Abstract
Postoperative pain, a post-surgery unwanted event contributing to functional limitations and psychological trauma, has become a significant clinical issue as more patients develop chronic pain after surgery. Opioids have become a gold standard in the management of severe pain; however, their use for the long-term in treating chronic pain is associated with side effects, including respiratory and gastrointestinal issues and complications, including addiction, drowsiness, dry mouth, urinary retention, and increased blood sugar levels. Non-opioid medications, physical therapy, occupational therapy, acupuncture, chiropractic, and cognitive behavioral therapy are used to decrease chronic pain. However, there is a need for other strategies to reduce opioid dependence postoperatively. Research on peripheral nerve blocks (PNBs), a procedure of injecting local anesthetics around the nerve, offers a promising alternative approach. This review explores various PNBs, their usage in decreasing opioid use, benefits, patient satisfaction, and limitations, followed by a discussion on increasing and optimizing PNB utilization in the clinical setting. PNBs offer a promising alternative to opioids for managing postoperative pain, significantly reducing opioid consumption, pain scores, and associated side effects. By integrating PNBs into multimodal analgesia strategies, healthcare providers can mitigate the risks of long-term opioid dependence, enhance patient outcomes and address a critical aspect of the ongoing opioid epidemic.
Keywords:Post-Operative Pain; Opioid Use; Peripheral Nerve Blocking; Alternative Approach
Abbreviations:PATHOS: Postoperative Analgesic Therapy Observational Survey; HPA: Hypothalamic-Pituitary-Adrenal; OE: Opioid Endocrinopathy; OPIAD: Opioid-Induced Androgen Deficiency; LH: Luteinizing Hormone; PNBs: Peripheral Nerve Blocks (PNBs); SPNB: Single-Injection Peripheral Nerve Block; CPNB: Continuous Peripheral Nerve Blocks; LAST: Local Anesthetic Systemic Toxicity; TAP: Transversus Abdominis Plane; MME: Morphine Milligram Equivalent; LOS: Length of Stay; RCTs: Randomized Controlled Trials; CACB: Continuous Adductor Canal Block; OFA: Opioid-Free Anesthesia; VAS: Visual Analog Scale
Introduction
The opioid epidemic represents a major public health crisis, exacerbated by the overprescription of opioids after surgical procedures, which can lead to prolonged use. An observational study evaluating the differences in risk of long-term opioid therapy after surgery among an opioid-naive population reported dramatically increased amounts of opioids prescribed for postoperative pain after surgery between 2005 and 2015, suggesting an increased opioid need for a longer duration [1]. Further, Postoperative Analgesic Therapy Observational Survey (PATHOS), a prospective, cross-sectional, observational, multicenter practice survey, conducted in 7 European hospitals between 2004-2005, concluded that current post-operative pain management remains suboptimal and there is a need to improve the strategies [2]. A hospital-based study by Ndebea et al. [3] reported that approximately 70% of patients who underwent general and orthopedic surgery reported experiencing moderate to severe pain within the first 48 hours postoperatively, with 15% to 16% of these cases with severe pain. In an Ethiopian cohort, 91.4% experienced intense post-operative pain [4]. A Western Cape hospital reported that 62% of patients undergoing surgery had moderate to severe pain [5]. A retrospective study by Oderda et al. [6] found that around 95% of over 300,000 patients undergoing surgery received opioid therapy across 380 US hospitals. These reports reflect the extent of this issue and the need for alternative strategies.
The mounting issue of postoperative opioid use in previously opioid-naïve patients has been studied using prescription databases. Alam et al. [7] reported that 7.7% of 391,139 opioidnaïve patients were still using opioids a year after minor surgeries and that 3.1% of 39,140 opioid-naïve patients filled opioid prescriptions 1-180 days post-abdominal, pelvic, or thoracic surgeries [7]. Brummett et al. [8] reported that 6% of 36,177 opioid-naïve patients filled opioid prescriptions 90-180 days after various surgeries, compared to 0.4% of 492,177 non-surgical controls. The issue is growing; Wunsch et al. [9] found an increase in opioid prescriptions from 75% to 81% in the first week postminor surgery between 2004 and 2012. Raebel et al. [10] noted that 8% of 11,719 bariatric surgery patients were opioid users before surgery, with 723 continuing a year later. Prospective studies also show ongoing opioid use post-surgery. Caroll et al. [11] found that 6% of 109 opioid-naïve patients continued opioid use 150 days post-surgery. Another study found 8.2% and 4.3% of 574 opioid-naïve patients continued opioid use six months after knee and hip arthroplasty, respectively. Armaghani et al. [12] reported that 26% of opioid-naïve patients continued opioid use one year after elective spine surgery. These findings highlight the persistent challenge of postoperative opioid use among initially opioid-naïve patients.
Opioids are favored because they effectively alleviate moderate to severe postoperative pain, have no ceiling effect, and come in various formulations. Their extensive history of use, familiarity, and accumulated clinical experience contribute to their prevalence. However, opioids have numerous dose-limiting side effects, ranging from nausea, vomiting, and constipation to oversedation, somnolence, and respiratory depression, which can be life-threatening [6]. Elderly patients, those with sleep apnea, the obese, and smokers are particularly at risk for oversedation and respiratory depression. Patients may reduce their opioid intake to avoid these side effects, potentially resulting in inadequate pain relief. This results in increased overall healthcare costs, longer hospital stays, and lower survival rates during in-hospital resuscitation. Despite these issues, opioid use has not decreased; in fact, it has risen in recent years in both inpatient and outpatient settings [13]. Despite strong evidence of their drawbacks, opioids remain the choice of postoperative pain treatment. Mounting evidence of long-term opioid side effects warrants an alternate strategy with minimal side effects for post-surgical pain to improve clinical outcomes. This review explores the role of PNBs in mitigating opioid consumption following surgery, aiming to identify and summarize the existing evidence on the reduction of opioid use.
Opioid Use: Side effects and complications
Opioid use has been shown to induce suppression of cellular immunity and reduced resistance to bacterial infection, contributing to higher infection rates in heroin addicts and potentially affecting HIV pathogenesis. Exogenous opioids can contribute to immunosuppression through central and peripheral mechanisms, including effects on the hypothalamic-pituitaryadrenal (HPA) axis and autonomic nervous system. Endogenous opioids like endorphins induce immune activation. Immune cells can release endogenous opioids and express opioid receptors, facilitating a dynamic interaction that modulates immune and inflammatory responses [14]. Opioid usage can also lead to opioid endocrinopathy (OE) or opioid-induced androgen deficiency (OPIAD), affecting hormonal function in both men and women. This hormonal disruption includes reduced levels of testosterone, estrogen, luteinizing hormone (LH), and other hormones, leading to side effects such as sexual dysfunction, depression, and decreased energy. In men, chronic opioid use results in lower testosterone levels, potentially contributing to hypogonadism. In women, opioids can cause hormonal imbalances, increasing the risk of osteoporosis and fractures, particularly in elderly populations. Although hormone replacement therapies show some promise in mitigating these side effects, further large-scale randomized controlled studies are warranted to evaluate their efficacy and safety [15].
Regarding managing the post-surgical pain, opioids have been a main staple in pain management as they do an excellent job of decreasing pain and improving patient outcomes; however, that does not come without side effects. Common side effects include nausea, vomiting, constipation, sedation, dizziness, physical dependence, tolerance, and respiratory depression, with addiction being the biggest issue [6]. Delayed gastric emptying, muscle rigidity, myoclonus, hyperalgesia, and immunologic and hormonal dysfunction are other less common side effects. Among these side effects, physical dependence and addiction are of clinical concern, posing hurdles in prescribing opioids, contributing in turn to inadequate pain management [15]. Another common issue with opioid use is tolerance. Over time, the reduction in effectiveness leads to increased dosage requirements and inadequate pain relief. This can later lead to physical dependence, causing the body to adapt to the opioids, consequently inducing withdrawal upon cessation [16].
Up to 80% of patients who use opioids experience constipation, with nausea in 40% of patients and vomiting in 15- 24% of patients, respectively [17,18]. Opioid-induced respiratory distress, a much more severe complication, occurs between 0.3% and 21% of patients [19]. Over 80% of patients receive opioids after low-risk surgery, and over 60% of people with 90 days of continuous opioid therapy remain on opioids years later. Patients receiving an opioid prescription after short-stay surgeries have a 44% increased risk of long-term opioid use [20]. Opioid usage has increased; nerve blocks and multimodal analgesia may be used to decrease the dependence on opioids. PNBs, a procedure of injecting local anesthetics around the nerve, offer a promising alternative approach. When examining 609,991 patients who underwent total knee arthroplasty, patients who were given PNBs had a decreased consumption of morphine, a commonly used opioid, compared to patients who did not receive PNBs [21]. However, not every surgery has seen similar results. When looking at 6695 patients who underwent shoulder arthroplasty, there was no statistically significant difference in opioid use between patients who had nerve block and those who did not [22]. This suggests the need for a better understanding and future research to optimize the protocols for improved clinical outcomes using PNB.
Surgical Anesthesia vs. Analgesia
Surgical anesthesia and analgesia are distinct but related concepts in pain management during and after surgery. Surgical anesthesia results in a complete sensory-motor block with more concentrated local anesthetics (e.g., 0.5% ropivacaine) and provides a state of unconsciousness, amnesia, and immobility. However, analgesia specifically addresses pain relief without necessarily inducing a loss of consciousness. Surgical analgesia prefers nociceptive blockade at lower concentrations (e.g., 0.2% ropivacaine) to preserve motor function for early rehabilitation. Anesthesia often includes analgesia, but analgesia can also be achieved independently using regional or local anesthesia techniques [23-25].
Peripheral Nerve Blocks
PNB, a regional anesthesia technique where a local anesthetic is injected near a peripheral nerve to numb a specific area of the body, blocking pain signals from reaching the brain. PNB used for surgical anesthesia and pain management offers advantages like reduced opioid use, faster recovery, and fewer side effects compared to general anesthesia. The nerve block can be administered as a single injection or with a catheter for continuous pain relief. Ultrasound or nerve stimulation may be used to guide needle placement for accuracy. Depending on the medication and technique used for nerve block, numbness and pain relief can last from a few hours to several days [26]. Cardwell et al. [27] concluded that perioperative peripheral nerve blocks (PNBs) significantly reduce opioid consumption both during surgery and in the postoperative period, with the most pronounced reduction observed within the first 24-72 hours after surgery. Additionally, patients receiving PNBs report lower pain scores compared to those who do not receive these blocks.
Single-Shot Peripheral Nerve Blocks
A single-injection peripheral nerve (sPNB) block is a one-time procedure of injecting numbing medication into a nerve or nerve plexus. The duration of its effect, which typically ranges from 3 to 18 hours, depends on the medication used, the injection site, and individual response. This procedure is intended to reduce surgical pain but may not eliminate it. While effective for shorter surgical procedures, sPNB may require supplemental pain management for longer-lasting pain [28].
Continuous Peripheral Nerve Blocks (CPNB)
CPNB involves the percutaneous insertion of a catheter near a peripheral nerve to continuously deliver a local anesthetic, providing prolonged pain relief after surgery or trauma. CPNBs are a valuable tool in acute pain management, offering benefits like reduced opioid use and improved patient recovery. Catheters can be plain plastic, echogenic, stimulating, or both echogenic and stimulating. Echogenic catheters have become increasingly popular due to their ability to be precisely placed near target nerves in real time [29]. In trauma care, CPNBs are valuable for managing pain throughout the perioperative period. They aid in patient transport, surgical anesthesia, postoperative analgesia, chronic pain prevention, and sympathetic blocks (e.g., tissue flaps). Patients with complex injuries requiring multiple surgeries, debridement, and/or skin grafting benefit significantly from CPNBs [29]. Compared to single-injection nerve blocks, CPNBs offer prolonged pain relief, while single-injection blocks typically last for a shorter duration. Compared to epidural anesthesia, CPNBs may be a safer alternative, especially in patients at higher risk of complications from epidurals, such as those on anticoagulants [30,31]. Minor complications of CPNBs include catheter dislodgement, occlusion, leakage, and infusion pump malfunction. Serious but rare complications include local anesthetic systemic toxicity (LAST), catheter knotting and shearing, and infection, especially in emergency rooms or intensive care units. Prolonged use of an indwelling catheter increases the risk of infection in trauma patients, while infection odds vary with the type of catheter [32].
Regional Anesthesia
Regional anesthesia numbs a specific part of the body, such as an arm, leg, or the area below the chest, by blocking nerve signals. Regional anesthesia can be used alone or in combination with other anesthetic techniques, like sedation or general anesthesia. Nerve block involves injecting local anesthetic medication near a group of nerves to interrupt pain signals from that area. Regional anesthesia may be spinal anesthesia, epidural anesthesia, or peripheral nerve blocks. Spinal and epidural anesthesia are often used for lower body procedures and pain management during childbirth or after abdominal/chest surgery. While PNBs are used for procedures on specific limbs, such as arm or leg surgeries. Major advantages include reduced postoperative pain, nausea, and the need for opioids [33-35]. Regional anesthesia for the upper limb is achieved by targeting the brachial plexus at various points along its pathway, including the roots (C5-T1), trunks, divisions, cords, and terminal branches. Understanding both the standard anatomy and the anatomic variation of the brachial plexus is crucial for clinicians performing regional anesthesia [36]. Targeting the brachial plexus can be used for clavicular, scapular, and humeral fractures as well as shoulder dislocations. Regional anesthesia for the lower limb can be performed by blocking branches of the lumbar plexus and/or the sacral plexus. Although these plexuses exhibit fewer anatomic variations compared to the brachial plexus, understanding these variations is still essential for clinical practice. Blocking the lumbar or sacral plexuses can be used for hip, knee, tibial, ankle, and foot fractures [37].
Clinical Applications and Anatomical Targets
Peripheral nerve blocks (PNBs) are employed in several anatomical sites to provide localized analgesia and anesthesia. Some examples of common applications are the use of brachial plexus blocks for upper limb surgery (for example, shoulder and elbow surgery), femoral/sciatic nerve blocks for lower limb surgery, and transversus abdominis plane (TAP) blocks for abdominal surgery [24,26]. In interventional radiology, PNBs are utilized for procedures such as radiofrequency ablation of hepatic tumors (paravertebral blocks) and percutaneous nephrolithotomy (intercostal nerve blocks) [24]. Accuracy is enhanced with ultrasound and fluoroscopic guidance, reducing complications like vascular puncture [24,38].
Efficacy of Peripheral Nerve Blocks and Opioid Sparing Effect
PNBs are effective in reducing opioid consumption following surgery or for other painful conditions. This reduction in opioid use can lead to fewer opioid-related side effects and improved patient satisfaction [23]. PNBs are superior to systemic opioids in producing postoperative pain relief, with 30-50% decreases in acute pain scores and a 40% reduction in opioid consumption during the first 24 hours [24]. Intercostal nerve blockade for percutaneous nephrolithotomy significantly reduced visual analog scale (VAS) scores (p<0.05) and reduced postoperative narcotic consumption. Similarly, paravertebral blocks for ablation of liver tumors produced mean pain scores of less than 2/10 in most patients [24]. Opioid-sparing action is important in preventing the risk of dependency and respiratory depression [26]. The reduced opioid consumption via PNBs is evidenced by various studies discussed below. A study with over 600k patients examined the differences in postoperative complications and opioid consumption associated with perioperative PNB utilization during primary total knee arthroplasty [21].
The study found that patients who received PNB exhibited an 8.12% lower average morphine milligram equivalent (MME) exposure compared to those who did not. Patients with a length of stay (LOS) of less than 1 day had higher MME exposure with PNB, while those with a 1-day LOS had lower overall and postoperative day (POD) zero opioid exposure. For LOS of 2 days, opioid exposure was similar overall but higher on POD1 and POD2 for PNB patients. For LOS of 3 days, PNB patients had lower POD0 but higher POD1, POD2, and POD3 opioid exposure. A meta-analysis of 19 articles concluded that CPNB extends postoperative pain relief beyond the duration of single injection techniques, making them beneficial in both outpatient and inpatient settings. A metaanalysis of randomized controlled trials (RCTs) revealed that perineural analgesia with local anesthetics provides significantly better postoperative pain control compared to opioids, with benefits lasting up to three days. CPNBs resulted in fewer side effects such as nausea, vomiting, pruritus, and sedation, and improved patient satisfaction. Although protocols varied, CPNBs consistently demonstrated superior analgesia and reduced opioidrelated side effects. This suggests that CPNBs are effective for managing postoperative pain, offering both enhanced pain relief and fewer complications compared to IV morphine, a traditional opioid analgesia. Further research is needed to optimize protocols and maximize the benefits of CPNB for different surgical and catheter sites [39].
A randomized, double-blind, placebo-controlled study [40] with 50 patients undergoing hip surgery compared a pericapsular nerve group using 25 mL of 0.5% ropivacaine with saline control for pain management. The study reported a significantly lower total opioid consumption 24 h postoperatively (440.72 ± 242.20 μg vs. 611.07 ± 313.89 μg) in the pericapsular nerve group compared to the control group. The pain scores at 30 minutes postblock and 6 hours postoperatively were significantly lower in the pericapsular nerve group, while the first opioid demand was significantly shorter in the control group. The study found that the sensory block effectiveness was better in the pericapsular nerve group 30 minutes postblock and 6 and 12 hours postoperatively. These findings suggest that PNB is effective in reducing opioid consumption. Another study compared CPNB (n=82) with singleshot peripheral nerve block (SPNB; n=114) in 196 patients undergoing surgery for anterior cruciate ligament reconstruction [41]. CPNB was delivered via the placement of an elastomeric reservoir ball. The study compared the need for opioid use and improved pain control in children and adolescents. Cryotherapy, oral acetaminophen, and ibuprofen were used for postoperative pain management, and hydrocodone/acetaminophen (5/325 mg) was given for 10 days if needed in case of uncontrolled pain. The study reported that 70% of patients (n=138) did not need opioids at home after surgery, and the remaining 30% (n=58) needed home opioids. Of these, 30.7% (n=35) were in SPNB and 28% (n=23) in CPNB. These results suggest that PNB may attenuate the need for opioid use at home efficiently, and CPNB may be better than SPNB [41]. In addition to these, other systematic reviews discussed the opioid sparing effects of peripheral nerve block or opioid free analgesia [42] (Table 1).

Immediate and significant pain relief, a noticeable reduction in pain scores, improved functional recovery, and a decreased need for opioid analgesics are common indicators of PNB’s efficacy. Reduced incidence of postoperative nausea and vomiting, faster recovery time, and enhanced patient satisfaction are other indicators of PNB’s efficacy. A decreased need for general anesthesia or local anesthetic agents for procedures, a significant decrease in pain intensity compared to those not receiving the block or receiving alternative pain management methods, reduced postoperative nausea and vomiting, faster transition through the recovery phases potentially leading to earlier discharge from the hospital, functional recovery allowing patients to engage in physical therapy and activities sooner by reducing pain and inflammation are characteristics of successful nerve block. Additionally, improved pain control and faster recovery, contributing to higher patient satisfaction with their overall experience and shorter hospital stays, particularly when combined with early mobilization and rehabilitation, are also indicators of successful nerve block efficacy [23,28]. The most notable reduction in opioid consumption is typically seen in the first 24-72 hours post-surgery [27,43-49].
Mechanisms and determinants for PNBs in Reducing Opioid Use
Local Anesthetic Action and Pain Pathway Interruption
PNBs work by injecting a local anesthetic into the area to inhibit impulse transmission distally in a nerve terminal, resulting in terminating the pain signal perceived by the cortex [50]. The local anesthetics, commonly lidocaine, bupivacaine, and ropivacaine, block sodium channels on nerve fibers, preventing depolarization and thus stopping action potentials. This results in a loss of pain and sensation in the targeted area [24]. Nociceptors are specialized sensory neurons that detect injurious stimuli, including temperature, mechanical, and chemical. Nociceptors use voltage-gated sodium channels to transmit the injurious signal from either muscle or skin to the dorsal horn of the spinal cord, which travels to the thalamus [51]. The sodium channel is composed of an alpha subunit along with a beta subunit. The alpha subunit contains four homologous domains (I-IV), each containing six transmembrane segments (S1-S6). Local anesthetics typically bind to the S6 segment of the sodium channel located in the inner pore of domains I, III, and IV during its depolarization stages. This enables local anesthetics to bind to nociceptors that are firing via their voltage-gated sodium channels. This binding will stop sodium from passing through the channel, thus stopping depolarization from propagating action potentials [52].
By blocking the transmission of pain signals from the nerves to the central nervous system, PNBs reduce postoperative pain as the first and most critical point of intervention to reduce the consumption of systemic opioids immediately after surgery [53]. PNB lowers acute postoperative pain and consumption of opioids within the first 24-72 hours after surgery, which is normally the peak period of systemic opioid requirement [27]. In the process of opioid sparing, PNBs contribute through pre-emptive as well as preventive analgesia. Preceding the surgical trauma, the PNBs provide an early interruption of nociceptive input, preventing the central sensitization. Central sensitization is defined as the state of hyper-responsiveness of the central nervous system towards pain after the injury [54]. The nerve blocks not only decrease the intensity and duration of postoperative pain by limiting central sensitization but also reduce the probability of chronic postsurgical pain development, which is a significant aspect of prolonged opioid use. Nerve blocks decrease central sensitization, decreasing the probability of chronic postsurgical pain, a major aspect of prolonged opioid use. Clinical evidence shows that patients are less likely to develop chronic postsurgical pain, thereby reducing the need for opioid usage [53]. The local anesthetics used in PNBs have anti-inflammatory properties, which further reduce pain. These agents can attenuate the release of pro-inflammatory cytokines and suppress excessive inflammatory response. This also serves as a dual mechanism for both immediate and extended analgesic effects [55-58]. When added as a single element of a multimodal analgesic regime, apart from reduced consumption of opioids, improved overall pain management and hence patient outcome, with lower tendencies of opioid dependency and chronic pain syndromes, are achieved [53].
Duration, concentration, and combination of analgesia
The duration of analgesia from PNBs varies depending on the type of local anesthesia used. The effect may last only a few hours, up to a day, or up to 72 hours for some formulations (lidocaine- typically 1-2 hours, bupivacaine and ropivacaine- 2-8 hours, and liposome bupivacaine- up to 72 hours). For instance, short-acting anesthetics like lidocaine have a shorter duration compared to long-acting ones like bupivacaine or ropivacaine. Higher concentrations and larger volumes of local anesthetic can prolong the duration of the block, but this may also increase the risk of side effects. Adding substances like epinephrine or other agents can help prolong the block’s duration by reducing local anesthetic absorption and prolonging its effect. Continuous PNBs, where a catheter is placed and local anesthetic is infused over time, can provide longer-lasting analgesia compared to single-shot PNBs. Factors like patient metabolism, blood flow to the nerve, and the specific nerve block site can also influence the duration. Understanding the duration of local anesthetics is crucial for choosing the appropriate agent and technique for PNBs, especially in the context of postoperative pain management. For surgeries where pain is expected to last longer than the duration of a single-shot block, continuous techniques or adjuvants may be necessary to provide adequate pain relief [56-58]. These aspects are supported by various studies discussed below.
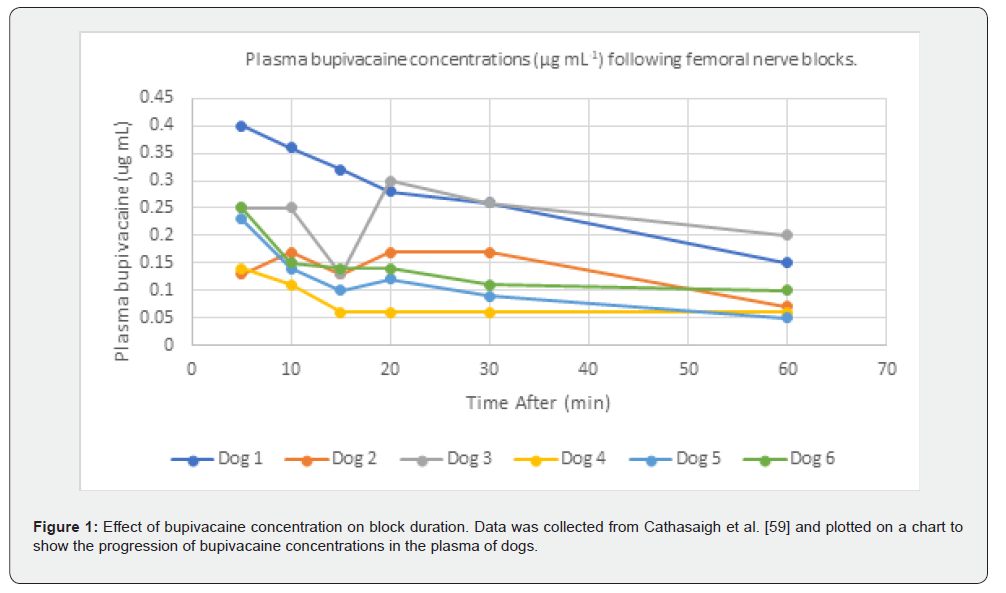
More than 80% of patients experience acute postoperative pain, and approximately 75% of those report the pain severity as moderate or greater [59]. Without proper management of the pain, it can lead to chronic pain and further complications [60]. The duration of analgesia is crucial for managing postoperative pain, which is often the most intense during the initial recovery period. This pain is typically the most intense for patients, and the usage of analgesia (local anesthetics) allows patients to have decreased opioid consumption and improved patient comfort [61]. Cathasaigh et al. [62] suggest that higher plasma concentrations of bupivacaine are linked to prolonged sensory blockade (Figure 1) in dogs. Further research comparing specific dosages of anesthetics for prolonged sensory blockage is warranted in humans to translate these findings. Reporting plasma drug concentration, not the drug around the nerves, is a limitation of this study for clinical effects and translational aspects of PNBs.
A double-blind, randomized, placebo-controlled trial evaluating the additional analgesic efficacy of continuous adductor canal block (CACB; 0.2% ropivacaine compared to normal saline) added to single-dose local infiltration analgesia (100 ml 0.2% ropivacaine, 10 mg oxycodone and 0.5 mg adrenaline) after medial unicondylar knee arthroplasty [63] found that CACB added to local infiltration analgesia provides better analgesia and ambulation without motor weakness. The study also reported delayed onset of pain, nearly 8 hours later in the CACB group than the placebo group. Another study looking at opioid usage 0-48 hours postoperatively found the need for a decreased dose of opioids 18.7 mg compared to 84.9 mg when bupivacaine was used [64]. Overall, the usage of analgesia for pain reduction has proved effective and has decreased the need for opioid usage postoperatively.
Adjuvants such as dexamethasone and clonidine are used more frequently to prolong block duration and enhance analgesia. The use of perineural and dexamethasone combined with ulnar nerve block in patients undergoing upper extremity surgery showed that the addition of dexamethasone to the nerve block significantly prolonged the duration of analgesia and reduced opioid consumption [65]. Dexamethasone extends sensory blockade by 4-8 hours but can cause hypotension with clonidine. More recently developed drugs such as cannabinoids have potential in preclinical studies but lack sufficient strong clinical evidence. The choice of local anesthetic (e.g., ropivacaine or bupivacaine) and concentration also affects efficacy and motorsparing effects. Adjuvants like dexamethasone and clonidine are frequently used with local anesthetics to extend nerve block duration and improve analgesia. Dexamethasone, when added to local anesthetics, extends sensory block duration by 4-8 hours, while clonidine also prolongs block duration but can cause hypotension. Combining dexamethasone and clonidine can lead to a longer overall block duration, but the potential for side effects like hypotension should be considered. Further, it should be noted that the duration of ropivacaine brachial plexus blocks is prolonged with dexamethasone and clonidine but not with epinephrine [23,24,66,67]. Duration of analgesia plays a major role in the reduction of pain and opioid reduction. Using anesthetics such as bupivacaine and ropivacaine, as well as the addition of adjunctive medications like dexamethasone, helps mitigate the risk of opioid misuse and contributes to better pain control.
Pre-emptive Analgesia
The timing of PNBs plays a pivotal role in optimizing postoperative pain management and minimizing opioid consumption. Administering PNBs as preemptive analgesia, before the initiation of the surgical stimulus, can interrupt nociceptive signaling at its onset, mitigating central sensitization and reducing pain intensity in the immediate postoperative period [68,69]. Studies have demonstrated that preoperative PNBs, compared to intraoperative or postoperative administration, are associated with superior outcomes in terms of pain relief and reduced opioid requirements. For instance, a meta-analysis reported that interscalene blocks provide optimal analgesia for shoulder surgery, significantly reducing postoperative pain and opioid use [70]. Moreover, preemptive blocks also improve patient satisfaction by facilitating smoother transitions through recovery phases [71].
Types of Local Anesthetic Drugs
The choice of anesthetic agent and its concentration is another critical factor influencing the efficacy and duration of PNBs. Long-acting local anesthetics such as bupivacaine, ropivacaine, and levobupivacaine are commonly used due to their extended duration of action, which aligns with the goal of sustained analgesia [72,73]. Concentration plays a dual role: higher concentrations provide a denser blockade, which is advantageous for high-pain surgeries, whereas lower concentrations minimize the risk of systemic toxicity and facilitate early motor recovery. Kirksey et al. [74] demonstrated that lower concentrations are also effective when combined with adjuncts such as epinephrine or dexamethasone. Table 2 lists commonly used anesthetic agents for PNBs. Key considerations for (Table 2) include
1) Commonly used concentration ranges in peripheral
nerve blocks.
2) Analgesic duration as documented in postoperative
settings, and
3) Unique characteristics, advantages, or risks of each agent.
Those with a duration of less than 30 minutes are considered ultrashort
acting, short acting has a duration of between 30 minutes to
1-hour, intermediate lasts from 1 to 2 hours, and long-acting lasts
longer [75-79].

Limitations and Drawbacks
PNBs, while generally safe when performed correctly, can have both short-term and long-term implications and limitations. PNB offers effective pain relief and reduces the need for opioids, nerve injury, infection, bleeding, and local anesthetic systemic toxicity (LAST) are potential associated risks. Although not frequent, complications may involve vascular puncture, bleeding, nerve injury, and local anesthetic systemic toxicity [80]. Technical difficulties can include the inability to visualize the target nerve, particularly in obese patients, and nerve injury can result from needle placement or local anesthetic injection. Complications like hematomas, infections, and issues with catheter placement can also arise, especially with continuous nerve blocks [28,50,80]. Complications, while rare, include nerve injury (0.22% incidence), Horner syndrome (e.g., stellate ganglion blocks), and motor weakness [24]. Success is very operator-dependent, with technique refinement being important for the outcome, just as electrodiagnostic testing is highly operator-dependent and should be used on a case-by-case basis [81]. Failure rates of 11-26% can result from anatomical variation, catheter dislodgment, or lessthan- ideal local anesthetic spread [24,26].
PNBs are contraindicated in the presence of severe coagulopathy or systemic infection. PNBs for chronic neuropathic pain remain controversial due to a lack of evidence for long-term benefits and risks of nerve damage. While acute headache disorders (e.g., occipital neuralgia) respond favorably in the short term to PNBs, chronic disorders with multiple blocks lack robust evidence [82,83]. In the long term, persistent neurological symptoms or neuropathies may occur in some patients, highlighting the need for careful evaluation and management. However, intraneural injection with a nerve stimulator or ultrasound-guided techniques is rarely associated with nerve injury [23,28,50,80]. A large series of studies with more than 7,000 patients demonstrates an overall success rate of merely 89%, i.e., about 1 in every 10 PNBs fails in clinical practice [84]. For continuous PNBs alone, first postoperative day failure rates are 19% for infraclavicular and 26% for supraclavicular techniques [85,86]. With regards to long-term effects, there is a much greater incidence of neurogenic symptoms in the PNB group (38.14%) versus controls (9.43%) (p<0.001), with 51% of the former group experiencing ongoing symptoms at 7-13 months following the procedure [81]. The administration of local anesthetic agents also demands careful consideration of the agent and dose to balance efficacy while avoiding side effects [84]. This suggests that despite the well-documented advantages, the success of PNBs is technique-dependent and variable, requiring practitioner proficiency and appropriate patient education on complications.
Nerve injury can occur due to needle trauma, intraneural injection, or compression from hematomas. The risk is higher in patients with pre-existing nerve conditions. Local Anesthetic Systemic Toxicity (LAST) occurs when local anesthetic enters the bloodstream, potentially causing seizures, cardiac arrhythmias, or even death. Infections are rare; however, injection site infection is a potential risk. If blood vessels are damaged during the block, hematomas can form. The block may not provide adequate pain relief, requiring additional interventions or alternative pain management strategies (block failure). Femoral nerve blocks, commonly used for knee and thigh surgeries, can cause quadriceps weakness, potentially leading to falls. Single-shot peripheral nerve blocks provide temporary pain relief, typically lasting for 12-24 hours. Continuous peripheral nerve blocks require more resources, including catheters, pumps, and 24/7 availability of healthcare providers, which can be costly. Further, patient-specific factors like obesity or pre-existing nerve conditions can increase the risk of complications or block failure [50, 86]. Obesity, edema, or subcutaneous emphysema can make it difficult to visualize nerves using ultrasound, potentially leading to block failure or complications. Inability to cooperate with the procedure (e.g., due to dementia or severe anxiety), pre-existing neurological deficits, and active infections at the site of the block are contraindications.
These issues may be mitigated for a better outcome. Using ultrasound to visualize nerves and surrounding structures during the block can help minimize the risk of nerve injury and improve block success. Ultrasound and nerve stimulation devices improve safety but do not completely reduce risk [24,38]. Identifying patients at higher risk for complications (e.g., those with preexisting nerve conditions or obesity) enables the appropriate adjustment of the block technique and the choice of local anesthetic (patient selection). Using appropriate needle types and techniques can minimize the risk of nerve injury. Monitoring patients closely for signs of complications (e.g., bleeding, infection, or LAST) is crucial. If the block fails or provides inadequate pain relief, alternative pain management strategies (e.g., opioid analgesics, other nerve blocks, or multimodal analgesia) should be considered. By carefully weighing the risks and benefits, utilizing appropriate techniques, and implementing mitigation strategies, healthcare professionals can optimize the use of peripheral nerve blocks and minimize the potential for complications [23,84,87].
Conclusion
Future Research, and Emerging Trends: Ultrasound-guided peripheral nerve blocks (PNBs) enhance accuracy (success rates >95%) and enable opioid-free anesthesia (OFA) with multimodal analgesia (e.g., NSAIDs, dexmedetomidine), reducing 24-hour opioid use by 40-60% and postoperative nausea [28]. More recent trends include fascial plane blocks (e.g., erector spinae) and longacting agents like liposomal bupivacaine, prolonging analgesia to 72 hours. Future directions include standardizing practice to control for variable catheter dislodgement rates (1.5-25%), describing surgery-specific benefits (e.g., thoracic surgery preserves good OFA outcomes with less pain and complications) and determining long-term neuropathic adverse effects. Cooperative efforts must delineate PNBs’ role in opioid-sparring Enhanced Recovery After Surgery pathways while keeping innovation in tandem with safety. In conclusion, the overreliance on opioids for postoperative pain management has contributed significantly to the opioid epidemic, with substantial risks including side effects, long-term dependence, and inadequate pain relief. Peripheral nerve blocks (PNBs) present a valuable alternative, effectively reducing opioid consumption, improving pain control, and minimizing adverse events. Incorporating PNBs into multimodal analgesia strategies not only addresses immediate postoperative pain but also plays a crucial role in reducing opioid exposure and dependence, ultimately enhancing patient outcomes and offering a safer approach to pain management.
References
- Young JC, Dasgupta N, Chidgey BA, Jonsson Funk M (2021) Postsurgical Opioid Prescriptions and Risk of Long-term Use: An Observational Cohort Study Across the United States. Ann Surg 273(4): 743-750.
- Benhamou D, Berti M, Brodner G, De Andres J, Draisci G, et al. (2008) Postoperative Analgesic THerapy Observational Survey (PATHOS): a practice pattern study in 7 central/southern European countries. Pain 136(1-2): 134-141.
- Ndebea AS, van den Heuvel SAS, Temu R, Kaino MM, Van Boekel RLM, et al. (2020) Prevalence and Risk Factors for Acute Postoperative Pain After Elective Orthopedic and General Surgery at a Tertiary Referral Hospital in Tanzania. J Pain Res 13: 3005-3011.
- Woldehaimanot TE, Eshetie TC, Kerie MW (2014) Postoperative pain management among surgically treated patients in an Ethiopian hospital. PLoS One 9(7): e102835.
- Murray AA, Retief FW (2016) Acute postoperative pain in 1 231 patients at a developing country referral hospital: incidence and risk factors. Southern African Journal of Anaesthesia and Analgesia 22(1): 19-24.
- Oderda GM, Gan TJ, Johnson BH, Robinson SB (2013) Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother 27(1): 62-70.
- Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, et al. (2012) Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med 172(5): 425-430.
- Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, et al. (2017) New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg 152(6): e170504.
- Wunsch H, Wijeysundera DN, Passarella MA, Neuman MD (2016) Opioids Prescribed After Low-Risk Surgical Procedures in the United States, 2004-2012. JAMA 315(15): 1654-1657.
- Raebel MA, Newcomer SR, Reifler LM, Boudreau D, Elliott TE, et al. (2013) Chronic use of opioid medications before and after bariatric surgery. JAMA 310(13): 1369-1376.
- Armaghani SJ, Lee DS, Bible JE, Archer KR, Shau DN, et al. (2014) Preoperative opioid use and its association with perioperative opioid demand and postoperative opioid independence in patients undergoing spine surgery. Spine (Phila Pa 1976) 39(25): E1524-E1530.
- Oderda GM, Said Q, Evans RS, Stoddard GJ, Lloyd J, et al. (2007) Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Annals of Pharmacotherapy 41(3): 400-406.
- Radulovic J, Jankovic BD (1994) Opposing activities of brain opioid receptors in the regulation of humoral and cell-mediated immune responses in the rat. Brain Res 661(1-2): 189-195.
- Benyamin R, Trescot AM, Datta S, Buenaventura RM, Adlaka R, et al. (2008) Opioid complications and side effects. Pain physician 11(2S): S105-S107.
- Collett B (1998) Opioid tolerance: the clinical perspective. British journal of anaesthesia 81(1): 58-68.
- Chang CY, Challa CK, Shah J, Eloy JD (2014) Gabapentin in acute postoperative pain management. Biomed Res Int 2014: 631756.
- Kong L, Wang J, Guan S, Chen X, Li M, et al. (2023) Nomogram for predicting opioid-induced nausea and vomiting for cancer pain patients. Support Care Cancer 31(12): 663.
- Khanna AK, Bergese SD, Jungquist CR, Morimatsu H, Uezono S, et al. (2020) Prediction of Opioid-Induced Respiratory Depression on Inpatient Wards Using Continuous Capnography and Oximetry: An International Prospective, Observational Trial. Anesth Analg 131(4): 1012-1024.
- Hah JM, Bateman BT, Ratliff J, Curtin C, Sun E (2017) Chronic Opioid Use After Surgery: Implications for Perioperative Management in the Face of the Opioid Epidemic. Anesth Analg 125(5): 1733-1740.
- Wang JC, Piple AS, Mayfield CK, Chung BC, Oakes DA, et al. (2023) Peripheral Nerve Block Utilization is Associated with Decreased Postoperative Opioid Consumption and Shorter Length of Stay Following Total Knee Arthroplasty. Arthroplast Today 20: 101101.
- Mueller KG, Memtsoudis SG, Mariano ER, Baker LC, Mackey S, et al. (2017) Lack of Association Between the Use of Nerve Blockade and the Risk of Persistent Opioid Use Among Patients Undergoing Shoulder Arthroplasty: Evidence from the Marketscan Database. Anesth Analg 125(3): 1014-1020.
- Jogie J, Jogie JA (2023) A Comprehensive Review on the Efficacy of Nerve Blocks in Reducing Postoperative Anesthetic and Analgesic Requirements. Cureus 15(5): e38552.
- Midia M, Dao D (2016) The Utility of Peripheral Nerve Blocks in Interventional Radiology. AJR Am J Roentgenol 207(4): 718-730.
- Chen YK, Boden KA, Schreiber KL (2021) The role of regional anaesthesia and multimodal analgesia in the prevention of chronic postoperative pain: a narrative review. Anaesthesia 76 Suppl 1(Suppl 1): 8-17.
- Chang A, Dua A, Singh K, White BA (2025) Peripheral nerve blocks.
- Cardwell TW, Zabala V, Mineo J, Ochner CN (2022) The Effects of Perioperative Peripheral Nerve Blocks on Peri- and Postoperative Opioid Use and Pain Management. Am Surg 88(12): 2842-2850.
- Joshi G, Gandhi K, Shah N, Gadsden J, Corman SL, et al. (2016) Peripheral nerve blocks in the management of postoperative pain: challenges and opportunities. Journal of clinical anesthesia 35: 524-529.
- Ilfeld BM (2017) Continuous peripheral nerve blocks and alternative regional analgesic modalities: clarification regarding relative superiority. Anesthesia & Analgesia 124(6): 2088.
- Ilfeld BM (2011) Continuous peripheral nerve blocks in the hospital and at home. Anesthesiol Clin 29(2): 193-211.
- Chelly JE, Ghisi D, Fanelli A (2010) Continuous peripheral nerve blocks in acute pain management. Br J Anaesth 105 Suppl 1: i86-i96.
- Bomberg H, Bayer I, Wagenpfeil S, Kessler P, Wulf H, et al. (2018) Prolonged catheter uses and infection in regional anesthesia: a retrospective registry analysis. Anesthesiology 128(4): 764-773.
- Torpy JM, Lynm C, Golub RM (2011) JAMA patient page. Regional anesthesia. JAMA 306(7): 781.
- Hutton M, Brull R, Macfarlane AJR (2018) Regional anaesthesia and outcomes. BJA Educ 18(2): 52-56.
- Folino TB, Mahboobi SK (2023) Regional anesthetic blocks. StatPearls Publishing, USA.
- Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, et al. (2014) Brachial plexus anesthesia: A review of the relevant anatomy, complications, and anatomical variations. Clin Anat 27(2): 210-221.
- Saranteas T, Koliantzaki I, Savvidou O, Tsoumpa M, Eustathiou G, et al. (2019) Acute pain management in trauma: anatomy, ultrasound-guided peripheral nerve blocks and special considerations. Minerva Anestesiol 85(7): 763-773.
- Pasnicki M, Krol A, Kosson D, Kolacz M (2024) The Safety of Peripheral Nerve Blocks: The Role of Triple Monitoring in Regional Anaesthesia, a Comprehensive Review. Healthcare (Basel) 12(7): 769.
- Richman JM, Liu SS, Courpas G, Wong R, Rowlingson AJ, et al. (2006) Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg 102(1): 248-257.
- Chung CJ, Eom DW, Lee TY, Park SY (2022) Reduced Opioid Consumption with Pericapsular Nerve Group Block for Hip Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. Pain Res Manag 2022: 6022380.
- Schlechter JA, Gornick BR, Harrah T, Sherman B (2022) Do Continuous Peripheral Nerve Blocks Decrease Home Opioid Use Following Anterior Cruciate Ligament Reconstruction in Children and Adolescents? The Envelope Please. J Pediatr Orthop 42(4): e356-e361.
- Gewandter JS, Smith SM, Dworkin RH, Turk DC, Gan TJ, et al. (2021) Research approaches for evaluating opioid sparing in clinical trials of acute and chronic pain treatments: Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials recommendations. Pain 162(11): 2669-2681.
- Nielsen S, Sabioni P, Trigo JM, Ware MA, Betz-Stablein BD, et al. (2017) Opioid-Sparing Effect of Cannabinoids: A Systematic Review and Meta-Analysis. Neuropsychopharmacology 42(9): 1752-1765.
- Nielsen S, Picco L, Murnion B, Winters B, Matheson J, et al. (2022) Opioid-sparing effect of cannabinoids for analgesia: an updated systematic review and meta-analysis of preclinical and clinical studies. Neuropsychopharmacology 47(7): 1315-1330.
- Powell VD, Rosenberg JM, Asanti A, Garpestad C, Lagisetty P, et al. (2021) Evaluation of Buprenorphine Rotation in Patients Receiving Long-term Opioids for Chronic Pain: A Systematic Review. JAMA Netw Open 4(9): e2124152.
- Chey WD, Webster L, Sostek M, Lappalainen J, Barker PN, et al. (2014) Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med 370(25): 2387-2396.
- Collaborative T (2024) Impact of opioid-free analgesia on pain severity and patient satisfaction after discharge from surgery: multispecialty, prospective cohort study in 25 countries. Br J Surg 111(1): znad421.
- Heron MJ, Zhu KJ, Zhu L, Davis AJ, Alahmadi S, et al. (2025) Impact of Peripheral Nerve Blocks on Opioid Use Following Flap Reconstruction Involving the Lower Extremity: A Systematic Review and Meta-Analysis. Journal of Plastic, Reconstructive & Aesthetic Surgery 105: 230-242.
- Niyonkuru E, Iqbal MA, Zeng R, Zhang X, Ma P, et al. (2024) Nerve blocks for post-surgical pain management: a narrative review of current research. Journal of Pain Res 2: 3217-3239.
- Wiederhold BD, Garmon EH, Peterson E, Stevens JB, O'Rourke MC, et al. (2025) Nerve block anesthesia.
- Bennett DL, Clark AJ, Huang J, Waxman SG, Dib-Hajj SD, et al. (2019) The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol Rev 99(2): 1079-1151.
- Korner J, Albani S, Sudha Bhagavath Eswaran V, Roehl AB, Rossetti G, et al. (2022) Sodium Channels and Local Anesthetics-Old Friends with New Perspectives. Front Pharmacol 13: 837088.
- Gottschalk A, Smith DS (2001) New concepts in acute pain therapy: preemptive analgesia. Am Fam Physician 63(10): 1979-1984.
- Cruz FF, Rocco PR, Pelosi P (2017) Anti-inflammatory properties of anesthetic agents. Crit Care 21(1): 67.
- Gola W, Zajac M, Cugowski A (2020) Adjuvants in peripheral nerve blocks - the current state of knowledge. Anaesthesiol Intensive Ther 52(4): 323-329.
- Gasteiger L, Kirchmair L, Hoerner E, Stundner O, Hollmann MW (2023) Peripheral Regional Anesthesia Using Local Anesthetics: Old Wine in New Bottles? J Clin Med 12(4): 1541.
- Chou R, Gordon DB, De Leon-Casasola OA, Rosenberg JM, Bickler S, et al. (2016) Management of Postoperative Pain: A Clinical Practice Guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 17(2): 131-157.
- Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, et al. (2012) The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg 115(2): 428-442.
- Kianian S, Bansal J, Lee C, Zhang K, Bergese SD, et al. (2024) Perioperative multimodal analgesia: a review of efficacy and safety of the treatment options. Anesthesiology and Perioperative Science 2(1): 9.
- M OC, Read MR, Atilla A, Schiller T, Kwong GPS, et al. (2018) Blood concentration of bupivacaine and duration of sensory and motor block following ultrasound-guided femoral and sciatic nerve blocks in dogs. PLoS One 13(3): e0193400.
- Lan F, Shen Y, Ma Y, Cao G, Philips N, et al. (2019) Continuous Adductor Canal Block used for postoperative pain relief after medial Unicondylar Knee Arthroplasty: a randomized, double-blind, placebo-controlled trial. BMC Anesthesiol 19(1): 114.
- Mont MA, Beaver WB, Dysart SH, Barrington JW, Del Gaizo DJ, et al. (2018) Local Infiltration Analgesia with Liposomal Bupivacaine Improves Pain Scores and Reduces Opioid Use After Total Knee Arthroplasty: Results of a Randomized Controlled Trial. J Arthroplasty 33(1): 90-96.
- Maagaard M, Stormholt ER, Nielsen LF, Baerentzen F, Danker J, et al. (2023) Perineural and Systemic Dexamethasone and Ulnar Nerve Block Duration: A Randomized, Blinded, Placebo-controlled Trial in Healthy Volunteers. Anesthesiology 138(6): 625-633.
- Singhal A, Taksande K (2024) Role of Adjuvants in Enhancing the Efficacy and Duration of Anesthesia Blocks: A Comprehensive Review. Cureus 16(9): e69880.
- Saied NN, Gupta RK, Saffour L, Helwani MA (2016) Dexamethasone and Clonidine, but not Epinephrine, Prolong Duration of Ropivacaine Brachial Plexus Blocks, Cross-Sectional Analysis in Outpatient Surgery Setting. Pain Med 18(10): 2013-2026.
- Gerbershagen HJ, Aduckathil S, Van Wijck AJ, Peelen LM, Kalkman CJ, et al. (2013) Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology 118(4): 934-944.
- Kehlet H, Jensen TS, Woolf CJ (2006) Persistent postsurgical pain: risk factors and prevention. Lancet 367(9522): 1618-1625.
- Hussain N, Goldar G, Ragina N, Banfield L, Laffey JG, et al. (2017) Suprascapular and Interscalene Nerve Block for Shoulder Surgery: A Systematic Review and Meta-analysis. Anesthesiology 127(6): 998-1013.
- Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL, et al. (2014) Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin 30(1): 149-160.
- Neal JM, Barrington MJ, Fettiplace MR, Gitman M, Memtsoudis SG, et al. (2018) The Third American Society of Regional Anesthesia and Pain Medicine Practice Advisory on Local Anesthetic Systemic Toxicity: Executive Summary 2017. Reg Anesth Pain Med 43(2): 113-123.
- Albrecht E, Kern C, Kirkham KR (2015) A systematic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia 70(1): 71-83.
- Kirksey MA, Haskins SC, Cheng J, Liu SS (2015) Local Anesthetic Peripheral Nerve Block Adjuvants for Prolongation of Analgesia: A Systematic Qualitative Review. PLoS One 10(9): e0137312.
- Getachew M, Tesfaye H, Yihunie W, Ayenew T, Alemu S, et al. (2024) Sustained release local anesthetics for pain management: relevance and formulation approaches. Front Pain Res (Lausanne) 5: 1383461.
- Zink W, Graf BM (2008) The toxicity of local anesthetics: the place of ropivacaine and levobupivacaine. Curr Opin Anaesthesiol 21(5): 645-650.
- Marhofer P, Chan VW (2007) Ultrasound-guided regional anesthesia: current concepts and future trends. Anesth Analg 104(5): 1265-1269.
- Popping DM, Elia N, Marret E, Wenk M, Tramer MR, et al. (2009) Clonidine as an adjuvant to local anesthetics for peripheral nerve and plexus blocks: a meta-analysis of randomized trials. Anesthesiology 111(2): 406-415.
- Weinberg L, Peake B, Tan C, Nikfarjam M (2015) Pharmacokinetics and pharmacodynamics of lignocaine: A review. World J Anesthesiol 4(2): 17-29.
- Jeng CL, Torrillo TM, Rosenblatt MA (2010) Complications of peripheral nerve blocks. Br J Anaesth 105 Suppl 1: i97-i107.
- Kern RZ (2003) The electrodiagnosis of ulnar nerve entrapment at the elbow. Can J Neurol Sci 30(4): 314-319.
- Fernandes L, Randall M, Idrovo L (2021) Peripheral nerve blocks for headache disorders. Practical Neurology 21(1): 30-35.
- Rajagopalan S, Siva N, Novak A, Garavaglia J, Jelsema C, et al. (2023) Safety and efficacy of peripheral nerve blocks to treat refractory headaches after aneurysmal subarachnoid hemorrhage - A pilot observational study. Front Neurol 14: 1122384.
- Bottomley T, Gadsden J, West S (2023) The failed peripheral nerve block. BJA Educ 23(3): 92-100.
- Ahsan ZS, Carvalho B, Yao J (2014) Incidence of failure of continuous peripheral nerve catheters for postoperative analgesia in upper extremity surgery. J Hand Surg Am 39(2): 324-329.
- O'Flaherty D, McCartney CJL, Ng SC (2018) Nerve injury after peripheral nerve blockade-current understanding and guidelines. BJA Educ 18(12): 384-390.
- Wang S, Li Y, Liang C, Han X, Wang J, et al. (2023) Opioid-free anesthesia reduces the severity of acute postoperative motion-induced pain and patient-controlled epidural analgesia-related adverse events in lung surgery: randomized clinical trial. Front Med (Lausanne) 10: 1243311.
- Feenstra ML, Jansen S, Eshuis WJ, Van Berge Henegouwen MI, Hollmann MW, et al. (2023) Opioid-free anesthesia: A systematic review and meta-analysis. J Clin Anesth 90: 111215.
- Dabbagh A, Madadi F, Ebrahimi M, Dabir S, Vosoughian M, et al. (2025) Experimental implementation of the peripheral nerve block clinical registry: an observational study. Front Med (Lausanne) 12: 1486300.
- D'Amico F, Barucco G, Licheri M, Valsecchi G, Zaraca L, et al. (2022) Opioid Free Anesthesia in Thoracic Surgery: A Systematic Review and Meta Analysis. J Clin Med 11(23): 6955.






























