Antimicrobial, Antiparasitic and Antioxidant Activities of Medicinal Plants from Sudan
Mohamed Gamaleldin Elsadig Karar1, Ahmed Rezk1, Omnia Tag Alkhatim Abdalla2, Amna Ahmed Yousif Ebrahim2, Matthias S Ullrich1 and Nikoli Kuhnert1*
1Department of Life Sciences and Chemistry, Jacobs University Bremen, Germany
2Sudan Atomic Energy Commission, Sudan
Submission: March 21, 2017; Published: June 20, 2017
*Corresponding author: Nikoli Kuhnert, Department of Life Sciences and Chemistry, Jacobs University Bremen, Campus Ring 8, 28759 Bremen, Germany, Tel: 49-421-200-3120; Fax: 49-421-200 3229, Email: n.kuhnert@jacobs-university.de
How to cite this article: Mohamed G E K, Ahmed R, Omnia T A A, Amna A Y E. Antimicrobial, Antiparasitic and Antioxidant Activities of Medicinal Plants from Sudan. J Complement Med Alt Healthcare. 2017; 2(5): 555597. DOI: 10.19080/JCMAH.2017.02.555597
Abstract
The rural population of Sudan has traditionally used medicinal plants for treatment of several ailments and microbial infections. In the present study, we have investigated the antibacterial, antitrypanosomal, antiplasmodial, and antioxidant properties of selected Sudanese medicinal plants using various in vitro assays. Methanolic extracts of various parts of the plants were tested against six bacterial strains (Bacillus subtilis, Bacillus aquimaris, Clavibacter michiganensis, Escherichia coli, Erwinia amylovora, and Pseudomonas syringae) using agar diffusion and minimal inhibitory concentration (MIC) methods. The anti plasmodial activity was tested against a chloroquine sensitive strain of Plasmodium falciparum NF54, whereas the antitrypanosomal activity was evaluated against Trypanosoma brucei rhodesiense STI900 (African strain). The antioxidant activity of the plant extracts was assessed by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging. Various extract showed antibacterial, antiparasitic and antioxidant activities. Acacia nilotica, Ocimum basilicam, Ziziphus spina-christi, Balanites aegyptiaca, Sonchus oleraceus, Punica granatum, Mimosa pigra and Ixora coccinea were the most interesting ones with antioxidative, antiplasmodial, antitrypanosomal and antibacterial activities. By means of preparative HPLC, HPLC-ESI-TOF, HPLC-ESI-MSn, 1H-NMR and 13C-NMR, thirteen phyto constituents were isolated and identified in the methanolic extracts of Z. spina-christi, S. oleraceus, and H. sabdariffa including chlorogenic acids, flavonoid glycosides, coumarins and derivatives. Sudanese plants represent an important alternative source of natural antioxidants, antiparasitic and antimicrobials. To our knowledge, this is the first report about the antibacterial, antiparasitic and antioxidant activities of some of these plants.
Keywords: Medicinal plants; Antiplasmodial; Antitrypanosomal; Antibacterial; Antioxidant; Preparative-HPLC
Introduction
Medicinal plants continue to play a vital role as therapeutic agents in primary health care in developing countries [1]. Sudan is located in tropical Africa and has high plant diversity and a multinational population. In Sudan and other developing countries, traditional medicine plays a major role particularly in rural regions due to both economic and cultural reasons [2]. Comprehensive ethnobotanical investigations on Sudanese folk medicine was reported previously [3-6]. (The ethnomedicinal data of selected plant materials are provided in (Table S1) in the Supporting information).
The frequent use of medicinal plants for treatment of different diseases has encouraged a number of researchers to study their biological activities [7,8]. Additionally, natural products can contribute to the discovery of novel antimicrobial [9], and antioxidant components [10]. A number of pharmaceutical studies have demonstrated the antibacterial, antimalarial, antitrypanosomal, and antioxidant activities of Sudanese medicinal plants [11,12,2,13-18]. It is well-known that plants, which are rich in a diversity of secondary metabolites such as polyphenols, tannins, terpenoids and alkaloids are usually interesting for their antiparasitic, antimicrobial, and antioxidant activities [19-24]. The antioxidant hypothesis is however under discussion, and recent evidence suggested that its role has heavily been overestimated [25]. Therefore, the aim of the present study was to evaluate the antibacterial, antiplasmodial, antitrypanosomal, and antioxidant activities of some Sudanese medicinal plants. The antimicrobial effect of some phenolic compounds extracted from edible and medicinal plants from Europe was also assessed. We have also isolated thirteen bioactive phenolic constituents from Z. spina-christi, S. oleraceus and H. sabdariffa by preparative-HPLC (PHPLC).
Materials and Methods
Chemicals and standards
All chemicals, solvents and authentic standards used in this study were analytical grade. 5-O-caffeoylquinic acid (>98%), 3-O-caffeoylquinic acid (>98%) and 4-O-caffeoylquinic acid (>96.45%) were purchased from Phytolab (Vestenbergsgreuth, Germany). Quercetin 3-O-(6-O-rhamnosyl-glucoside) (rutin) (≥94%), quercetin 3-O-glucoside (90%), esculin (≥98%), luteolin 7-O-glucoside (≥98%), gallic acid (≥99%), methanol (≥99.9%), methanol-d4 (99.96%) and DMSO (≥95%) were purchased from Sigma-Aldrich (Steinheim, Germany). Ultrapure water with a resistance of 18.2M was deionized in a Milli-Q system (Sartorius Stedim Biotech GmbH, Germany).
Plant materials and preparation
All Sudanese plant samples were freshly collected June and July 2010 from their natural habitats in Omdurman (15°38' North, 32° 26' East) and Khartoum (15°33' North, 32°31' East), Sudan. The voucher specimens were identified by Dr. Hayder Abdel Gadir of Herbarium of Medicinal and Aromatic Plants Research Institute (MAPRI), Khartoum, Sudan, where the specimens were also deposited. All plant species, part used and voucher specimen numbers are presented in (Table 1). The plants were selected based on previous studies [3-6,17,26-35]. (See also Table S1 in the Supporting information).
The flowers of Catharanthus roseus were purchased from Wassenaar Living Garden (Bremen, Germany). Quince fruits (Cydonia oblonga), green coffee (Coffea arabica) beans and black tea (Camellia sinensis) leaves were purchased from local markets in Bremen, Germany, while the cocoa (Theobroma cacao) beans and Bobgunnia madagascariensis pods were donated by Barry Callebaut, Wieze, Belgium and Prof. Dr. Philip Stevenson, Royal Botanic Gardens, Kew, Richmond, Surrey, UK, respectively
The sample preparation was achieved as described in our previous work [36] (Table 1).
Evaluation of antibacterial activities
Bacterial susceptibility determinations: The minimal inhibitory concentration (MIC) was defined as the lowest concentration of antimicrobial that will inhibit the visible growth of a micro-organism after overnight incubation. The MIC was determined by a twofold dilution assay in Mueller-Hinton broth (MHB) (Becton Dickinson, Heidelberg, Germany). Three Gram-positive bacterial strains (Bacillus subtilis S168, Bacillus aquimaris MB-2011, and Clavibacter michiganensis GSPB 390 and three Gram-negative bacterial strains (Escherichia coli DH5a, Erwinia amylovora 1189, and Pseudomonas syringae pv tomato DC300) were selected as model organisms to evaluate the antibacterial activity of the crude plant extracts and phenolic compounds. The plates were incubated overnight at 28 °C except for E. coli, for which incubation was done at 37 °C. All tests were done in triplicate following the National Center for Clinical Laboratory Standards recommendations (National Committee for Clinical Laboratory Standards (NCCLS), 2000).
Agar diffusion assays: Agar diffusion assays were performed as in our previous study [37].
Evaluation of antiplasmodial and antitrypanosomal activities
The antiplasmodial and antitrypanosomal activities were evaluated in vitro against a chloroquine sensitive strain of Plasmodium falciparum NF54 and Trypanosoma brucei rhodesiense STI900 (African strain), respectively. Both assays were carried out at two different concentrations (2 and 10 |ig/ mL). These experiments were conducted in collaboration with Swiss Tropical and Public Health Institute (Swiss TPH), Basel, Switzerland as previously described [38].
Evaluation of antioxidant activities
The scavenging activity of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals of the crude plant extracts was measured according to a method previously described [39]. Assays were carried out in 3mL reaction mixtures containing 2mL of 0.1mM DPPH-ethanol solution, 0.9ml of 50mM Tris-HCl buffer (pH 7.4) and 0.mL of plant extracts at two different concentrations (10 and 100|ig/mL). The reaction mixture was vortexed and left in the dark at room temperature (27 °C) for 30min. The absorbance was measured spectrophotometrically at 517nm. Gallic acid was used as a positive control, while ethanol was used as a blank sample. The inhibitory effect of DPPH was calculated using the standard equation [40]. The experiments were conducted in triplicates, and the data are given as mean values ± standard deviation (SD).
HPLC & HPLC-MSn
The HPLC separation and HPLC-MSn analysis was achieved as previously described [41,42].
Preparative-HPLC isolation
Preparative-HPLC isolation of compound 1-13 was carried out as in our previous study [37].
NMR
1H NMR and 13C NMR spectra were acquired on a JEOL ECX- 400 spectrometer operating at 400 MHz for *H NMR and 100 MHz for 13C NMR in CD3OD using a 5mm probe. The chemical shifts (5) are reported in parts per million (ppm) and were referenced to the residual solvent peak. The coupling constants (J) are quoted in hertz (Hz).
Esculin (5): 1H-NMR (400 MHz, CD3OD) δH 6.28 (d, J 9.62 Hz, H-3), 7.82 (d, J 9.16 Hz, H-4), 7.18 (s, H-5), 7.0 (s, H-8), 4.96 (d, J 7.33 Hz, H1'), 3.54 (dd, J 9.27 and 7.3 Hz, H2'), 3.50 (dd, J 9.2 and 7.3 Hz, H-3'), 3.40 (dd, J 9.62 and 8.7 Hz, H-4'), 3.51 (m, H-5'), 3.92 (dd, J 12.36 and 2.29 Hz, H6'a), 3.70 (dd, J 11.91 and 5.5 Hz, H6'b); 13C-NMR (100 MHz, CD3OD) δC 61.02 (C-6'), 69.93 (C- 4'), 73.36 (C-2'), 76.03 (C-3'), 77.16 (C-5'), 101.55 (C-1'), 104.18 (C-8), 112.49 (C-3), 113.25 (C-10), 114.04 (C-5), 144.40 (C-6), 145.10 (C-4), 147.71 (C-7), 149.52 (C-9), 162.29 (C-2). These data were in agreement with those reported in the literature [43,44].
Luteolin 7-O-glucoside (6): 1H-NMR (400 MHz, CD3OD) δH 6.28 (1H, d, J 7.0 Hz, H-1''), 3.35 - 3.55 (4H, m, H-2",3'',4",5"), 3.91 (1H, dd, J 11.91 and 1.83 Hz, H-6"a), 3.70 (1H, dd, J 11.91 and 5.5 Hz, H-6"b), 6.59 (1H, s, H-3), 6.78 (1H, d, J 2.29 Hz, H-6), 6.48 (1H, d, J 2.29 Hz, H-8), 7.38 (1H, d, J 1.83 Hz, H-2'), 6.87 (1H, d, J 8.70 Hz, H-5'), 7.40 (1H, dd, J 8.24 and 2.29 Hz, H-6'); 13C-NMR (100 MHz, CD3OD) δC 61.11 (C-6"), 69.93 (C-4''), 73.39 (C-2”), 76.52 (C-3”), 77.05 (C-5'), 102.61 (C-1"), 165.55 (C-2), 102.61 (C-3), 182.69 (C-4), 161.55 (C-5), 99.79 (C-6), 163.66 (C-7), 94.70 (C-8), 157.50 (C-9), 105.73 (C-10), 122.20 (C-1'), 112.70 (C-2'), 146.02(C-3'), 145.21 (C-4'), 115.53 (C-5'), 119.14 (C-6') (45,46)
Results and Discussion
Phytochemical profiling and characterization of the isolated constituents
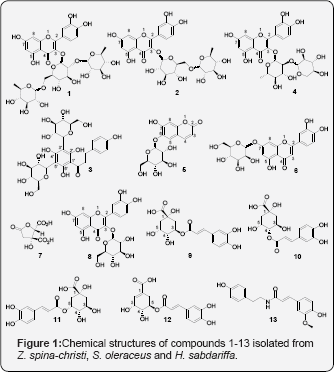
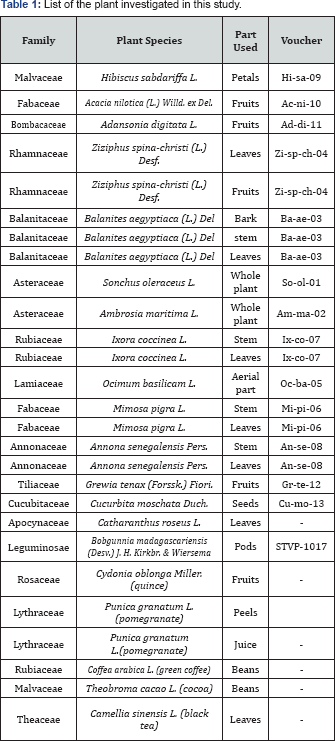
A detailed phytochemical analysis using HPLC-MS was carried out on Z. spina-christi, S. oleraceus and H. sabdariffa in previous work [47-50] From these plants in our present study, preparative HPLC was used to isolate four compounds (1-4) from the methanolic extracts of Z. spina-christi, two compounds (5 and 6) from S.oleraceus and seven compounds (7-13) from H. sabdariffa. The structures of the isolated compounds (Figure 1) were elucidated by HRMS, tandem MS, UV chromatograms, retention times (RT), authentic standards and data obtained from literature (Figure 2 & Table 2). Chromatographic resolution and MS data were considered for isolated compounds showing a poor NMR spectral resolution. Tandem MS data and preparative HPLC chromatograms are provided in the supporting information. With these agreements compounds (1-13) were identified as quercetin 3-O-(2,6-di-O-rhamnosyl-glucoside), quercetin 3-O-(6-O-rhamnosyl-glucoside) (rutin), phloretin 3',5' di-C-glucoside, quercetin 3-O-(2-O-rhamnosyl-pentoside), esculin, luteolin 7-O-glucoside, hibiscus acid, quercetin 3-O-glucoside, 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, 5-O-caffeoylshikimic acid and N-feruloyltyramine, respectively. The purity of these compounds (Table 1) was determined as in our previous study [37] by total ion chromatograms in negative ion mode and UV chromatograms at 280nm. (Table 2) Retention times, high resolution MS data and amounts of the isolated constituents.

Evaluation of antibacterial activities
The antibacterial activities of the crude plant extracts and some selected phenolic compounds isolated by preparative HPLC from the plant material analysed in this study were evaluated for their efficacies against Gram-positive and Gram-negative bacteria. As suitable model organisms B. subtilis S168, B. aquimaris MB- 2011, and C. michiganensis GSPB 390 were chosen for Gram- positive and E. coli DH5a, E. amylovora 1189, and P. syringae pv tomato DC300 for Gram-negative. The organisms chosen can be viewed as suitable model organisms for both pathogenic Grampositive and Gram-negative bacteria. Ampicillin and DMSO were used as positive and negative controls, respectively. The MIC values of the plant extracts obtained using the micro-dilution methods are presented in (Table 3). Interestingly, the plant extracts tested herein showed antibacterial activity only against Gram-positive bacteria, with MIC values varying from 195 to 1562|ig/mL, while Gram-negative strains were not affected at all. The higher sensitivity of the Gram-positive bacteria compared to Gram-negative bacteria could be attributed to their differences in cell envelope components. Gram-positive bacteria have an external peptidoglycan layer, which only is a permeable and thus ineffective barrier against toxic compounds Mallik [51]. Extracts of Acacia nilotica and Punica granatum (pomegranate) peals showed the highest activities against B. aquimaris MB 2011 with a MIC value of 195|ig/mL The antibacterial activities of these two plants against Streptococcus viridans, S. aureus, E. coli, B. subtilis, Shigella sonnei and Salmonella typhimurium were reported in previous studies [52,53] However, the reference antibiotic as a positive control showed variable inhibitory activity on the all strains of bacteria with MIC values ranging from 3.9 to 250|ig/ mL (Table 3). No inhibition zone was detected for the negative control (DMSO).
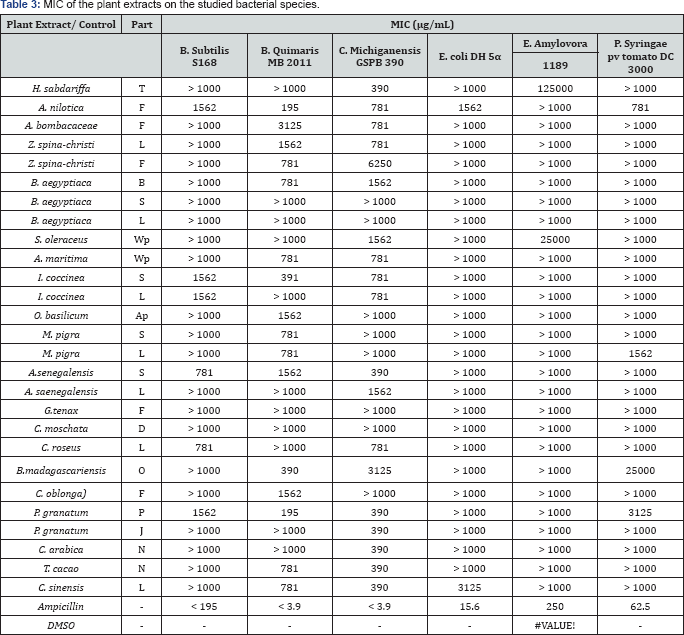
(-): Inactive; L: Leaves; S: Stem; B: Bark; Wp: Whole plant; F: Fruits; P: Peels; D: Seeds; T: Petals; O: Pods; Ap: Aerial part; J: Juice; N: Beans.
Using agar diffusion assay, we have also investigated the some of the crude extracts and the the positive control ampicillin against the Gram-negative antimicrobial activities of bacterium E. coli DH5a and Gram-positive bacterium B. subtilis S168. Surprisingly, some of the plant extracts showed growth inhibitory effects on both tested strains (Figure 3). Regarding the Gram-positive bacterium, B.aegyptiaca bark extract exhibited the highest activity (4.65±0.07mm). These findings were in agreement with previous reports [54,55]. While, for Gramnegative bacterium, the arial part extract of O. basilicum showed the strongest inhibitory effect (4.23±0.03mm). The positive control (ampicillin, 50mg/mL) showed zone of inhibition 18.5±0.7mm against B. subtilis and 16.0±0.6mm against E. coli. No inhibition zone was detected for the negative control (DMSO).
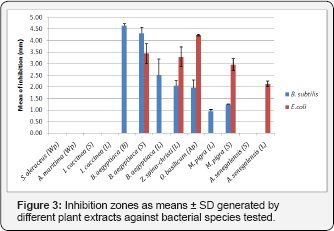
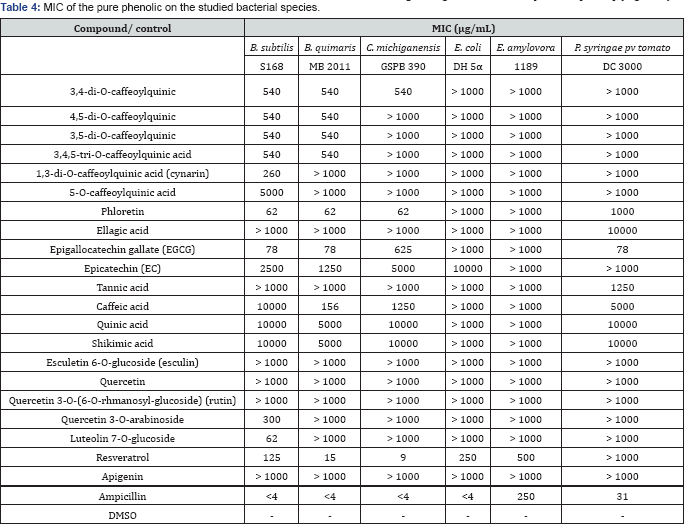
Phenolic compounds found in medicinal plants have been extensively studied against a wide range of microorganisms, and among them chlorogenic acids, flavanols and tannins received more interest due to their broad spectrum and the fact that most of them process antimicrobial properties [54-56]. Consequently, we have evaluated the in vitro antibacterial activities of some phenolic compounds against the selected bacterial strains (Table 4). Generally, the antibacterial activities the pure compounds were found to be comparatively higher than that of crude extracts. Additionally, Gram-positive bacteria were found to be more susceptible to the phenolic compounds than Gram-negative bacteria. Among the tested bioactive compounds, phloretin and resveratrol showed the strongest inhibitory activities against the all Gram-positive bacteria with MICs ranged from 9 to 125pg/mL (Table 4), followed by luteolin 7-O-glucoside and then epigallocatechin gallate (EGCG) (MICs 62 to 625pg/mL), whereas the MIC values of chlorogenic acids ranged from 260 to 540pg/mL, and therefore showed comparably low inhibitory activity. Nevertheless, the antibacterial activity of chlorogenic acids was already documented in previous studies [55,57]. The hydroxyl groups in the polyphenols are believed to be play an important role in the antimicrobial activity [58] because these groups can inactivate the microbial enzymes and interact with the cell membrane of bacteria to disrupt membrane structures and causing leakage of cellular components [59,60] (Figure 3).
(-): Inactive
Evaluation of antiplasmodial and antitrypanosomal activities
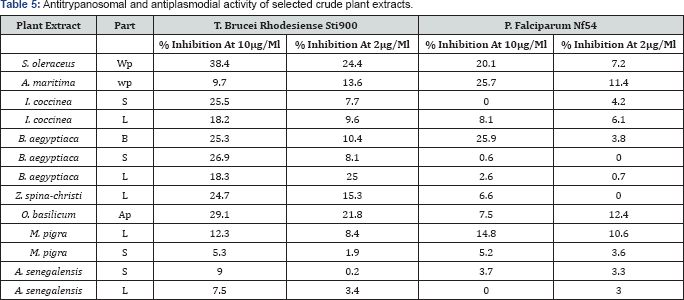
Table 5 shows the antiplasmodial and antitrypanosomal activities of selected Sudanese medicinal plants against a chloroquine sensitive strain P. falciparum NF54 and T brucei rhodesiense ST1900 (African strain), respectively. For most of these plants, no specific studies of antitrypanosomal and antitrypanosomal activities exist in the literature. Most of the plant extracts exhibited dose dependent antiparasitic activities. These plants (Table 4) are traditionally used for the treatment of many ailments, parasitic and microbial infections including malaria, virus infections, digestive disorders, weakness, hepatic diseases, obesity, diabetes, skin infections, fever, diarrhea, insomnia, heart problems, colds, toothaches, hypertension, bronchial asthma, spasms, frequent urination, urinary tract infections and elimination of kidney stones [5,17,26-35]. This could explain the good observed inhibitory activities of the most of these extracts against the tested parasites. The extracts S. oleraceus and B. aegyptiaca were found to be the most promising ones. The plant extracts showed however weaker antiparasitic activity than that reported for Chrysanthemum cinerariifolium flower extract (86% inhibition against P. falciparum and 99% inhibition against T. brucei rhodesiense at test concentrations of 4.8|ig/mL) [38]. Nevertheless, our findings were close to that reported by Karou et al. [61] which showed significant antimalarial activity for methanolic extract of B. aegyptiaca against P falciparum (1C50 24.56|ig/mL). I. coccinea, A. senegalensis and Z. spina-christi extracts showed very low or no activities against P falciparum NF54. On the other hand, T. brucei rhodesiense was sensitive towards the methanolic extracts of S. oleraceus (whole plant) and I. coccinea (stem) (38.4 and 25.5% inhibition activity at 10|ig/mL, respectively), when relatively compared to the reported antitrypanosomal drug suramin (1C50 0.03±0.02|ig/mL) [62] (Table 5).
Evaluation of antioxidant activities
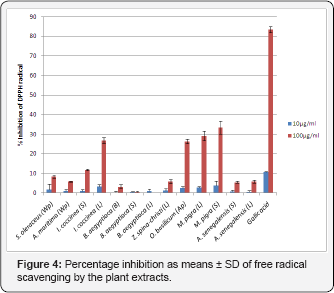
1t is believed that the antioxidant activity of plant extracts rich phenolic phytoconstituents is due to their ability to be donors of hydrogen atoms or electrons and to capture the free radicals. Scavenging activity for free radicals of 1.1-diphenyl-2- picrylhydrazyl (DPPH) has been commonly used to assess the antioxidant activity of medicinal plants and natural products. Plant extracts from Sudan were prepared for investigation of their antioxidant activities. They showed significant free radical scavenging activity at the high concentration (100|ig/mL) on DPPH (Figure 4). The extracts of M. pigra stem, M. pigra leaves, O. basilicam arial part and I. coccinea leaves were the most effective radical scavengers with the inhibition of 33.4 ± 3.3%, 29.1±2.4%, 26.3 ± 1.2% and 26.7 ± 1.4%, respectively, as compared to 83.5±1.5% for gallic acid standard. Nevertheless, these crude plant extracts showed better DPPH scavenging activity than that reported for Crataegus monogyna fruits extract (15±1% scavenging activity at a test concentration of 100|ig/mL) a flavonoid drug included in most European pharmacopeia [20]. At lower concentration (10 |ig/mL) the extracts were not effective as the positive standard (Figure 4), indicating that the activity was concentration dependent. Many studies have demonstrated that the antioxidant activity is significantly affected by the phenolic constituents of the sample [20,21]. Thus, the radical-scavenging activity of the plant extracts may be attributed to their phenolic and flavonoid contents. Furthermore, the antioxidant property of M. pigra was in agreement with those mentioned in the literature [61,63-70] (Figure 4). Percentage inhibition as means±SD of free radical scavenging by the plant extracts.
Conclusion
In conclusion, our findings strongly support the traditional use of the studied Sudanese plants in the treatment of bacterial and parasitic infections. The results revealed that some of these plants, such as A. nilotica, O. basilicam, Z. spina-christi, B. aegyptiaca, S. oleraceus, P. granatum, M. pigra and I. coccinea have the potential to be investigated further to identify the antioxidative, antiplasmodial, antitrypanosomal, and antibacterial metabolites in these plants. The study also reports on the antibacterial activities ofsome naturally occurring phenolic compounds. Among the tested phytochemicals, phloretin, resveratrol, luteolin 7-O-glucoside and epigallocatechin gallate showed the highest antimicrobial activities. By means of preparative HPLC, HPLC-ESI-TOF, HPLC-ESI-MS", 1H-NMR and 13C-NMR, thirteen phytoconstituents were isolated and identified in the methanolic extracts of Z. spina-christi, S. oleraceus and H. sabdariffa including chlorogenic acids, flavonoid glycosides, coumarins and derivatives. The results of this study highlight the importance of the Sudanese medicinal plants as potential source of plant derived antimicrobial and antiparasitic drugs. However, pharmacological and toxicological studies will be necessary to confirm this hypothesis.
Conflict of Interest
The authors disclose that there is no conflict of interest.
Acknowledgement
This work was supported by Jacobs University Bremen and Deutscher Akademischer Austausch Dienst (DAAD). The authors would like to thank Prof. Dr. Sami A. Khalid, Department of Pharmacognosy and Phytochemistry, Faculty of Pharmacy, University of Science
References
- Champ M, Langkilde AM, Brouns F, Kettlitz B, Yle CB (2003) Advances in dietary fibre characterisation. 1. Definition of dietary fibre,physiological relevance, health benefits and analytical aspects. Nutr Res Rev 16(1): 71-82.
- Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, et al. (2008) Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 31(Suppl 1): S61-S78.
- Marangoni F, Poli A (2008) The glycemic index of bread and biscuits is markedly reduced by the addition of a proprietary fiber mixture to the ingredients. Nutr Metab Cardiovasc Dis 18(9): 602-605.
- Wannamethee SG, Whincup PH, Thomas MC, Sattar N (2009) Associations between DFand inflammation, hepatic function, and risk of type 2 diabetes in older men: potential mechanisms for the benefits of fiber on diabetes risk. Diabetes Care 32(10): 1823-1825.
- Higgins JA (2004) Resistant starch: metabolic effects and potential health benefits. J AOAC Int 87(3): 761-768.
- Theuwissen E, Mensink RP (2008) Water-soluble dietary fibers and cardiovascular disease. Physiol Behav 94(2): 285-292.
- Tungland BC, Meyer D (2002) Nondigestible Oligo- and polysaccharides (dietary fiber): their physiology and role in human health and food. Comprehensive Rev in Food Science and Food Safety 1: 73-92.
- Meiselman HL, Macfie HJH (1996) Food Choice Acceptance and Consumption. Glasgow UK, Backie Academic and Professional p. 239.
- Lawless, HT, Heymann H (1998) Sensory Evaluation of Food: Principles and Practices. Chapman& Hall, Newyork, USA.
- Srilakshmi B (2007) Food Science (4) Sensory evaluation. New Age International (p) Lt., pp. 297-298.
- Gannam N (1986) The antidiabetic activity of aloes: preliminary clinical and experimental observations. Horm Res 24(4): 288-294.
- Laura Shane McWhorter, BCPS, FASCP, BC-ADM, CDE (2009) Dietary Supplements for Diabetes: An Evaluation of Commonly Used Products. Diabetes Spectrum 22(4): 206-213.
- Abubakar NS, Florence IO, Iyanu OO (2014) Phytochemical Screening and Hypoglycemic effect of Methanolic fruit extract of Cucumis sativus in alloxan induced diabetic rats. Journal of Medicinal Plant Research 8(39): 1173-1178.
- Galgani J, Aguirre C, Diaz E (2006) Acute effect of meal glycemic index and glycemic load on blood glucose and insulin responses in humans. Nutr J 5: 22.
- Ball SD, Keller KR, Moyer-Mileur LJ, Ding YW, Donaldson D, et al. (2003) Prolongation of satiety after low versus moderately high glycemic index meals in obese adolescents. Pediatrics 111(3): 488-494.
- Grover JK, Vats V, Rathi SS (2000) Antihyperglycemic effect of Eugenia jambolana and Tinospora cordifolia in experimental diabetes and their effects on key metabolic enzymes involved in carbohydrate metabolism. J Ethnopharmacol 73(3): 461-470.
- Ezekwe, Sunday A, Ify E, Uzoma OK (2014) Hypoglycemic, hypolipidemic and body weight effects of unripe pulp of Carica papaya using diabetic Albino rat model. Journal of Pharmacognosy and Phytochemistry 2(6): 109-114.
- Agrawal Supriya, Katare Charu (2015) Antioxidant activity, total phenolic compound and flavonoid content of vaccum dreid extract of Ll Siceraria. Global journal of multidisciplinary studies 4(6): 302-308.
- Prakongpan T, Nitihamyong A, Luangpituksa P (2002) Extraction and application of DFand cellulose from pineapple cores. J Food Sci 267(4): 1308-1313.
- Eshak ES, Iso H, Date C, Kikuchi S, Watanabe Y, et al. (2010) Dietary fiber intake is associated with reduced risk of mortality from cardiovascular disease among Japanese men and women. J Nutr 140(8): 1445-1453.
- Hopping BN, Erber E, Grandinetti A, Park SY, Kolonel LN, et al. (2010) Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multiethnic cohort in Hawaii. J Nutr 140(1): 68-74.
- Nazare JA, Sauvinet V, Normand S, Guerin DL, Gabert L, et al. (2011) Impact of a resistant dextrin with a prolonged oxidation pattern on day-long ghrelin profile. J Am Coll Nutr 30(1): 63-72.
- Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, et al. (2004) Glycemic index, glycemic load, and DFintake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr 80(2): 348-356.






























