the administration of highly diluted medicationoffered by gavage, altered the evolution course of the experimental infection by the strain Y of T. cruzi, a strain partially sensitive to conventional allopathic treatment.
The authors say that administered for 20 consecutive days, daily from the 5th day of the infection, the CM was harmful, causing 100% of death in the infected animals, in a short period. This result was also observed in this study, for the GI-16CM group treated by gavage. It was interesting to notice that, along with the results described above, the animals in the GI-16CM group, as well as all the others that received the CM showed increase in PPP, and anticipation in the parasitemia peak, as observed in the CI-9CM group, which showed the best performance [44]. This result leads to the discussion that the CM really alters host- parasite relationship in experimental infection. This conclusion may suggest that in clinical approach using CM, the practitioner must be careful in relation to the best treatment scheme for the patient.
Pupulin et al. [30] using the CM treatment by gavage and the murine infection with the Y strain of T cruzi, observed an increase in parasitemia, and a clear change in the patterns of tissue tropism for the treated group. In this work, different administration schemes of the CM interfered in the infection evolution, with differences in parasitological and histopathological parameters, in relation to the Control group. The treatment initiated at different periods of the infection, resulting in different effects, and suggesting alterations of the host-parasite relationship. The CM is considered an immunomodulator, and the infection by T. cruzi is described as intense and widespread inflammatory process, with strong immunosuppression component, expressed by changes in the levels of several cytokines and their receptors [5]. The tissue distribution of T. cruzi and its consequences are important tools to study the relationship parasite/host in this infection [32,34]. It is a direct reflex of the levels of parasites in the blood [35,45] being, additionally, one of the parameters frequently used to evaluate the effects of drugs on experimental infection induced by the T. cruzi [31-34].
The best performance was observed with the CI-9CM group where animals were only treated after the detection of the infection. For the AI-24CM group, the treatment started 3 days before the infection. The animals in this group showed a more intense reaction when in contact with the parasite, probably by heart failure, because as observed in the AI-24CM group, the presence of inflammation in the heart was significantly higher compared to the Control group, a fact also observed in the liver. In the present article, the wide variation of IL-17A calls attention. In the Control group, it is observed greater concentration compared to that in the treated groups. In the CI-9CM group, which presented the best performance, its concentration is equal to the group that presented early mortality and worse performance (AI-24CM), being seven times higher than in the DI-20CM group that presented ascending parasitemia. Shortage of IL-17 response results in higher mice susceptibility to T cruzi infection [46]. Deficiency on this cytokine response leads to a severe inflammatory response, increasing the IFNy and TNFα production, with liver damage and death [47]. According to Guedes et al. [48], the IL-17 controls the resistance to the T cruzi infection modulates the differentiation of Th1 cells, having a strong correlation between low levels of IL-17 and high levels of IFNγ and TNFα, with severe myocarditis in humans. According to Miyazaki et al. [46], the dosage of IL-17A in splenocytes of infected animals with T. cruzi showed maximum peak on the 21st day of infection. In the present study, in the group of animals with the worst performance (AI-24CM) the dosage of IL-17A in the serum was low on the 21st day of infection.
Accentuated heart inflammation was also observed with premature death of the animals, as reported by Guedes et al. [49]. For the best performance group (CI-9CM), it was also observed a low production of IL-17A, but with the presence of statistically lower inflammation in the heart compared to the Control group, and with an animal surviving indefinitely. This difference between the groups AI-24CM and CI-9CM can be explained by the modulation of the immune system by the CM with the same direction to results for IL-17A, INFy and TNFα but with IL-10 in the opposite direction, with significantly reduced concentration in AI-24CM compared to Control group. Comparison between CI- 9CM and AI-24CM was not significant. The intra-group variation was very high so the standard deviation was so big. Others experiments with more animals in groups will be necessary to confirm these data. In the murine infection with the Colombian strain of T. cruzi, the control of the parasitemia, even for a period and with some survival can be considered extremely relevant data [36,37] suggesting deeper studies related to the treatment schemes with Canova.
Conclusion
The CM modulates the production of cytokines in experimental infection of mice by T cruzi, the modulation is dependent on the treatment scheme. It is a fact that the treatment scheme used determined the behavior of each group. The concurrent administration of drugs with the infection promoted increase of the second peak of parasites (D1-20CM). The administration of CM before the infection (A1-24CM) promoted early death of animals; when administered after confirmation of the infection (C1-9CM) it was beneficial, however, it is still not the ideal. Although the CM is used successfully in human diseases involving infection and immune suppression, our results alert to the indiscriminate use of ultra diluted medication and to the need for more studies, considering the animal species versus doses and treatment schemes.
References
- Chagas C (1909) Nova Tripanosomiase Humana. Mem Inst Oswaldo Cruz 1(2): 159-218.
- (2007) WHO (World Health Organization). WHO expands fight against Chagas disease with support from Bayer.
- Hotez PJ, Dumonteil E, Colburn WL, Serpa JA, Bezek S, et al. (2012) Chagas disease: The New H1V/A1DS of the Americas. PLoS Negl Trop Dise 6(5): e1498.
- Chmunis GA, Yadon ZE (2010) Chagas disease: A Latin American health problem becoming a world health problem. Acta Trop 115(1-2): 14-21.
- Araujo JTC (2000) Resposta do hospedeiro a infecfäo: Resposta inume in at a, inflamatoria e de fase aguda na doença de Chagas. In: Araujo JTC, Castro SL (Eds.), Doenfa de Chagas-Manual para experimentado animal. Rio de Janeiro, Editora Fiocruz, Brazil, pp. 39-47.
- Brener Z (2000) Terapéutica Experimental na Doenfa de Chagas. In Trypanosoma cruzi e Doença de Chagas. Rio de Janeiro, Guanabara Koogan, Brazil 2: 379-388.
- Cançado JR (2002) Long term evaluation of etiological treatment of Chagas disease with benznidazole. Rev Inst Med Trop Sao Paulo 44(1): 29-37.
- Toledo MJO, Bahia MT, Veloso VM, Carneiro CM, Machado CGLL, et al. (2004) Effects of specific treatment on parasitological and histopatological parameters in mice infected with different Trypanosoma cruzi clonal Genotypes. J Antim Chemot 53(6): 10451053.
- Pupulim ART, Neto 1R, Gabriel M, Kaneshima EM, Santana RG, et al. (2004) Avaliafäo dos efeitos do Medicamento Canova sobre a infecçäo experimental de camundongos pelo Trypanosoma cruzi. In: Proceedings XX Reuniao Anual de Pesquisa Aplicada em doenfa de Chagas e V111 de Leishmanioses, pp. 22-24.
- Sánchez SF, Campillo NE, Páez JA (2010) Chagas disease: progress and new perspectives. Curr Med Chem 17(5): 423-452.
- Silva FD, Esperandim VR, Toldo MP, Kuehn CC, Prado JC, et al. (2013) In vivo activity of ursolic and oleanolic acids during the acute phase of Trypanosoma cruzi infection. Exp Parasitol 134(4): 455-459.
- Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, et al. (1993) Unconventional medicine in the United States: prevalence, costs and patterns use. N Eng J Med 328(4): 246-252.
- Teixeira MZ, Lin CA, Martins MA (2005) Homeopathy and acupuncture teaching at Faculdade de Medicina da Universidade de Sao Paulo: the undergraduates attitudes. Sao Paulo Med J 123(2): 77-82.
- Correa AD, Leite SQM (2008) Ensino da homeopatia na gradualo em farmácia: concepföes e práticas pedagógicas em institutes do estado do Rio de Janeiro. Interface Comunicado, Saúde, Educafäo 12(25): 267-280.
- Bin HRH, Suhel P, Suwarna P, Iqbal S, Raisuddin S, et al. (2003) Immunomodulatory effects of fenugreek (Trigonella foenum gaecum L) extract in mice. Int Immunopharmacol 3(2): 257-265.
- Haque R, Bin HB, Parvez S, Pandey S, Sayeed I, et al. (2003) Aqueous extract of walnut (Juglans regia L) protects mice against cyclophosphamide-induced biochemical toxicity. Hum Exp Toxicol 22(9): 473-480.
- Yan A (1998) Hot Tea or Hot Air? Immunomodulatory Effects of Panax ginseng in the Prevention of Cancer. Nutrition Bytes 4(1): 1-5.
- Mungantiwar AA, Nair AM, Shinde UA, Dikshit VJ, Saraf MN, et al. (1999) Studies on the immunomodulatory effects of Boerhaavia diffusa alkaloidal fraction. J EthnoPharmacol 65(2): 125-131.
- The Canova Medication®.
- Piemont MR, Buchi DF (2002) Analysis of IL-2, IFN-γ and TNF-α production, α5β1 integrins and actin filaments distribution in peritoneal mouse macrophages treated with homeopathic medicament. J Submic Cytol Pathol 34(3): 255-263.
- Lopes L, Godoy LM, De Oliveira CC, Gabardo J, Schadeck RJ, et al. (2006) Phagocytosis, endosomal/lysosomal system and other cellular aspects of macrophage activation by Canoca medication. Micron 37(3): 277287.
- Moreira COC, Costa JFFB, Leal MF, Andrade EF, Rezende AP, et al.(2012) Lymphocyte proliferation stimulated by activated Cebus apella macrophages treated with a complex homeopathic immune response modifiers. Homeopathy 101(1): 74-79.
- Pereira WK, Lonardoni MV, Grespan R, Caparroz Assef SM, Cuman RK, et al. (2005) Immunomodulatory effect of Canova medication on experimental Leishmania amazonensis infection. J Infect 51(2): 157164.
- Takahashi G, Maluf MLF, Svidzinski TIE, Dalalio MMO, Bersani CA, et al. (2006) In vivo and in vitro effects of the Canova medicine on experimental infection with Paracoccidioides braziliensis in mice. Indian J Pharmacol 38(5): 350-354.
- Sasaki MGM (2001) Estudo Clínico randomizado placebo controlado para avaliar a eficácia e seguranza do medicamento Canova na terapeutica de pacientes portadores de HIV/AIDS em uso de antiretrovirais.
- Castanheira P, Brito JR, Fischer I, Feliú D Qualidade de vida e tratamento de cancer ou AIDS com imunomodulador Canova®.
- Di Bernardi RP (2005) Recuperado de pacientes com HIV/AIDS em Botswana, África, com o uso do medicamento homeopático Canova.
- Aleixo DL, Ferraz FN, Ferreira EC, Lana M, Gomes ML, et al. (2012) Highly diluted medication reduces parasitemia and improves the experimental infection evolution by Trypanosoma cruzi. BMC Res Notes 5: 352.
- Ferraz FN, Simoni GK, Nascimento A, Melo CS, Aleixo DL, et al. (2011) Different forms of administration of biotherapy 7dH in mice experimentally infected by Trypanosoma cruzi produce different effects. Homeopathy 100(4): 237-243.
- Pupulim ART, Marques AS, Toledo MJO, Gomes ML, Kaneshima EN, et al. (2010) Canova medication modifies parasitological parameters in mice infected with Trypanosoma cruzi. Exp Parasitol 126(4): 435-440.
- Brener Z (1961) Atividade terapeutica do 5-nitro-2-furaldeido, semicarbazona nitrofurazona em esquema de duraçäo prolongada na infecçäo experimental do camundongo pelo Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo 3: 43-49.
- Andrade ZA, Lopes E (1963) A histochimical study of experimental Chagas' disease. Rev Inst Med Trop Sāo Paulo 5(5): 236-242.
- Toledo MJO, Guilherme ALF, Silva JC, Gasperi MV, Mendes AP, et al. (1997) Trypanossoma cruzi: chemotherapy with benznidazole in mice inoculated with strains from Paraná State and from different endemic areas of Brazil. Rev Inst Med Trop Sao Paulo 39(5): 283-290.
- Lima ES, Zelton AA, Andrade SG (2001) TNF-α is expressed at sites of parasite and tissue destruction in the spleen of mice acutely infected with Trypanosoma cruzi. Int J Exp Pathol 82(6): 327-336.
- Brener Z, Chiari E (1963) Variaçoes morfológicas observadas em diferentes amostras de Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo 5: 220-224.
- Federici EE, Abelman WB, Neva FA (1964) Chronic and progressive myocarditis and myositis in C3H mice infected with Trypanosoma cruzi. American J Trop Med Hyg 13: 272-280.
- Andrade SG, Carvalho ML, Figueira RM (1970) Caracterizaçao morfobiológica e histopatológica de diferentes cepas do Trypanosoma cruzi. Gazeta Médica da Bahia 70: 32-42.
- Filardi LS, Brener Z (1987) Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans R Soc of Trop Med Hyg 81(5): 755-759.
- Monteiro WM, Margioto TAP, Gruendling AP, Reis D, Gomes ML, et al. (2013) Trypanosoma cruzi I and IV stocks from Brazilian Amazon are divergent in terms of biological and medical properties in mice. PLoS Negl Trop Dis 7(2): e2069.
- Teston APM, Monteiro WM, Reis D, Bossolani GDP, Gomes ML, et al.(2013) In vivo susceptibility to benznidazole of Trypanosoma cruzi strains from the western Brazilian Amazon. Tropical Medicine and International Health 18(1): 85-95.
- Brener Z (1962) Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Revista do Instituto de Medicina Tropical Sao Paulo 4: 389-396.
- Andrade SG, Silva AA, Carvalho ML, Figueira RM (1972) Comportamento de uma cepa do Trypanosoma cruzi em hospedeiros com baixa resistencia. Revista do Instituto de Medicina Tropical Sao Paulo 14: 154-161.
- The Ethical Principles of Animal Experimentation established by the National Council for the Control of Animal Experimentation.
- Camandaroba ELP, Thé TS, Pessina DH, Andrade SG (2006) Trypanosoma cruzi: clones isolated from the Colombian strain, reproduce the parental strain characteristics, with ubiquitous histotropism. Int J Exp Path 87(3): 209-217.
- Postan M, Dvorak JA, Mcdaniel JP (1983) Studies of Trypanosoma cruzi clones in inbred mice I. A comparison of the course of infestation of C3H/HEN- mice with two clones isolated form a common source. Am J Trop Med Hyg 32(3): 497-506.
- Miyazaki Y, Hamano S, Wang S, Shimanoe Y, Iwakura Y, et al. (2010) cIL- 17 Is Necessary for Host Protection against Acute-Phase Trypanosoma cruzi Infection. J Immunol 185: 1150-1157.
- Tosello BJ, Amezcua VMC, Bermejo DA, Ramello MC, Montes CL, et al. (2012) IL-17RA Signaling Reduces Inflammation and Mortality during Trypanosoma cruzi Infection by Recruiting Suppressive IL-10- Producing Neutrophils. PLoS Pathog 8(4): e1002658.
- Guedes PMM, Gutierrez FRS, Maia FL, Milanezi CM, Silva GK, et al. (2010) IL-17 Produced during Trypanosoma cruzi Infection Plays a Central Role in Regulating Parasite-Induced Myocarditis. PLoS Negl Trop Dis 4(2): e604.
- Guedes PMM, Gutierrez FRS, Silva GK, Dellalibera JR, Rodrigues GJ et al. (2012) Deficient Regulatory T Cell Activity and Low Frequency of IL-17-Producing T Cells Correlate with the Extent of Cardiomyopathy in Human Chagas' Disease. PLoS Negl Trop Dis 6(4): e1630.






























