Abstract
The shortage of available organs for transplantation has led to an increase in the utilization of organs from donors who are dead by cardiac criteria, rather than dead by neurological criteria. The process of salvaging organs from patients who suffered a cardiac arrest from which they could not be resuscitated is an uncontrolled donation after cardiac death (uDCD). This involves placing the donor on extra-corporeal membrane oxygenation (ECMO), to provide circulation for the abdominal organs to be salvaged. In some cases, the flow which the ECMO system can deliver may be too low due to hypovolemia, large volume of distribution, extreme vasodilation, or ongoing fluid loss. We describe a case in which the application of a unique device to exsanguinate the legs and block the return of blood to the legs (arterial tourniquet) led to a substantial increase in ECMO flow, thus improving the perfusion of the abdominal organs.
Keywords:Donation After Circulatory Death; Uncontrolled DCD; Normothermic Regional Perfusion; ECMO Auto-Transfusion Tourniquet (or Exsanguination Tourniquet)
Abbreviations:ECMO: Extra-Corporeal Membrane Oxygenation; DCD: Donation After Circulatory Death; NRP: Normothermic Regional Perfusion; EBOA: Endovascular Aortic Balloon
Introduction
The scarcity of organs for transplantation has led to an increase in the utilization of organs from patients whose death was determined by cardiac criteria (DCD). Essentially, the process of saving organs from donors whose mechanism of death is cardiac, and not death by neurological criteria, can be done by one of two tracks. Controlled DCD, whereby a patient with a devastating unrecoverable injury, neurologic or otherwise, after consent of next of kin, is taken to the OR, disconnected from mechanical ventilation, and once cardiac arrest ensues, is placed on ECMO for organ support until the organs can be harvested for transplantation. A second process is uncontrolled DCD. In this scenario, a patient who suffered a cardiac arrest and the treating team realize that ongoing CPR is futile and declare death. In this scenario, there is a brief time period during which the family can be approached regarding consent for organ donation. If they agree, the patient can be placed on an ECMO system to provide perfusion for the abdominal organs, the liver, and kidneys, to reduce the warm ischemic time as much as possible. This is termed Normothermic Regional Perfusion (NRP). Utilizing uDCD may have a significant beneficial impact on the availability of organs [1-3].
The NRP is performed in a manner that will optimize blood flow to the abdominal viscera and will not perfuse the upper body. Perfusion of the brain and coronaries is prevented by inflating an endovascular balloon in the aorta above the diaphragm so that the arterial blood flow is obstructed at that level. The optimal flow of the NRP is 2.0-3.0 LPM [4]. However, this is not always achievable. When there is a low flow, fluids are often required to increase the preload for the NRP circuit. This may lead to organ edema and possibly impact graft function [5]. We describe a unique method of increasing the effective core blood volume in this scenario, using a device to provide a tourniquet after draining the blood volume of the legs into the central circulation.
Case Report
A 50-year-old man collapsed while engaging in sports. Bystander CPR was immediately initiated. An EMS team was called and arrived within 5 minutes. Advanced life support was performed with chest compressions, multiple attempts at defibrillation for VF, medications, and intubation. He was brought to the ER while mechanically ventilated and with mechanical chest compressions (LUCAS 3, Jolife AB, Lund, Sweden) Despite prolonged CPR, there was no return of spontaneous rhythm, and the patient was declared dead. Since he was a donor card carrier, his family immediately agreed to organ donations, and cannulation was performed for NRP. Cannulation was difficult because of the patient’s anatomy. A right venous femoral cannula was inserted to the level of the right atrium. A left arterial cannula was inserted to the level of the mid-abdominal aorta. He was connected to an ECMO machine.
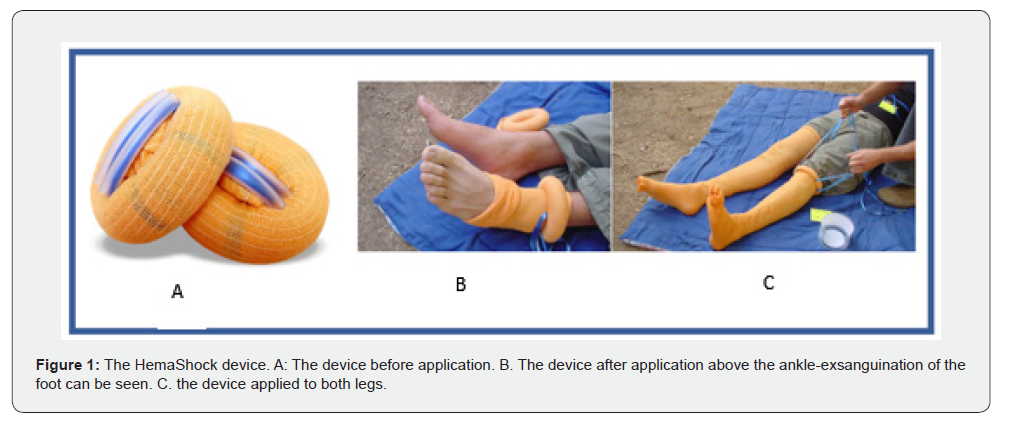
An endovascular balloon was inserted into the aorta via the right femoral artery and was inflated at the level just above the diaphragm. Its location at the level of the diaphragm was verified by x-ray. When the ECMO machine flow was initiated, the maximal flow was only 0.8-1.0 LPM. We administered a balanced salt solution with albumin, but the response was minimal. On examination, the patients’ legs also appeared perfused, despite ligatures on both femoral arteries, as evidenced by their color and venous filling. To improve the central blood volume and decrease flow to the legs, we used a device that squeezes all the blood out of a limb and then obstructs the blood flow, acting as a tourniquet (HemaShock, OHK Medical Devices Ltd. Tirat Carmel, Israel) (Figure 1). When the device was applied to the first leg, the flow in the ECMO system immediately increased from 1.1 LPM to 1.8 LPM. Applying the device to the other leg increased flow to 2.8 and intermittently even 3.0. (Figure 2). The application of the HemaShock on each leg took less than 30 seconds. The patient was taken to the OR where the kidneys were procured. One kidney was anatomically unfit for transplantation. One kidney was transplanted and has recovered good renal function.
Discussion
Organ transplantation represents the optimal and often the sole therapeutic intervention for individuals with end-stage organ failure. However, its widespread application is hampered by the limited availability of donor organs. While most transplants currently utilize organs from brain-dead individuals, the global shortage of donors has spurred the exploration of alternative strategies to expand the donor pool. This has led to a focus on donation after circulatory death (DCD). In contrast to controlled donation after circulatory death, uncontrolled donation refers to a donation from a person who dies following an unexpected and unsuccessfully resuscitated cardiac arrest. A recent European study across 27 countries highlighted the significant potential of uncontrolled DCD (uDCD), revealing that within a single month, 7142 patients experienced out-of-hospital cardiac arrest requiring advanced cardiopulmonary resuscitation. Return of spontaneous circulation occurred in 28.6% of these cases. These numbers emphasize the potential of uDCD donors [6]. Despite this considerable potential, uDCD remains uncommon in Europe and elsewhere. In addition to ethical issues [7], there are concerns regarding the quality of organs which have been subjected to prolonged periods of ischemia, during the no-flow, and low flow periods in the process of uDCD.
Nonetheless, while organs procured from uDCD donors exhibit a higher incidence of primary nonfunction and delayed graft function, overall patient and graft survival rates in kidney, liver, and lung transplantation are acceptable [4]. This is due to the use of Normothermic Regional Perfusion (NRP). NRP involves the selective perfusion of abdominal and thoracic organs, achieved by occluding major blood vessels, usually using an endovascular aortic balloon (EBOA). In situ NRP, pioneered in Spain [8], counteracts the detrimental effects of ischemia by replenishing ATP reserves and reducing oxygen free radicals [4]. Maintaining adequate pump flow throughout NRP is, therefore, essential for successful DCD donation. However, insufficient pump flow during NRP is a prevalent and clinically significant challenge. This inadequacy can arise from drainage issues, pump outflow, or donor-related factors such as vasoplegia, hypovolemia and ongoing fluid losses. All these factors can contribute to suboptimal in-situ organ preservation and subsequent graft dysfunction [5]. Another parameter contributing to low flow is fluid leakage, a consistent complication of DCD in-situ organ preservation. The combination of hydrostatic pressure and impaired endothelium resulting from death-related processes, compounded by the inflammatory stress from the extracorporeal device, leads to increased vascular permeability. While the use of oncotic fluids (e.g., albumin or colloids) may sometimes counteract this, the application of colloid solutions remains controversial, particularly regarding their impact on kidney function [5].

In our patient, a unique kind of stocking-HemaShock-was applied to the patient’s legs, elevating the blood flow perfusing the abdominal organs. Once applied to the first leg, the pooled blood it contained was drained upwards, immediately increasing the flow rate from 1.1 LPM to 1.8 LPM. The other leg added yet another 1-1.2 liters, bringing the total to 3.0 LPM. This flow rate improved organ perfusion during the period before organ procurement. HemaShock, an elastic silicone ring encased in stockinet fabric, is indicated for patients experiencing a very low systolic blood pressure due to hemorrhagic shock or cardiac arrest. Here we suggest a novel utilization of this device. As we applied the HemaShock tourniquet to the patient’s lower limbs, it promptly elevated the effective circulatory blood volume with the patient’s own fresh blood. By acting as an auto-transfusion device, we redirected a substantial amount of pooled blood towards the core organs [9], thus improving the effective volume for the ECMO circuit and allowing for a significant increase in blood flow. In addition, by impeding the arterial blood flow to the legs, there is an immediate and direct decrease in the circulatory bed perfused by the blood flow generated by the ECMO machine. These two complementary effects -increased volume and flow and reduced vascular bed -led to an improved perfusion of the abdominal organs. The HemaShock device has been shown to increase blood volume in volunteers [10], and is also used to decrease blood loss during orthopedic surgery [11,12]. In our case, the unique implementation of the HemaShock stockinet in the setting of uDCD for the salvage of core organs is the first report of its kind. It enabled a swift and efficient increase in ECMO flow without requiring external fluid administration. Further research should be done to fully evaluate its potential in improving organ perfusion in uDCD.
Data Availability Statement: The data supporting the findings of this study are available from the corresponding author upon request. The authors declare no conflict of interest. The research did not receive specific funding.
References
- Chocron R, Laurenceau T, Soumagnac T, Beganton F, Jabre P, et al. (2024) Potential kidney donors among patients with out-of-hospital cardiac arrest and a termination of resuscitation rule. Resuscitation 201: 110318.
- D’Aragon F, Lachance O, Lafleur V, Ortega-Deballon I, Masse MH, et al. (2022) Program of Uncontrolled Donation After Circulatory Death as Potential Solution to the Shortage of Organs: A Canadian Single-Center Retrospective Cohort Study. Open Access Emerg Med 14: 413-420.
- Río F del, Andrés A, Padilla M, Sánchez-Fructuoso AI, Molina M, et al. (2019) Kidney transplantation from donors after uncontrolled circulatory death: the Spanish experience. Kidney Int 95(2):420-428.
- Richards JA, Gaurav R, Butler AJ, Watson CJE (2022). The 6 C’s of normothermic regional perfusion. Progress in Transplantation, 32(2): 192-193.
- Squiccimarro E, Colombaro C, Civita A, Rociola R, Buys D, et al. (2023) Addressing inadequate blood flow during normothermic regional perfusion for in-situ donation after circulatory death grafts preservation. Perfusion 38(1_suppl): 54-58.
- Gräsner JT, Lefering R, Koster RW, Masterson S, Böttiger BW, et al. (2016) EuReCa ONE 27 Nations, ONE Europe, ONE Registry: A prospective one-month analysis of out-of-hospital cardiac arrest outcomes in 27 countries in Europe. Resuscitation 105: 188-195.
- Wall A, Testa G (2024) The ethics surrounding normothermic regional perfusion in donors following circulatory death. Clin Liver Dis 23(1): e0193.
- Hessheimer AJ, Coll E, Torres F, Ruíz P, Gastaca M, et al. (2019) Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J Hepatol 70(4): 658-665.
- Tang D, Olesnicky, Eby M, Heiskell (2013) Auto-transfusion tourniquets: the next evolution of tourniquets. Open Access Emerg Med 5: 29-32.
- Gavriely O, Nave T, Sivan A, Shabtai-Musih Y, Gavriely N, Dsc, et al. (2004) Auto Transfusion and Blood Pressure Elevation by Elastic Leg Compression in Normal Subjects.
- Bourquelot P, Levy BI (2016) Narrow elastic disposable tourniquet (Hemaclear®) vs. traditional wide pneumatic tourniquet for creation or revision of hemodialysis angioaccesses. J Vasc Access 17(3): 205-209.
- Brin YS, Feldman V, Ron Gal I, Markushevitch M, Regev A, Stern A, et al. (2015) The Sterile Elastic Exsanguination Tourniquet vs. the Pneumatic Tourniquet for Total Knee Arthroplasty. J Arthroplasty 30(4): 595-599.






























