Abstract
Introduction: Right ventricular dysfunction represents a diagnostic challenge, particularly in the absence of invasive monitoring using a pulmonary artery catheter (Swan-Ganz). In this setting, echocardiography is considered the noninvasive method of choice for the assessment of right ventricular function, making it necessary to standardize the most reliable echocardiographic parameter in the postoperative period of cardiac surgery.
Objective: To evaluate the agreement between fractional area change (FAC) and tricuspid annular plane systolic excursion (TAPSE) measurements as echocardiographic markers of right ventricular function in the postoperative period of adult patients undergoing cardiac surgery.
Methods: A Descriptive agreement study was conducted including 22 patients older than 18 years who underwent cardiac surgery at the Hospital Cardiovascular del Niño de Cundinamarca. Agreement between fractional area change (FAC) and tricuspid annular plane systolic excursion (TAPSE) was evaluated, and their correlation with the central venous pressure/pulmonary artery occlusion pressure index (CVP/PAOP), measured by thermodilution, was analyzed.
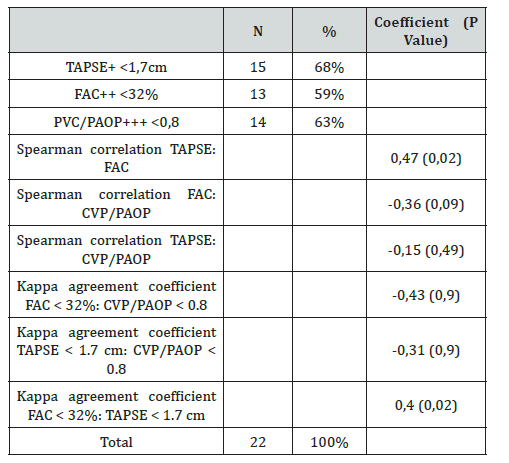
Results: Of the study population, 63.5% (n = 14) presented postoperative right ventricular dysfunction, defined by a CVP/PAOP ratio < 0.8. Likewise, 68.2% (n = 15) showed right ventricular dysfunction assessed by TAPSE < 1.7 cm, and 59.1% (n = 13) by FAC < 32%. When analyzing agreement, the kappa index between the CVP/PAOP ratio and TAPSE was -0.31 (p = 0.97), while that between CVP/PAOP and FAC was -0.43 (p = 0.92). In contrast, agreement between TAPSE and FAC showed a kappa index of 0.41 (p = 0.02).
Conclusion: Echocardiographic evaluation of the right ventricle (RV) allows the diagnosis of right ventricular dysfunction, demonstrating good agreement between its two main parameters, TAPSE and FAC. However, these measurements do not replace the hemodynamic information obtained using a Swan-Ganz catheter, which is considered the gold standard in the absence of cardiac magnetic resonance imaging.
Keywords:Echocardiography; Right Ventricular Dysfunction; Cardiac Surgical Procedures; Swan-Ganz Catheterization; Standards
Abbreviations:FAC: Fractional Area Change; TAPSE: Tricuspid Annular Plane Systolic Excursion; RV: Right Ventricle; LV: Left Ventricle, RVEF: Right Ventricular Ejection Fraction; ICU: Intensive Care Unit; RIMP: Myocardial Performance Index; LVEF: Left Ventricular Ejection Fraction; CVP: Central Venous Pressure; PAOP: Pulmonary Artery Occlusion Pressure; FUCS: Fundación Universitaria De Ciencias De La Salud
Introduction
Traditionally, right ventricular (RV) function has been considered to play a limited role in overall cardiovascular homeostasis [1]. In fact, several authors have stated that its contribution to systemic hemodynamics becomes relevant mainly in the presence of reduced diastolic compliance of the left ventricle (LV), [2] secondary to volume or pressure overload, a phenomenon known as ventricular interdependence [3]. Nevertheless, a more detailed study of RV function has led to its progressive recognition as a hemodynamically less powerful but functionally comparable pump to the LV [4]. Thus, the RV has been assigned a fundamental role in cardiovascular homeostasis, concluding that its primary function is to maintain effective cardiac output by minimizing impedance to venous return while simultaneously promoting adequate left ventricular filling. Changes in left ventricular function following cardiac surgery have been widely documented for decades. In this context, reversible ischemic injury, known as “hibernating myocardium,” has been described as one of the main underlying pathophysiological mechanisms [5]. However, deterioration of right ventricular function following cardiopulmonary bypass has been less extensively studied [6], despite its well-established impact on clinical outcomes and longterm prognosis.
This condition has been associated with mortality rates ranging from 37% to 90% [7], as well as an incidence of up to 20% after coronary revascularization surgery [7]. Additionally, RV dysfunction has been identified as a key predictor of longterm survival in patients undergoing mitral valve replacement [8]. Among the mechanisms proposed to explain postoperative right ventricular dysfunction are loss of pericardial restraint secondary to pericardiotomy, RV ischemia related to inadequate myocardial protection, and the formation of adhesions between the RV and adjacent tissues, which alter its geometry and normal contractile patterns [8]. Several studies have identified male sex, elevated pulmonary artery pressures [8], reduced LV ejection fraction [9], the presence of concomitant coronary artery disease, atrial fibrillation, and low systolic arterial pressure [10] as the main predictors of right ventricular dysfunction.
In an observational study conducted by Bootsma et al. [8], a significant increase in morbidity was observed in patients with a right ventricular ejection fraction (RVEF) below 20%, manifested by longer stays in the intensive care unit (ICU), increased need for mechanical ventilation, greater use of inotropic agents, and more pronounced increases in creatinine levels, compared with patients whose RVEF was greater than 30%.
Cardiac magnetic resonance imaging is considered the gold standard for noninvasive assessment of right ventricular function [11]. However, due to limitations related to portability, availability, and cost, echocardiography remains the method of choice in clinical practice, owing to its wide accessibility and ease of use [12]. It has been established that longitudinal contraction contributes approximately 80% of RV cardiac output, which is why echocardiographic evaluation has focused on this functional component [13]. Nevertheless, there is still no consensus regarding which echocardiographic parameter is optimal for diagnosing right ventricular dysfunction, particularly in the postoperative setting of cardiac surgery [14]. Recent recommendations from the European Society of Echocardiography and the European Association of Cardiovascular Imaging [15] suggest that global RV function should be assessed using at least one of the following parameters [16]:
• Fractional area change (FAC)
• Tricuspid annular plane systolic excursion (TAPSE)
• Tissue Doppler imaging: systolic velocity (S’) of the
tricuspid annulus
• Myocardial performance index (RIMP)
TAPSE is an M-mode measurement that quantifies the longitudinal displacement of the tricuspid annulus during the cardiac cycle, with values below 17 mm considered abnormal [15]. However, this measurement has important limitations. Translational motion of the heart may lead to overestimation of this parameter, and it evaluates only a single RV segment; therefore, regional wall motion abnormalities may yield inaccurate results. Additionally, it has been demonstrated that TAPSE and other longitudinal contraction parameters, such as tricuspid annular S’ velocity on tissue Doppler imaging and longitudinal strain, decrease significantly during cardiac surgery, even in the absence of concomitant changes in global echocardiographic parameters such as right ventricular ejection fraction (RVEF) and FAC [17,18]. This reduction has been documented in both coronary revascularization surgery and aortic valve surgery and may persist for up to one year after aortic valve replacement, without necessarily being associated with clinical signs of right ventricular dysfunction [17-19].
In contrast, fractional area change (FAC) measures the endocardial area variation of the RV between systole and diastole. One of its main advantages is that, in addition to reflecting longitudinal contraction, it incorporates assessment of radial contraction components, allowing a more comprehensive evaluation of RV function [20]. A value below 32% is considered abnormal [15]. However, this parameter is highly dependent on image quality, as inadequate visualization of the endocardium precludes accurate measurement. Given the importance of timely diagnosis of postoperative right ventricular dysfunction, and considering the potential alterations in echocardiographic parameters secondary to cardiac surgery, it is necessary to evaluate the agreement between the two echocardiographic measurements used in our institution, namely FAC and TAPSE. The latter has historically been the most widely used parameter and has been correlated with RV dysfunction; however, recent studies, particularly in patients undergoing cardiac surgery, have demonstrated lower sensitivity and a potential loss of validity compared with other echocardiographic markers. Therefore, the present study aims to perform echocardiographic measurements of FAC and TAPSE, evaluate the agreement between these two parameters, and analyze their correlation with the central venous pressure/pulmonary artery occlusion pressure (CVP/PAOP) ratio, a hemodynamic parameter widely used and standardized in guidelines for right ventricular dysfunction and failure. This approach may contribute in the future to standardizing the postoperative echocardiographic diagnosis of right ventricular dysfunction and to optimizing therapeutic strategies and early interventions that positively impact patient morbidity.
Methods
Study Design
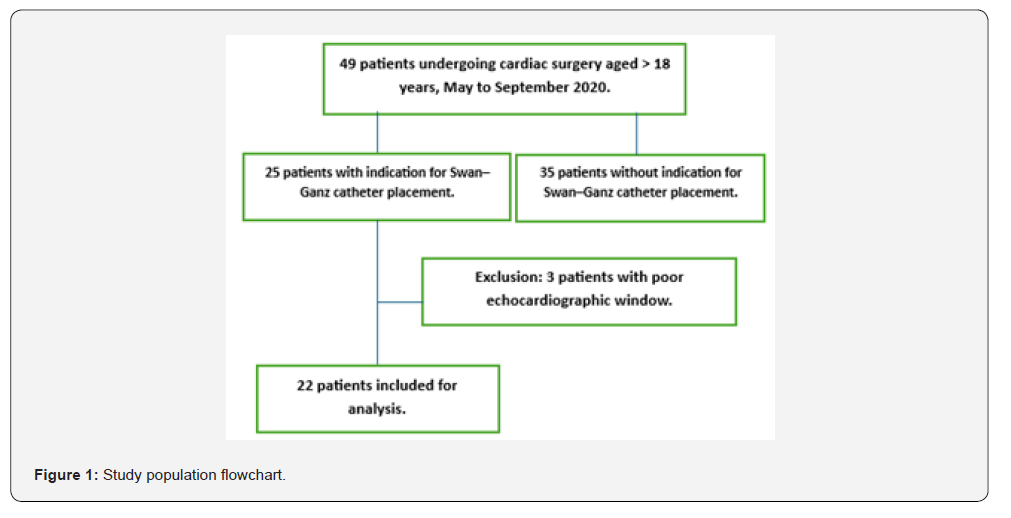
An observational, descriptive, cross-sectional study with prospective data collection was conducted in patients undergoing cardiac surgery during the period between May and September 2020. Of a total of 49 surgically treated patients, 27 were excluded due to the absence of postoperative hemodynamic monitoring with a pulmonary artery catheter (Swan-Ganz), and an additional 3 patients were excluded because of inadequate echocardiographic windows. Consequently, the final analysis included 22 patients. All included patients underwent preoperative and postoperative transthoracic echocardiography according to the hospital cardiology service protocol. The echocardiographic study was performed by obtaining parasternal and apical four-chamber views using an S5 linear transducer.
Values of left ventricular ejection fraction (LVEF), fractional area change (FAC), and tricuspid annular plane systolic excursion (TAPSE) were recorded as parameters of right ventricular function. Hemodynamic variables, including central venous pressure (CVP) and pulmonary artery occlusion pressure (PAOP), were obtained from nursing records, routinely documented by the head nursing staff in the Cardiovascular Unit at 1 and 24 hours after patient admission to the immediate postoperative period. This study was approved by the Hospital Ethics Committee, as well as by the Research and Ethics Committee of the Fundación Universitaria de Ciencias de la Salud (FUCS) and the Hospital de San José de Bogotá. No additional informed consent was required beyond that already obtained for routine clinical and surgical procedures.
Variables and Outcomes
Sample characterization included the following variables: age, sex, body mass index (BMI), type of surgical procedure performed, comorbidities, LVEF, FAC, and TAPSE in the preoperative and postoperative periods, as well as calculation of the CVP/PAOP ratio. The primary outcome was the presence or absence of postoperative right ventricular dysfunction, defined by the following criteria: TAPSE < 1.7 cm, CVP/PAOP ratio < 0.8, and FAC < 32%.
Statistical Análisis
Data were analyzed using basic descriptive statistics. Agreement between variables of interest was assessed using Cohen’s kappa coefficient, and correlation was analyzed using Spearman’s correlation coefficient. A p value < 0.05 was considered statistically significant.
Results
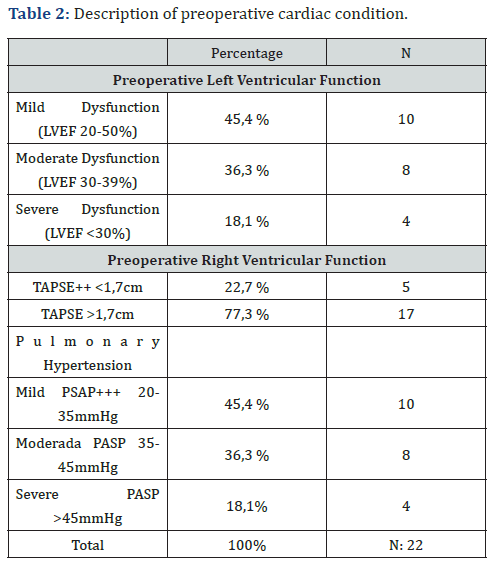
During the period between May 1 and September 30, 2020, a total of 22 patients older than 18 years were evaluated. All patients underwent cardiac surgery under cardiopulmonary bypass (CPB) and hemodynamic monitoring with a pulmonary artery catheter (Swan-Ganz). The analyzed sample showed a mean age of 59.4 ± 14.5 years. Male sex was predominant, accounting for 54.5% of the patients (n = 12). The most prevalent comorbidity was arterial hypertension, present in 77.2% (n = 17), followed by diabetes mellitus and chronic obstructive pulmonary disease, both with a prevalence of 27.2% (n = 6). Regarding the type of surgical procedure, 54.5% of the patients (n = 12) underwent valve replacement surgery; of these, 22.7% (n = 5) corresponded to mitral valve replacement, 4.5% (n = 1) to aortic valve replacement, and 27% to surgery involving more than one valve. Nine percent of the patients (n = 2) underwent a Bentall procedure, and 4.5% (n = 1) underwent a Bentall procedure combined with coronary revascularization (Table 1). Preoperative echocardiographic assessment showed a left ventricular ejection fraction (LVEF) of 40-50% in 45.4% of the patients (n = 10), followed by an LVEF of 30-39% in 36.3% (n = 8), and an LVEF < 30% in 18.1% (n = 4). Right ventricular function was assessed using TAPSE, with values < 1.7 cm observed in 22.7% of cases (n = 5) and ≥ 1.7 cm in 77.3% (n = 17).
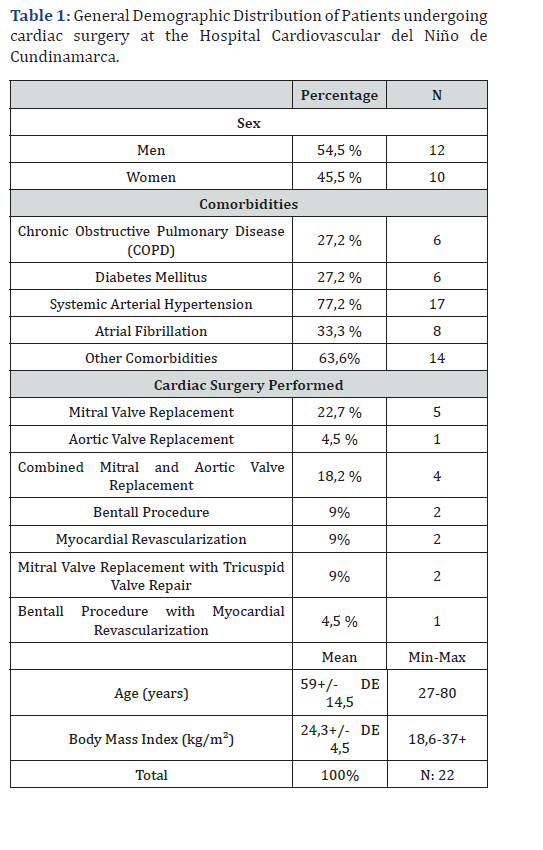
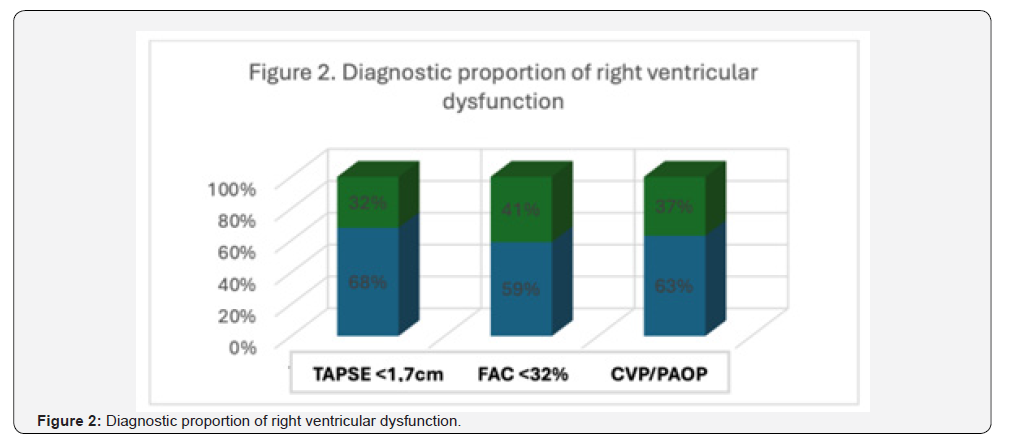
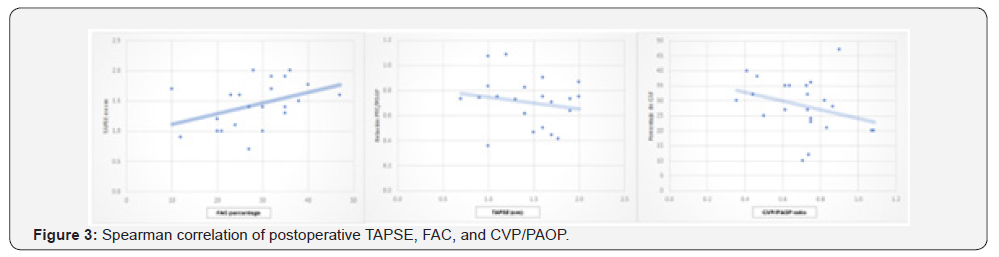
All patients presented some degree of pulmonary hypertension, with a pulmonary artery systolic pressure (PASP) of 20-35 mmHg in 45.4% (n = 10), 35-45 mmHg in 36.3% (n = 8), and > 45 mmHg in 18.1% (n = 4) of cases (Table 2). Postoperative echocardiographic evaluation identified a reduction in TAPSE, with values < 1.7 cm, compatible with right ventricular dysfunction, in 68% of patients (n = 15) (Figure 1). Similarly, fractional area change (FAC) values < 32% were observed in 59% of cases (n = 13). In addition, the CVP/PAOP ratio, obtained using a Swan- Ganz catheter and considered diagnostic of right ventricular dysfunction when < 0.8, was observed in 63% of patients (n = 14) (Figure 2). Correlation analysis using Spearman’s coefficient showed a moderate positive correlation between TAPSE and FAC (r = 0.47; p = 0.02). In contrast, the correlation between FAC and the CVP/PAOP ratio was negative and not statistically significant (r = -0.36; p = 0.09), as was the correlation between TAPSE and CVP/PAOP (r = -0.15; p = 0.49). When diagnostic agreement for right ventricular dysfunction was evaluated using Cohen’s kappa coefficient, a negative agreement was observed between FAC and CVP/PAOP (kappa = -0.43; p = 0.9), as well as between TAPSE and CVP/PAOP (kappa = -0.31; p = 0.9). In contrast, agreement between FAC and TAPSE was moderate, with a kappa index of 0.4 (p = 0.02) (Figure 3).
+ Left Ventricular Ejection Fraction.
++ Tricuspid Annular Plane Systolic Excursion.
+++ Pulmonary Artery Systolic Pressure.
Discussion
Right ventricular dysfunction and, consequently, secondary right ventricular failure are increasingly frequent entities in the postoperative period of cardiac surgery. An incidence of up to 20% has been reported in patients undergoing myocardial revascularization, with mortality rates reaching up to 90% [7]. In our series, 100% of patients who underwent coronary revascularization were diagnosed with right ventricular dysfunction assessed by thermodilution. It is well established that the right ventricle is highly sensitive to changes in afterload; therefore, pulmonary hypertension has been identified as one of the main causes of right ventricular dysfunction [21,22]. In our cohort, this factor was present in 95% of patients and in 100% of those who presented right ventricular dysfunction diagnosed by thermodilution (Table 3). Likewise, male sex has been reported as an independent risk factor in different series [23,24]. In the present study, 64.2% of patients with right ventricular dysfunction diagnosed by thermodilution were men, whereas this proportion was 46% when the diagnosis was established using TAPSE and FAC.
Table 3: Frequency of right ventricular dysfunction according to Diagnostic Modality.
Left ventricular dysfunction has also been associated with a higher incidence of right ventricular dysfunction [25], mainly through mechanisms of ventricular interdependence. In the context of cardiac surgery, this phenomenon has been closely related to pericardiotomy, by reducing pericardial restraint and altering longitudinal right ventricular motion, with a reported incidence of up to 33% in patients with reduced left ventricular ejection fraction (LVEF). Concordantly, Damy et al. [26] reported postoperative TAPSE abnormalities after cardiac surgery in 20% of patients with preserved LVEF versus 47% in those with reduced LVEF. In our series, patients with LVEF < 40% accounted for 50% of cases of right ventricular dysfunction diagnosed by thermodilution, 46% of those diagnosed by FAC, and 53% of those diagnosed by TAPSE. Atrial fibrillation has been described as one of the main predictors of right ventricular dysfunction [10]. In our study, the frequency of this factor ranged between 28% and 40% across echocardiographic measurements and Swan-Ganz catheter-based assessment.
Bitcon et al. [17] described significant differences between preoperative and postoperative TAPSE values, particularly after sternal closure, attributable to pericardiotomy and anatomical changes that condition alterations in this echocardiographic parameter without necessarily reflecting true right ventricular dysfunction. In agreement with these findings, our series showed a prevalence of preoperative right ventricular dysfunction of 22.7%, defined by a TAPSE < 1.7 cm. In the postoperative evaluation, right ventricular dysfunction was diagnosed in 68.1% of patients using TAPSE < 1.7 cm, in 63% using a CVP/PAOP ratio < 0.8, and in 59% using a FAC < 32%. When analyzing the correlation between postoperative TAPSE and FAC using Spearman’s coefficient, a moderate and statistically significant correlation was observed. In contrast, correlations between FAC and CVP/PAOP, as well as between TAPSE and CVP/PAOP, were weak. Several studies have reported that TAPSE shows low correlation in the diagnosis of right ventricular dysfunction when compared with FAC.
In our study, this diagnostic discordance was evident when analyzing the kappa coefficient between CVP/PAOP and TAPSE, as well as between CVP/PAOP and FAC (Table 3). However, when evaluating diagnostic agreement between FAC and TAPSE, a moderate agreement was observed, with a kappa index of 0.4 and a statistically significant p value (p = 0.02). These findings suggest that anatomical variations secondary to cardiac surgery not only affect measurements of longitudinal right ventricular contraction but also influence the global assessment of right ventricular contractility. This is reflected in the low correlation and agreement observed between echocardiographic measurements and hemodynamic parameters obtained by thermodilution, in contrast to the better concordance found between the two echocardiographic parameters evaluated.
Conclusion
Right ventricular dysfunction is a highly prevalent entity in patients undergoing cardiac surgery, and its timely diagnosis and treatment constitute a fundamental pillar for reducing morbidity and mortality and improving clinical outcomes. In recent years, measurement of tricuspid annular plane systolic excursion (TAPSE) has been considered an echocardiographic marker of limited reliability in the postoperative period of cardiac surgery. However, in the present series, a moderate correlation between TAPSE and fractional area change (FAC), measured using Spearman’s coefficient, was demonstrated, as well as a moderate diagnostic agreement assessed using the kappa index, both of which are validated methods for diagnosing right ventricular dysfunction in the postoperative setting. Nevertheless, when these echocardiographic parameters were compared with the central venous pressure/pulmonary artery occlusion pressure (CVP/ PAOP) ratio, considered a hemodynamic parameter with high validity and specificity, no significant agreement was found.
These findings suggest that cardiac surgery may produce a global alteration of right ventricular echocardiographic measurements, rather than affecting exclusively the parameters that assess longitudinal contraction. Additionally, a review of the available literature did not identify studies directly comparing echocardiographic measurements with parameters obtained by thermodilution. It is important to consider that echocardiography is an operator-dependent technique and that appropriate placement and accurate measurement of pulmonary artery catheter wedge pressure are essential to ensure the precision of hemodynamic data.
Limitations
The present study has limitations mainly related to the sample size. During the COVID-19 pandemic, the cancellation of scheduled surgical procedures as a contingency measure hindered data collection and the acquisition of a more representative sample. In this regard, further studies including a larger number of patients are required to strengthen and validate the results obtained. Likewise, increasing the sample size could contribute to improving the concordance indices observed among the evaluated variables.
References
- Pinsky MR (2016) The right ventricle: Interaction with the pulmonary circulation. Critical Care 20(1): 266.
- Taylor RR, Corell JW, Sonnenblick EH, Ross JR (1967) Dependence of ventricular distensibility on filling the opposite ventricle. Am J Phisiol 213: 711-718.
- Sibbad WJ, Driedger A (1983) Right ventricular function in disease states: pathophysiologic considerations. Crit Care Med 11: 339-346.
- Lawson WE, Seifert FA (1988) Effect of coronary artery bypass grafting on left ventricular diastolic function. Am J Cardiol 61(4): 283-287.
- Deschamps A, Nozza A, Pagé P, Tardif J, Lambert J, et al. (2015) Right Ventricular Depression After Cardiopulmonary Bypass for Valvular Surgery. J Cardiothorac Vas Anesth29(4): 836-844.
- Reichert CL, Visser CA, Van Den Brink RB (1992) Prognostic value of biventricular function in hypotensive patients after cardiac surgery as assesed by transesophageal echocardiography. J cardiothorac Vasc Anesth 6(4): 429-432.
- Imada T, Kamibayashi t, Ota C, Sho Carl Shibata S, Iritakenishi T, et al. (2014) Intraoperative Right Ventricular Fractional Area Change Is a Good Indicator of Right Ventricular Contractility: A Retrospective Comparison Using Two- and Three-Dimensional Echocardiography. J cardiothorac Vasc Anesth 29(4): 831-835.
- Bootsma IT, De Lange F, Koopmans M, Haenen J, Boonstra PW, et al. (2017) Right Ventricular Function After Cardiac Surgery Is a Strong Independent Predictor for Long-Term Mortality. J Cardiothorac Vasc Anesth 31(5): 1656-1662.
- Maslow AD, Regan MM, Panzica P (2002) Precardiopulmonary bypass right ventricular function is associated with poor outcome after coronary artery bypass grafting in patients with severe left ventricular systolic dysfunction. Anesth Analg 95(6): 1507-1508.
- Melenovsky V, Hwang SJ, Lin G (2014) Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 35(48): 3452-3462.
- Judge O, Ji F, Fleming N, Liu H (2015) Current use of the pulmonary artery catheter in cardiac surgery: a survey study. J cardiothorac Vasc Anesth 29(1): 69-75.
- Lancelotti P, Edvardsen T, Cosyns B, Neskovic AN, Dulgheru R, et al. (2015) The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular imaging and the Acute Cardiovascular care association. Eur Heart J acute Cardiovasc Care 4(1): 3-5.
- Brown SB, Raina A, Katz D, Szerlip M, Wiegers SE, et al. (2011) Longitudinal Shortening Accounts for the Majority of Right Ventricular Contraction and Improves After Pulmonary Vasodilator Therapy in Normal Subjects and Patients with Pulmonary Arterial Hypertension. Chest 140(1): 27-33.
- Grønlykke L, Korshin A, Holmgaard F (2019) Severe loss of right ventricular longitudinal contraction occurs after cardiopulmonary bypass in patients with preserved right ventricular output. Int J Cardiovasc Imaging 35(9): 1661-1670.
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, et al. (2015) Recommendations for Cardiac Chamber Quantification. J Am Soc Echocardiogr 28(1): 1-39. e14.
- Venkatachalam S, Wu G, Ahmad M (2017) Echocardiographic assessment of the right ventricle in the current era: Application in clinical practice. Echocardiography 34(12): 1930-1947.
- Bitcon CJ, Tousignant C (2017) The effect of pericardial incision on right ventricularsystolic function: a prospective observational study. Can J Anesth 64(12): 1194-1201.
- Gronlykke L, Ihlemann N, Ngo AT (2017) Measures of right ventricular function after transcatheter versus surgical aortic valve replacement. Interact Cardiovasc Thorac Surg 24(2): 181-187.
- Tamborini G, Muratori M, Brusoni D (2009) Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur J Echocardiogr 10(5): 630-634.
- Anavekar N, Gerson D, Skali H (2007) Two-dimensional assessment of right ventricular function: an echocardiographic-MRI correlative study. Echocardiography 24(5): 452-456.
- Lam CS, Roger VL, Rodeheffer RJ, Bourlaug BA, Enders FT, et al (2009) Pulmonary hypertension in heart failure with preserved ejection fraction: a community study. J AM coll Cardiol 53(13): 1119-1126.
- Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CS, et al. (2012) Pulmonary pressures and death in heart failure: a community study. J AM coll Cardiol 59(3): 222-231.
- Kawut SM, Lima JA, Barr RG, Chahal H, Jain A, et al. (2011) Sex and race differences in right ventricular structure and function: The Milti-Ethnic study of atherosclerosis-right ventricular study. Circulation 123(22): 2542-2551.
- Haddad F, Doyle R, Murphy DJ, Hunt SA (2008) Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance. and management of right ventricular failure. Circulation 117(13): 1717-1731.
- Puwanant S, Priester TC, Mookadam F, Bruce CJ, Redfield MM, et al. (2009) Right ventricular function in patients with preserved and reduced ejection fraction heart failure. Eur J Echocardiogr 10(6): 733-737.
- Damy T, Kallvikbacka-Bennett A, Goode K (2012) Prevalence of, Associations With, and Prognostic Value of Tricuspid Annular Plane Systolic Excursion (TAPSE) Among Out-Patients Referred for the Evaluation of Heart Failure. J Card Fail 18(3): 216-225.






























