Abstract
Aim: Systemic inflammatory index (SII) is an indicator with prognostic value that has been previously shown to be associated with inflammatory processes and prognosis in malignancies. In our study, SII and related factors were examined in the postoperative surveys of patients who underwent craniotomy for various reasons.
Methods: In January-December 2024, 144 patients who underwent craniotomy with the diagnoses of intracranial mass, spontaneous intracerebral haemorrhage and traumatic intracerebral haemorrhage were included in the study. Inflammatory, metabolic indicators and survey statuses were recorded as study data. Systemic inflammatory indices were calculated, and the results were statistically analysed.
Results: No significant relationship was observed between systemic inflammatory index (SII) and mortality (p=0,670). There was no significant difference in survival between genders and age groups. Hemoglobin and platelet values were lower in patients with mortality. PT, APTT, and INR values were significantly higher in patients with mortality. AST, BUN, and creatinine values were also higher in patients with mortality. Preoperative and postoperative CRP values were found to be higher in patients with mortality.
Conclusion: Although we say that SII has no effect on prognosis, our study has revealed very important findings that will affect prognosis. There is always a need for new studies that will provide close follow-up of the prognosis and the patient.
Keywords:Systemic Inflammatory Index; Craniotomy; Immune Response; Cytokines; Inflammation
Abbreviations:SII: Systemic Inflammatory Index; CRP: C-Reactive Protein; NLR: Neutrophil-To-Lymphocyte Ratio; TPN: Total Parenteral Nutrition; PLR: Platelet-To-Lymphocyte Ratio
Introduction
The systemic inflammatory index (SII) is a marker calculated by combining platelet, neutrophil and lymphocyte count. Studies have demonstrated that elevated SII values are associated with disease severity and poor prognosis in numerous diseases and malignancies [1,2]. It is crucial for indicating inflammation and immune response. Several studies have demonstrated that a range of inflammatory biomarkers, including C-reactive protein (CRP), the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR), are associated with prognosis in various types of cancer, as well as in several diseases characterised by inflammation [3-7]. The surgical procedure of craniotomy, or the creation of a burr-hole in the skull, has been observed since the Paleolithic and Neolithic periods [8]. Craniotomy represents the foundation of neurosurgical practice, encompassing the fundamental techniques employed to access the requisite location in intracranial procedures. Infections are a common and potentially fatal complication in the neurosurgical intensive care unit, particularly in the context of craniotomy. Impaired immune response after central nervous system surgery is associated with an elevated risk of infection and postoperative complications [9].
Systemic inflammation is induced as a biological response to craniotomy. The resulting immune response causes the release of intracellular antigens from leukocytes that are not normally secreted [10]. During neurosurgical procedures, such as the placement of cranial pins and the creation of surgical incisions in the scalp, noxious stimulation can precipitate a sudden increase in blood pressure and heart rate [11]. Furthermore, numerous neurohormonal responses give rise to the release of cytokines, which can ultimately result in tissue damage. Nevertheless, to the best of our knowledge, no study has been conducted on SII in patients who have undergone craniotomy surgery for any reason, including the removal of an intracranial mass, the treatment of an intracerebral haemorrhage, or the repair of traumatic injuries. Considering the considerations, the objective of our study was to evaluate patients who were admitted to the postoperative neurosurgical intensive care unit over the past year. This was done to ascertain the prognostic value of SII in patients who underwent craniotomy.
Material and Methods
Study Design
This study is a retrospective clinical study, conducted by Kayseri City Hospital Neurosurgery Clinic with taken of Ethical Approval by Kayseri City Hospital (Approval Number: 273/10.12.2024). The retrospective review of patients was conducted in accordance with the ethical standards established by the institutional or regional responsible committee and in accordance with the principles set forth in the 1975 Declaration of Helsinki, as revised in 1983. Owing to the retrospective design and use of fully anonymized data, the requirement for individual patient informed consent was formally waived by the “Kayseri City Hospital Ethics Committee”, in line with the Regulation on Clinical Researches of the Ministry of Health of T… (Official Gazette No. 28617, 13 April 2013). The study included 144 patients who met the appropriate criteria.
Inclusion Criteria: Patients between the ages of 18-80 who underwent surgery due to tumour, spontaneous intracerebral haemorrhage, and trauma-related bleeding were included in the study regardless of gender.
Exclusion Criteria: Patients who underwent surgery with a diagnosis of intracranial infection or abscess, patients with known autoimmune and inflammatory disease. Patients who underwent burr-hole surgery alone due to hydrocephalus, and patients who underwent burr-hole craniotomy due to chronic subdural effusion.
Data Collection
Age, gender, reason for surgery, systemic inflammatory response markers, duration of intensive care unit stay, and blood laboratory tests were retrospectively evaluated in patients who underwent craniotomy with the diagnosis of intracranial mass, intracerebral haemorrhage or posttraumatic haemorrhage in the neurosurgery intensive care unit of our hospital in 2024 (January-December). Of these variables, CRP value was recorded preoperatively and, on the 2nd, postoperative day. Hemogram, coagulation tests: Pt- aPTT-INR, kidney (GFR, Creatinine) and liver (AST, ALT) function tests were recorded on the 2nd postoperative day. The duration of intensive care unit stays and the feeding methods (enteral, total parenteral nutrition) during this period were recorded. The surveys (Discharge, Exitus) at the end of the hospitalization period were recorded.
Statistical Analysis
Data were recorded and analyzed using SPSS 22 programme. We used frequencies, percentages, mean values, standard deviations, median values, and maximum and minimum values for descriptive statistics. For statistical analysis of categorical data, Fisher’s Exact test was used for Pearson’s chi-square (continuity correction) values below 5. The Kolmogorov-Smirnov test was used to check the data’s conformity to a normal distribution. Because quantitative data did not conform to a normal distribution in independent groups, the Mann-Whitney U test was used for two groups and the Kruskall-Wallis test (post hoc: Dunn’s test) was used for groups greater than two. Statistical significance was considered at p<0.05.
Results
Of the 144 patients included in the study, 52 (36.1%) were female and 92 (63.9%) were male. The mean age was 57.03±17.51 (min-max: 18-80) years. The majority of patients (n: 93, 64.6%) were diagnosed with malignancy, while 30 patients (20.8%) had trauma-related bleeding and 21 patients (14.6%) had spontaneous bleeding. Thirty-five (24.3%) of the patients died, and 109 (75.7%) were discharged. Total Parenteral Nutrition (TPN) was the preferred feeding method for 9 (6.3%) patients. General patient information and some variables that may be related to their progression are presented in Table 1. There was no significant difference in survival between genders and age groups (p>0.05). Mortality in patients fed TPN was lower in those with a high history of malignancy (p<0.05) (Table 1). Patients with mortality as a progression had a higher number of days in the intensive care unit. Hemoglobin and Platelet counts were lower in patients with mortality. PT, APTT, and INR values were significantly higher in patients with mortality.
No significant relationship was observed between systemic inflammatory index (SII) and mortality (p=0,670). AST, BUN, and Creatinine values were higher in patients with mortality, while GFR and ALB were lower. Patients with mortality also had higher preoperative and postoperative CRP values, and higher sodium and chloride levels (Table 2). Length of stay was significantly lower in patients undergoing surgery for malignancy. In patients with traumatic bleeding, hemoglobin and platelet counts were significantly lower, while PT, AP, INR, and AST values were higher compared to patients undergoing surgery for malignancy. Preoperative creatinine and CRP values were significantly higher in patients with spontaneous bleeding compared to patients with malignancy, while GFR was lower. Postoperative CRP and Na values were significantly lower in patients with malignancy compared to the other groups. Cl was significantly different between patients with tumor-related and trauma-related bleeding (Table 3).
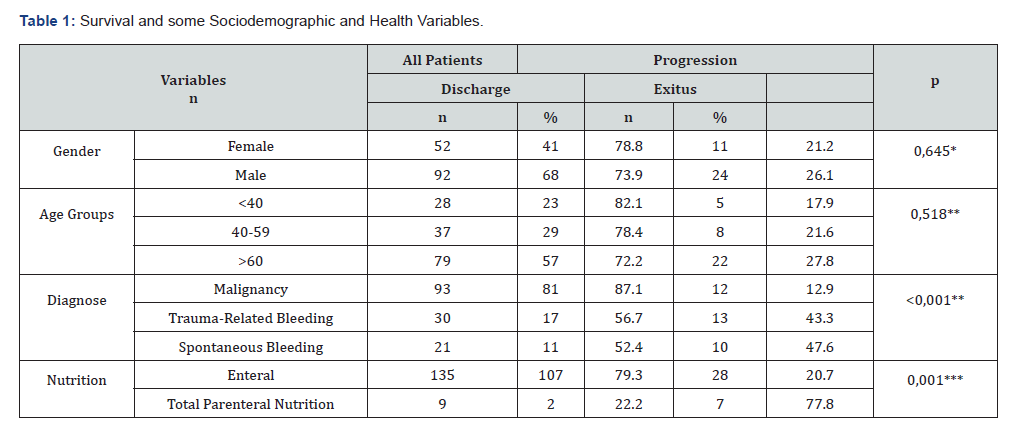
Row Percentage Was Used. *Continuity Correction, ** Pearson Chi Square, *** Fisher’s Exact Test
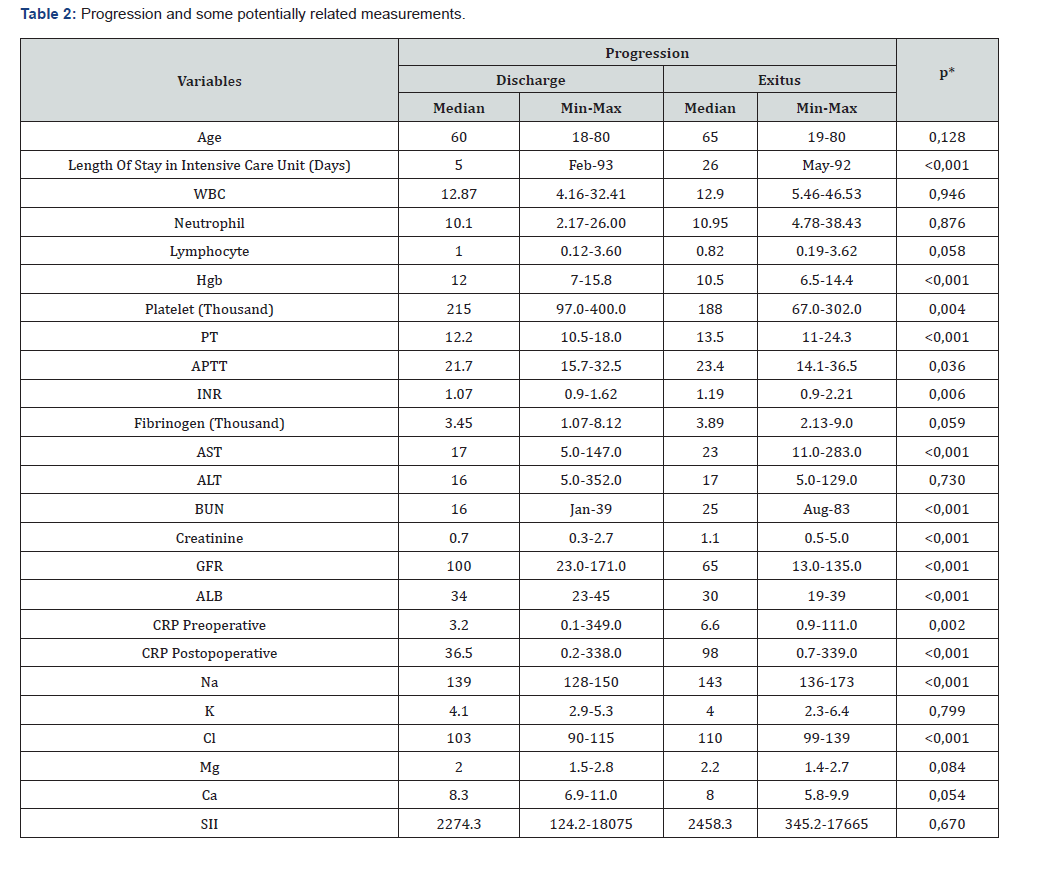
WBC: White Blood Cell, HGB: Hemoglobin, PLT: Platelet, PT: Prothrombin Time, APTT: Activated Partial Thromboplastin Time, INR: International Normalized Ratio, AST: Aspartate Aminotransferase, ALT: Alanine Aminotransferase, BUN: Blood Urea Nitrogen, GFR: Glomerular Filtration Rate, ALB: Albumin, CRP: C-Reactive Protein, NA: Sodium, K: Potassium, CL: Chloride, MG: Magnesium, CA: Calcium, SII: Systemic Inflammatory Index *: Mann Whitney U Test
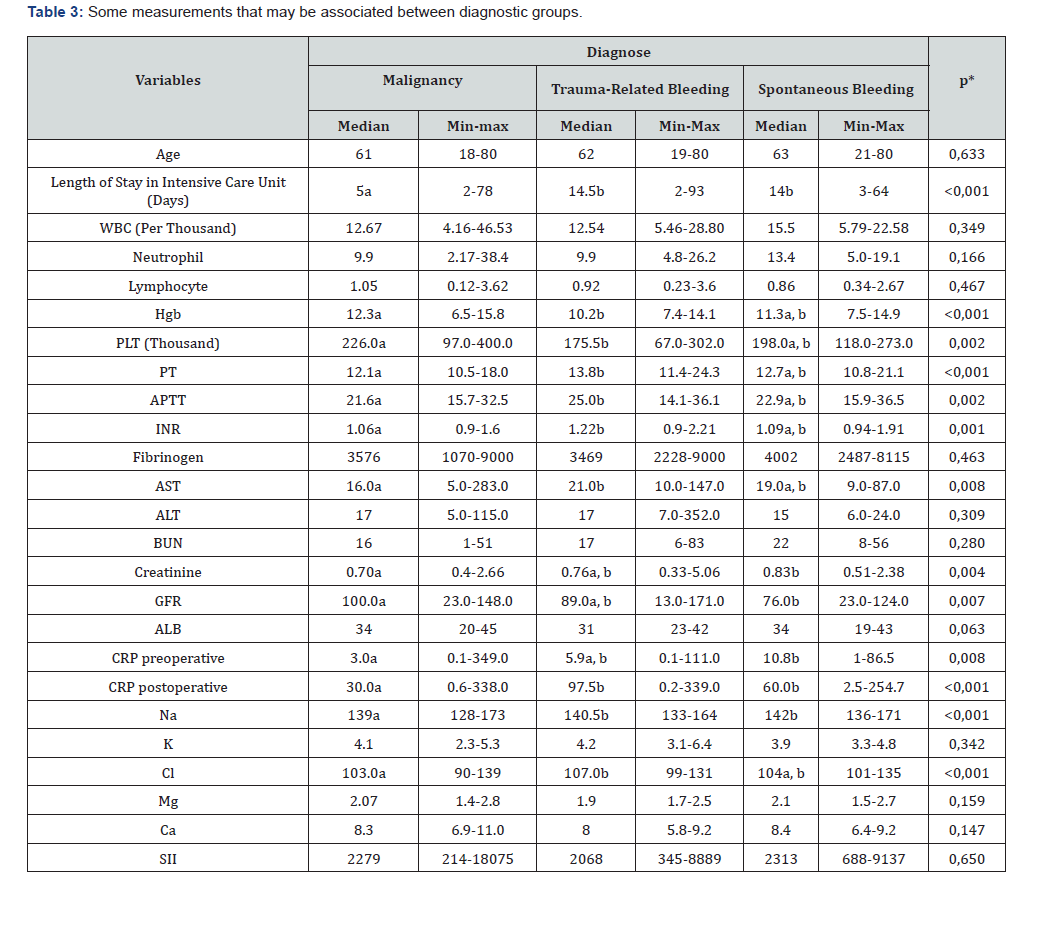
WBC: White Blood Cell, HGB: Hemoglobin, PLT: platelet, PT: Prothrombin Time, APTT: Activated Partial Thromboplastin Time, INR: International Normalized Ratio, AST: Aspartate Aminotransferase, ALT: Alanine Aminotransferase, .BUN: Blood Urea Nitrogen, GFR: Glomerular Filtration Rate, ALB: Albumin, CRP: C-Reactive Protein, NA: Sodium, K: Potassium, CL: Chloride, MG: Magnesium, CA: Calcium, SII: Systemic Inflammatory Index *: Kruskall Wallis Test (Post hoc: Dunn’s Test a, b, The difference between groups that do not have the same letter on the same line is significant)
Discussion
To our knowledge, this is the first report showing the prognostic role of SII in patients who underwent craniotomy for various reasons. This study revealed that SII was an independent significant factor but could not predict the prognosis (discharge or exitus) in patients who underwent craniotomy (p=0,670). There is a strong link between surgical incision, craniotomy, and inflammation in particular. In previous SII studies conducted on patients with various inflammatory diseases or cancer patients, a clear relationship between SII and the disease was frequently detected. For example, Hu B, et al. worked on patients with hepatocellular carcinoma, and he concluded that SII was a powerful prognostic indicator in patients with hepatocellular carcinoma [12]. Again, Hong X, et al. worked on small cell lung cancer and concluded that SII was an independent factor for patients with small cell lung cancer [13]. In our study, although the mortality rate was lower in patients with brain tumour from bleeding patients. We found that SII had no effect on prognosis, i.e. survival. But mortality was higher in patients who underwent craniotomy; were older, and had a longer history of stay in intensive care unit. Length of stay was significantly lower in patients undergoing surgery for malignancy, because tumor patients often undergo elective surgery, preoperative compliance is carefully considered before undergoing a planned operation. However, these prerequisites may not be met in patients with spontaneous or traumatic bleeding requiring emergency surgery. Therefore, hospital stays for tumor-related patients are shorter.
Hgb and PLT values were significantly lower in patients with trauma-related bleeding, which suggests that these data should be evaluated not only in patients with head trauma but also in patients with multiple trauma. Patients undergoing traumarelated surgery also have higher PT, APTT, and INR values than those undergoing tumor surgery, suggesting that tumor surgery is performed under more selective conditions. The lower creatinine and higher GFR in malignant patients compared to those undergoing bleeding-related surgery may be due to the consultation with the internal medicine clinic before surgery and the provision of optimal preoperative conditions. Because the Na and Cl balance in bleeding patients is adjusted under emergency conditions, a higher level in tumor patients is a predictable and preventable outcome. Maintaining fluid and electrolyte balance is crucial, as mortality rates are higher in bleeding patients. However, when nutrition was evaluated, the aim of nutritional support is to reduce the negative effects of critical illness on nutritional status; it can positively influence outcomes and prevent or reverse malnutrition [14,15]. It is also extremely important to start nutritional therapy as early as possible and certainly within the first week of critical illness. In our study, mortality was found to be higher in patients who underwent craniotomy and were 60 years of age or older and were fed with TPN. While 80.4% of our patients fed enterally were discharged, 28.6% of our patients fed TPN were discharged. Enteral, that is, natural nutrition, gave much better results in terms of prognosis. In or study, low albumin levels have also been found to have a negative effect on prognosis.
In studies conducted on various diseases accompanied by inflammation, SII was found to be significantly effective on prognosis. Liu B, et al. found that when SII is higher than 578.25, the risk of rheumatoid arthritis will increase significantly [16]. Various studies have been conducted to reveal the relationship between SII and diseases such as psoriatic arthritis and ankylosing spondylitis, which are accompanied by systemic inflammation. Keleşoglu, et al. showed that SII levels were significantly higher in PsA patients with moderate to severe disease (p<0.001) in 106 psoriatic arthritis patients [17]. Wu, et al. confirmed that SII was increased in patients with ankylosing spondylitis [18]. They also stated that SII could be a new indicator to monitor disease activity in AS. Systemic immune-inflammation index (SII) is an indicator used to assess the degree of systemic inflammation in an individual. It is calculated by platelet count, neutrophil count, and lymphocyte count (PLT×N/L ratio) [12]. Some researchers think that it can be used as an indicator of the progression of a certain disease. The study by Li, et al. suggested that high SII levels may increase overall mortality and cardiovascular disease mortality in the general population [19]. He L, et al. found that increased SII was associated with poor survival in patients with atherosclerotic cardiovascular disease [20]. Guo, et al. found that SII levels were associated with the development of diabetic kidney disease [21]. In fact, SII can be used to follow the prognostication of many diseases and can be a low-cost and simple method. Adanır, et al. found that SII levels were significantly higher in patients with ulcerative colitis and similarly higher in in patients with active disease. They found that SII levels were statistically significantly higher in those with widespread colitis [22].
The main component of the biological response to craniotomy is the induction of inflammation. This type of immune response occurs through the release of intracellular antigens from leukocytes [10]. Craniotomy and surgery of malignant patients are usually planned and controlled. The most used non-invasive method for the evaluation of the inflammatory process is the CRP level [22]. However, in our patients who come to craniotomy with spontaneous or traumatic bleeding, the immune response and CRP increase due to stress may be present preoperatively. However, in our study, the inflammatory response and CRP levels were found to be higher than normal on the second postoperative day in all groups and were shown to have an effect on prognosis. Despite this, it was observed that the calculated SII value had no effect on prognosis. (p=0, 670). Also, in our study, impaired renal function tests, liver tests, and high prothrombin time increased mortality (<0,001).
Limitations of The Study
This study was conducted for only one year and the limited number of patients meeting the criteria constitutes the limitations of the study. In addition, since it is a single-centre study, it would not be right to generalize. Our own clinical knowledge and experiences were intended to be conveyed to the readers.
Conclusion
Neurosurgical intensive care usually hosts individuals whose general condition has suddenly deteriorated and who are in deep coma with a low Glasgow Coma Score. In the evaluation, followup and treatment of patients, ensuring hemodynamic, metabolic and neurological stability is of vital importance. Although we say that SII has no effect on prognosis, our study has yielded very important findings that will affect the prognosis. New treatment algorithms and paradigms are still very much needed. Our work continues to monitor the prognosis of patients who have undergone craniotomy and to level up of treatments for improving their surveys.
Ethical Approval
Ethical Approval for this Study (Approval Number: 273/10.12.2024) was provided by the Ethical Committee of Kayseri City Hospital, .
Contribution of Authors
Exp. Dr. ŞG has contributions detailing the work; Project Preparation, Data Collection and Writing. Exp.Dr.BO did the statistical analysis. Full approval of the manuscript by all authors should be explicitly stated by including the following statement.
References
- Yang R, Chang Q, Meng X, Gao N, Wang W, et al. (2018) Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J Cancer 9(18): 3295-3302.
- Zhong JH, Huang DH, Chen ZY (2017) Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget 8(43): 75381-75388.
- Nakamura T, Matsumine A, Matsubara T, Asanuma K, Uchida A, et al. (2013) The combined use of the neutrophil-lymphocyte ratio and C-reactive protein level as prognostic predictors in adult patients with soft tissue sarcoma. J Surg Oncol 108(7): 481-485.
- Karakiewicz PI, Hutterer GC, Trinh QD, Jeldres C, Perrotte P, et al. (2007) C-reactive protein is an informative predictor of renal cell carcinoma-specific mortality: a European study of 313 patients. Cancer 110(6): 1241-1247.
- Jiang N, Deng JY, Liu Y, Ke B, Liu HG, et al, (2014) The role of preoperative neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after radical resection for gastric cancer. Biomarkers 19(6) :444-451.
- Sharaiha RZ, Halazun KJ, Mirza F (2011) Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 18(12): 3362-3369.
- Sato H, Tsubosa Y, Kawano T (2012) Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg 36(3): 617-622.
- Dinesh Rao, Rebecca Tuan Le, Peter Fiester, Jeet Patel, Gazanfar Rahmathulla, et al. (2020) Journal of Clinical Imaging Science. Journal of Clinical Imaging Science 10(81).
- Liu S, Wang B, Li S, Zhou Y, An L, et al. (2011) Immune cell populations decrease during craniotomy under general anesthesia. Anesth Analg 11393): 572-577.
- Chen GY, Nunez G (2010) Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10(12): 826-837.
- Pinosky ML, Fishman RL, Reeves ST, Harvey SC, Patel S, et al. (1996) The effect of bupivacaine skull block on the hemodynamic response to craniotomy. Anesth Analg 83(6): 1256-1261.
- Hu B, Yang XR, Xu Y, Sun YF, Sun C, et al. (2014) Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 20(23): 6212-6222.
- Hong X, Cui B, Wang M, Yang Z, Wang L, wet al. (2015) Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med 236(4): 297-304.
- McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, et al. (2016) Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N). J Parenter Enter Nutr 40(2): 159-211.
- Singer P, Reintam A, Berger MM, Alhazzani W, Calder PC, et al. (2019) ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 38(1): 48-79.
- Liu B, Wang J, Li Y, Li K, Zhang Q, et al. (2023) The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from NHANES 1999-2018. Arthritis Res Ther 25(1): 34.
- Kelesoglu Dincer AB, Sezer S (2022) Systemic Immune Inflammation Index as a reliable disease activity marker in psoriatic arthritis. J Coll Physicians Surg Pak 32(6): 773-778.
- Wu J, Yan L, Chai K (2021) Systemic Immune Inflammation Index is associated with disease activity in patients with ankylosing spondylitis. J Clin Lab Anal 35(9): e23964.
- Li H, Wu X, Bai Y, Wei W, Li G, et al. (2021) Physical activity attenuates the associations of systemic immune inflammation index with total and cause specific mortality among middle aged and older populations. Sci Rep 11(1): 12532.
- He L, Xie X, Xue J, Xie H, Zhang Y, et al. (2022) Association of the systemic immune inflammation index with all-cause mortality in patients with arterioscle rotic cardiovascular disease. Front Cardiovasc Med 9: 952953.
- Guo W, Song Y, Sun Y, Du H, Cai Y, et al. (2022) Systemic immune inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011 2018. Front Endocrinol (Lausanne) 13: 1071465.
- Adanır H, Akıncıoğlu P (2022) The importance of the systemic immune-inflammation index in ulcerative colitis. Dicle Med J 49 (3): 521-528.






























