An Integrated Approach to Treatment of Chronic Low Back Pain: Based on Neuroscience of Pain
Yu-Jen Chen, En-Dien Shang, Pei-Fang Tang and Shwu-Fen Wang*
Graduate Institute and School of Physical Therapy, National Taiwan University, Taiwan
Submission: February 14, 2017; Published: April 21, 2017
*Corresponding author: Shwu-Fen Wang, Graduate Institute and School of Physical Therapy, National Taiwan University, Taiwan, Email: sfwang@ntu.edu.tw
How to cite this article: Yu-Jen C, En-Dien S, Pei-Fang T, Shwu-Fen W. An Integrated Approach to Treatment of Chronic Low Back Pain: Based on Neuroscience of Pain. J Complement Med Alt Healthcare. 2017; 2(2): 555581. DOI: 10.19080/JCMAH.2017.02.555581
Abstract
Prevalence and recurrent rate of chronic low back pain is high and causes work incapacity,increasing the cost of both medical resources and the economic burden on society. The neuroscience of pain and chronic pain indicated by the ascending and descending pathways and the pain matrix in the brain is altered. Mesolimbic and prefrontal areas altered neuronal responses to pain. Furthermore, attention and emotional modulatory pathways have mechanisms on pain modulation related to different areas of the pain matrix. Neurophysiological changes in somatosensory and motor cortex changes lead to changes in the motor control of the impaired laterality recognition and body images. Additionally, physical pain and virtual pain share the same areas of activation in the pain matrix, but activate differently. Changes in ascending and descending pain modulatory systems, the PAG-RVM pathway, the dorsal horns of the spinal cord and peripheral receptors, influence central sensitization through different mechanisms.
Current peripheral mechanisms of pain describe low back pain as changes in muscle, joint, and movement patterns, related to a deficit in proprioception and motor control. Traditional approaches, including modality and bio-mechanical theory with treatment result in room for improvement. Fascia and its attached core muscle is the target of new treatment strategies for improving trunk stability for patients with chronic low back pain. Fascia expansion of various muscles from proximal to distal parts forms a myofascial chain that resembles the Chinese Meridian, serving functions in force transmission, protection, circulation and homeostasis. Fascia lesion stimulating nociceptors is considered the source of low back pain. Top-down mechanism approaches for pain management, including motor control, motor imagery, cognitive behavior therapy, and pain education demonstrate promising results. The need of an integrating model for dealing with chronic pain is urgent.
Keywords: Low back pain; Fascia; Pain management; Chinese meridian
Abbreviations: ACC: Anterior Cingulate Cortex; PFC: Prefrontal Cortex; RVM: Rostral Ventromedial Medulla; S2: Secondary Somatosensory Cortex; AMY: Amygdala; S1: Primary Sensory Cortex
Introduction
Prevalence and recurrent rate of chronic low back pain
The high recurrence rate and prevalence rate of chronic low back pain causes work incapacity and increases the cost of both medical resources and the economic burden on society. The incidence of low back pain is about 84%, with a high recurrence rate and prevalence rate Nielens [1]. Low back pain has been considered as a kind of disability Airaksinen et al. [2]. Treatments are mainly focused on decreasing pain through traction and exercise, but with only short-term effects. Chronic fascial low back pain is a common muscular-skeletal disease, and is categorized according to the source of pain: muscle, ligament, and soft tissue-related, neurological, joint-related, and others Cohen et al [3], (Morlion [4]. There are 65% of patients with muscle-related low back pain. Muscle-related low back pain is considered an early stage of the disease, and has potential of reversal. With the recurrence of pain, however, dysfunction, decrease in quality of life, increased consumption of social resources, and loss of productivity may be inevitable.
Neuroscience of pain and chronic pain: the ascending and descending pathways and the pain matrix in the brain
A recent review examined the area of neuroplasticity changes of the pain matrix associated with chronic musculoskeletal disorders Pelletier et al. [5]. The pain matrix includes the associated sensory cortex, the ACC, and the insula cortex. Changes in certain areas of the brain are associated with specific signs/symptoms of patients with chronic pain.
Changes in mesolimbic and prefrontal areas altered neuronal responses to pain
Changes in mesolimbic, prefrontal and pain matrix, including the insula, cingulate cortex, and nucleus accumbens lead to the altered response to pain, regard to the "unpleasantness” and spontaneous pain, change ineffective, cognitive, and/or motivational aspects of pain, may be presented as signs and symptoms (Bushnell, Ceko, & Low, 2013). Besides, changes in psychological aspects of pain, such as fear avoidance and catastrophization, are also affected Wertli et al. [6].
Attention and emotional modulatory pathways have mechanisms on pain modulation related to different area of pain matrix
Attentional pathways include the anterior cingulate cortex (ACC), prefrontal cortex (PFC), periaqueductal gray Lee et al. [7], and rostral ventromedial medulla (RVM) while emotional pathways include the Nucleus SpirifornisLateralis (SPL), secondary somatosensory cortex (S2), Insula, and Amygdala (AMY). When focusing on pain, the activity in primary sensory cortex (S1), insula, and ACC, is stronger than when distracting from the pain. As for the emotional pathway, negative emotional states produced by looking at emotional faces, listening to unpleasant music, or smelling unpleasant odors Villemure & Bushnell [8], altered pain-evoked cortical activation in ACC consistently. These researches suggest the existence of different mechanisms and modulations of the pain matrix behind attentional and emotional modulatory pathways Woo et al. [9].
Neurophysiological changes in somatosensory and motor cortex changes lead to changes in the motor control of the impaired laterality recognition and body images
Changes in the somatosensory cortex include expansion, restriction, or shifting of the representation of the somatosensory map, therefore, increase in 2-point discrimination, underperformed lateral recognition and changes in body image perception may be presented Heinricher et al. [10]; Moseley [11]; Pijnenburg et al. [12]. When the primary motor cortex is influenced, altered brain mapping of motor areas is identified. [11]; Tsao, et al. [13] Changes in corticospinal excitability were also noted. Therefore, impaired ability to selectively recruit individual muscles, and the ability to perform cocontraction were presented. Brain functional connectivity of the sensorimotor network in patients with low back pain by using fMRI has revealed significant decrease in the left supplementary motor area, left precentral gyrus, and lobules IV and V of the cerebellum. In contrast, an increase in the right middle frontal gyrus and superior frontal gyrus has been discovered [12]. Therefore, it is likely that both the peripheral and the central neural systems should be considered as important factors when discussing low back pain.
Physical pain and virtual pain shared the same areas of activation in the pain matrix, but activate differently
"Shared representation theory of social pain” suggests that rejection and related experiences piggyback on brain systems evolve to represent physical pain Macdonald & Leary [14]; Eisenberger [15]; Eisenberger et al. [16], and the overlapping activity in previous studies have strengthened this theory.However, a recent study that examined the "core pain processing region”, dorsal anterior cingulate cortex with fMRI revealed different patterns in activation evoked by physical pain and rejection, suggesting a different pathway of signal transmission and processing for physical pain and rejection [9]. A possible explanation may be that while physical pain is a bottom-up stimulus, rejection is a top-down signal received by the brain. It is possible that chronic low back pain could be induced physically, psychologically, and socially.
Changes in ascending and descending pain modulatory systems, PAG-RVM pathway, dorsal horn of the spinal cords and peripheral receptors, influence central sensitization through different mechanisms
Not only changes in central but also in peripheral receptors contribute to the neuronal changes. When changes occur at peripheral receptors,there is an increased release of neurotransmitters from the peripheral through the projection of the dorsal horn to the upper neural system (Boadas-Vaello et al. [17], and changes about the input and output characteristics are presented, contributing to central sensitization. Lee et al. [7] Changes in descending pain modulatory systems, PAG-RVM pathway decrease, and descending inhibition of pain (disturbed conditioned pain modulation), therefore, contribute to central sensitization. Ossipov et al. [18]; Porreca et al. [19] Changes in the dorsal horn of spinal cord lead to increased transmission of nociceptive and neuropathic stimuli Bushnell et al. [20]; [5]; Woolf [21] as well as changes in membrane permeability. Through the changes of descending pain modulatory systems and the dorsal horn of spinal cord, pain threshold decreased, and central sensitization may be presented.
Bottom-up Approaches to Treating Chronic LBP
Current peripheral mechanism of pain describes low back pain in the changes of muscle, joint, movement pattern, related to deficit in proprioception and motor control
To explore the possible cause of low back pain, one possible theory is the bottom up theory. When there is injury to the muscle, the body must compensate to maintain its functional ability, which leads to altered joint position and movement pattern. As a result, under-stretched or tension-concentrated muscles or joints will stimulate the pain receptors within, and the signals will be transmitted to the higher neural system. A recent review about lumbo-pelvic kinematics in people with and without back pain suggested that though there are no differences between lordosis curves, reduced lumbar ROM, speed, and proprioception has been discovered. Changes in proprioception and motor control have also been thought as a possible reason behind low back pain Laird et al. [22]. As a previous review suggested, patients with low back pain performed more error during a reposition test, and when asked to reposition with a slower speed, patients with low back pain required more practice to achieve the target, indicating altered motor control and compromised proprioception. Besides, a recent study has shown that when performing a sit-to-stand-to sit task, patients with low back pain needed more time to finish the task compared to those without Pijnenburg et al. [12].
Traditional approaches including modality and bio-mechanical theory with treatment result in room for improvement
Traditional therapy is focused on the relief of symptoms. Both heat and cryotherapy are typically applied for short-term, immediate effects on patients. Using electrical stimulation alone cannot increase the chances of recovery of function. Traction applied to patients with low back pain only helped to decrease the pain, but with mild effect. A 2013 Cochrane Database systemic review about the effect of mechanical traction on low back pain patients without sciatica pain revealed that there was moderate-quality evidence suggesting that in the aspect of pain intensity, there was mild or no decrease in pain when comparing traction to non-treatment Wegner et al. [2 3]. Furthermore, manual therapy and exercise are recommended for treatment of low back pain in the 2016 treatment guideline. Chen et al. [24]; Saragiotto et al. [25]; Saragiotto et al. [26]; Steffens et al. [27].
Fascia and its attached core muscle is the target of new treatment strategy for improving trunk stability for patients with chronic low back pain
New treatment strategy focusing on training and motor learning of core muscle, and clinical guideline has suggested that it can effectively decrease pain and increase function. In a study, including 414 participants in 5 trials revealed that core muscle exercise was superior to other type of exercise, but the effects only last for 6 months. Wang et al. [28] Locating in the deepest layer of muscle around the spine, and connects to the fascia, core muscle contains high amount muscle spindle to be responsible for proprioception of spine. Most of core muscle contains muscle, tendons, such as multifidus has more than 50% of tendinous ingredients. Transverse abdominis connects to fascia to form a natural brace providing the stability of the lumbar spine Willard et al. [29].
Thoracolumbar fascia consists of anterior, middle, and posterior parts to separate erector spinae, abdominal muscle, Quadratus lumborum, and Psoas major, connects back muscles to form a supportive belt, and expands upward to thoracic, neck and finally occipital area, downward to sacrum. The myofascial system with the vertebral spines forms a tension-compression balance system, tensegrity. The change of the tension of the muscle fascia system results in morphological change and movement. Wong et al. [30].
Fascia expansion of various muscles from proximal to distal parts form myofascial chain resembles Chinese Meridian, serving functions in force transmission, protection, circulation and homeostasis
Recent research explores the connection between deep fascia and muscle. The anatomical structure of fascia Myers, [31], mechanical interaction with muscles, proprioceptive role in protection and pain processing, their role in circulation in maintaining homeostasis may provide us new ideas about how do low back pain occur. Deep fasciaare well-organized dense fibrous layers that interact with muscles, and provide sliding and gliding mechanism. Stecco & Hammer [32] Myofascial expansion is the connection from a skeletal muscle or tendon and inserts into the aponeurotic fascia, functioning in stabilizing tendons, and reducing the stress of bony attachment [32]. Fascia retinacula or reinforcement areas are richly innervated, and are considered to be a dynamic pulley system, which mechanically change the line of pull of muscles during motion, and proprioception providing sensory input for coordination. Fascia is well vascularized and with lymphatic channels to serve functions in circulation and fluid hemostasis
Fascia lesions stimulating nociceptor is considered the source of low back pain
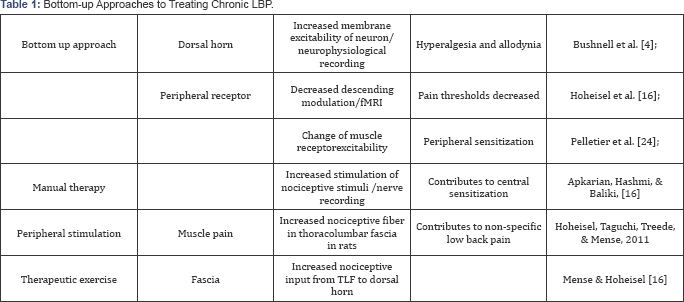
It is believed that within muscles are many muscle spindles, so when contracting muscles, signals will be detected and received. As a result, injury to muscle may stimulate nociception and cause pain. But recent studies suggest that muscle spindles lay not within the muscle, but in the fascia-particularly at the junction of the perimyosium and epimyosium [32]. Therefore, it is possible that the pull of the fascia stimulates certain receptors causing proprioceptive response of muscles. Abundant free and encapsulated nerve endings have been found in the thoracolumbar fascia. Nerves are more numerous in the superficial and intermediate sublayers, but not in the deep layers.Thus, when muscle contracts fascia is being stretched. When the force is prolonged or excessive, it stimulates the nociceptors at fascia and lead to pain. A recent study inducing experimental inflammation at the thoracolumbar fascia of rats showed increased density of nociceptive receptors on fascia, suggesting a possible connection to low back pain. Mense & Hoheisel [33] Injecting inflammatory substances into thoracolumbar fascia tissue in in-vivo condition resulted in strong pain, in comparison to injecting into muscle tissue, which supports the concept that the nociception from fascia is the source of low back pain Langevin et al. [34] (Table 1).
Top-down Mechanism Approaches for Pain Management Including Motor Control, Motor Imagery, Cognitive Behavior Therapy and Pain Education Demonstrate Promising Results
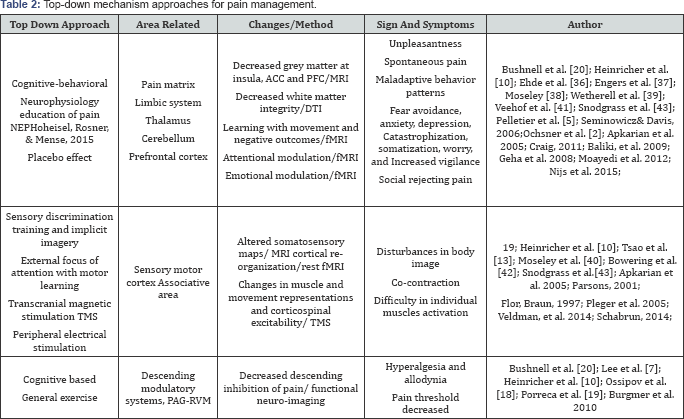
Motor control training for 2 weeks induced a shift in the motor area controlling trunk muscles in patients with chronic low back pain, indicating the post-trainingneuroplasticity of the motor cortex in patients with chronic pain. [13]; Tsao et al. [35] To address the behavioral change in fear avoidance as well as other symptoms associated with chronic low back pain, intervention targeting cognitive-behavioral approach has been developed and applied, with promising results [20]. To promote neuroplasticity, repetition, and intensity are required for adaptive change. Re- conceptualizing pain, neurophysiology education of pain focusing on anatomical and physiological information, education as well as increasing the understanding of noxious stimuli processing of patients, which has been demonstrated with promising effects in decreasing pain, are associated with changes in brain activation.
Ehde et al. [36]; Engers et al. [37]; Moseley [38] Addressing maladaptive thoughts has been demonstrated with the effects of improving mood, decreasing pain intensity for up to 6 months in patients with chronic pain [36], Wetherell et al. [39] while acceptance-based interventions such as acceptance commitment therapy has been applied to healthy subject and with the effects of neuroplasticity in the insula and S1 Moseley et al. [40] as well as the functional connectivity between the medial PFC and the insula. Veehof et al. [41]; [39] Cognitive-based intervention such as motor imagery can influence brain function and cortical processes Bowering et al. [42], including sensorimotor areas, though with positive results, it can only optimize its effects if subjects are able to sustain their attention Snodgrass et al. [43] (Table 2).
Conclusion
The need of an integrating model for dealing with chronic pain is urgent
Chronic low back pain may have multiple causes, therefore, single model (e. g. Bottom-up) can only partially explain the mechanism behind LBP. The need of integration includes the structural and physiological basis of myofascial trigger points and myofascial expansion,force transmission, neuroplasticity and neuroscience in pain management and pain prevention. Chronic low back pain is a bio-psychosocial issue further complicated by different cultural factors; requiring multilevel classification and identification of the biomarker and risk factors for optimal management and prevention. Therefore, when dealing with chronic low back pain, various soft tissues could be considered as targets, while the education of pain as well as other approaches dealing with plastic changes in the central neural system should be addressed to maximize the effect of the treatment.
References
- Nielens H, Van ZJ, Mairiaux P, GaillyJ, Van DH, et al. (2006) Chronic low back pain. Belgian Health Care Knowledge Centre KCE, Belgium.
- Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, et al. (2006) Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J 15(Suppl 2): S192-S 300.
- Cohen SP, Argoff C, Carragee EJ (2008) Management of low back pain. BMJ, doi: 10.1136/bmj.a2718.
- Morlion B (2013) Chronic low back pain: Pharmacological, interventional and surgical strategies. Nature Reviews Neurology 9(8): 462-473.
- Pelletier R, Higgins J, Bourbonnais D (2015) Addressing Neuroplastic Changes in Distributed Areas of the Nervous System Associated With Chronic Musculoskeletal Disorders. Phys Ther 95(11): 1582-1591.
- Wertli MM, Eugster R, Held U, Steurer J, Kofmehl R, et al. (2014) Catastrophizing-a prognostic factor for outcome in patients with low back pain: a systematic review. Spine J 14(11): 2639-2657.
- Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, et al. (2011) Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res Hoboken 63(3): 320-327.
- Villemure C, Bushnell MC (2009) Mood influences supraspinal pain processing separately from attention. J Neurosci 29(3): 705-715.
- Woo CW, Koban L, Kross E, Lindquist MA, Banich MT, et al. (2014) Separate neural representations for physical pain and social rejection. Nat Commun 5: 5380.
- Heinricher MM, Tavares I, Leith JL, Lumb BM (2009) Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev 60(1): 214-225.
- Moseley GL (2011) Cognitive neuroscience: swapping bodies in the brain. Curr Biol 21(15): R583-585.
- Pijnenburg M, Brumagne S, Caeyenberghs K (2015) Resting-State Functional Connectivity of the Sensorimotor Network in Individuals with Nonspecific Low Back Pain and the Association with the Sit-to- Stand-to-Sit Task. Brain Connect 5(5): 303-311.
- Tsao H, Danneels LA, Hodges PW (2011) ISSLS prize winner: Smudging the motor brain in young adults with recurrent low back pain. Spine (Phila Pa 1976) 36(21): 1721-1727.
- Macdonald G, Leary MR (2005) Why does social exclusion hurt? The relationship between social and physical pain. Psychol Bull 131(2): 202-223.
- Eisenberger NI (2012) The neural bases of social pain: evidence for shared representations with physical pain. Psychosom Med 74(2): 126-135.
- Eisenberger NI, Lieberman MD, Williams KD (2003). Does rejection hurt? An FMRI study of social exclusion. Science 302(5643): 290-292.
- Boadas-Vaello P, Castany S, Homs J, Älvarez-Perez B, Deulofeu M, et al. (2016) Neuroplasticity of ascending and descending pathways after somatosensory system injury: reviewing knowledge to identify neuropathic pain therapeutic targets. Spinal Cord 54(5): 330-340.
- Ossipov MH, Dussor GO, Porreca F (2010) Central modulation of pain. J Clin Invest 120(11): 3779-3787.
- Porreca F, Ossipov MH, Gebhart GF (2002) Chronic pain and medullary descending facilitation. Trends in Neurosciences 25(6): 319-325.
- Bushnell MC, Ceko M, Low LA (2013) Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14(7): 502511.
- Woolf CJ (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152(Suppl 3): S2-S15.
- Laird RA, Gilbert J, Kent P, Keating JL (2014) Comparing lumbo-pelvic kinematics in people with and without back pain: a systematic review and meta-analysis. BMC Musculoskelet Disord 15: 229.
- Wegner I, Widyahening IS, van Tulder MW, Blomberg SE, de Vet HC, et al. (2013) Traction for low-back pain with or without sciatica. Cochrane Database Syst Rev (8): CD003010.
- Chen YH, Chai HM, Shau YW, Wang CL, Wang SF (2016) Increased sliding of transverse abdominis during contraction after myofascial release in patients with chronic low back pain. Man Ther 23: 69-75.
- Saragiotto BT, Maher CG, Yamato TP, Costa LO, Costa LC, et al. (2016) Motor Control Exercise for Nonspecific Low Back Pain: A Cochrane Review. Spine Phila Pa 1976 41(16): 1284-1295.
- Saragiotto BT, Maher CG, Yamato TP, Costa LO, Menezes Costa LC, et al. (2016) Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst Rev (1): Cd012004.
- Steffens D, Maher CG, Pereira LS, Stevens ML, Oliveira VC, et al. (2016) Prevention of Low Back Pain: A Systematic Review and Meta-analysis. JAMA Intern Med 176(2): 199-208.
- Wang XQ, Zheng JJ, Yu ZW, Bi X, Lou SJ, et al. (2012) A meta-analysis of core stability exercise versus general exercise for chronic low back pain. PLoS One 7(12): e52082.
- Willard FH, Vleeming A, Schuenke MD, Danneels L, Schleip R (2012) The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat 221(6): 507-536.
- Wong KK, Chai HM, Chen YJ, Wang CL, Shau YW, et al. (2017) Mechanical deformation of posterior thoracolumbar fascia after myofascial release in healthy men: A study of dynamic ultrasound imaging. Musculoskelet Sci Pract 27: 124-130.
- Myers TW (2014) Anatomy trains: myofascial meridians for manual and movement therapists (3rd edn), Edinburgh: Elsevier, Scotland.
- Stecco C, Hammer, WI (2015) Functional atlas of the human fascial system. Edinburgh: Elsevier Ltd, Scotland.
- Mense S, Hoheisel U (2016) Evidence for the existence of nociceptors in rat thoracolumbar fascia. J Bodyw Mov Ther 20(3): 623-628.
- Helene ML, James RF, Cathryn K, Gary JB, Greenan- Naumann AC, et al. (2011). Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet Disord 12: 203.
- Tsao H, Galea MP, Hodges PW (2010) Driving plasticity in the motor cortex in recurrent low back pain. Eur J Pain 14(8): 832-839.
- Ehde DM, Dillworth TM, Turner JA (2014). Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol 69(2): 153-166.
- Engers A, Jellema P, Wensing M, Windt DA, Grol R, et al. (2008) Individual patient education for low back pain. Cochrane Database Syst Rev (1): CD004057.
- Moseley GL (2004) Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic low back pain. Eur J Pain 8(1): 39-45.
- Wetherell JL, Afari N, Ayers CR, Stoddard JA, Ruberg J, et al. (2011) Acceptance and Commitment Therapy for generalized anxiety disorder in older adults: a preliminary report. Behav Ther 42(1): 127-134.
- Moseley GL, Zalucki NM, Wiech K (2008) Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain. Pain 137(3): 600-608.
- Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET (2011). Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain 152(3): 533-542.
- Bowering KJ, O'Connell NE, Tabor A, Catley MJ, Leake HB, et al. (2013) The effects of graded motor imagery and its components on chronic pain: a systematic review and meta-analysis. J Pain 14(1): 3-13.
- Snodgrass SJ, Heneghan N, Tsao H, Stanwell PT, Rivett DA, et al. (2014) Recognising neuroplasticity in musculoskeletal rehabilitation: a basis for greater collaboration between musculoskeletal and neurological physiotherapists. Man Ther 19(6): 614-617.






























