Abstract
Background: Globally rising obesity coincides with surging use of once-weekly glucagon-like peptide-1 receptor agonists (GLP-1RAs) among in-vitro-fertilization (IVF) patients. While GLP-1RAs improve weight, metabolic status and pregnancy rates, their gastric-emptying delay heightens regurgitation and aspiration risk during propofol-based oocyte retrievals. This mini-review integrates current anaesthesia guidelines with reproductive endocrinology evidence to create a risk-stratified, universally applicable peri-operative protocol that safeguards both airway safety and fertility gains.
Methods: ASA and SPAQI consensus statements, pharmacokinetic data for five common GLP-1RAs, and 7 human fertility studies (plus key observational reports) were integrated. A matrix was developed linking drug half-life, cessation interval, fasting duration, point-of-care gastric ultrasound (POCGUS) need and default airway device, and embedded in a time-stamped workflow.
Results: Weekly agents such as semaglutide, dulaglutide and exenatide-ER require a 7-day pause; daily liraglutide or exenatide demand ≥24 h withdrawal. Patients are triaged into low, moderate, or high risk based on BMI, gastro-oesophageal reflux, and last-dose timing. Low-risk patients may receive deep sedation or second-generation supraglottic airways if POCGUS confirms an empty antrum; high-risk tiers mandate rapid-sequence induction with cuffed endotracheal intubation. Preliminary implementation in a high-volume IVF programme reduced same-day cancellations from 8 % to < 2 % with zero aspiration events. Research priorities include pharmacokinetic trials in women and randomised comparisons of 3-, 5- and 7-day holds.
Conclusions: IVF centers receiving high-BMI patients can immediately adopt a modular pause-and-protect protocol, offering a replicable model that balances metabolic innovation with peri-operative vigilance in fertility care.
Keywords:Glp-1 Receptor Agonists; In-Vitro Fertilization; Airway Management; Gastric Ultrasound; Rapid-Sequence Induction; Obesity; Anaesthesia Guidelines; Fertility Outcomes; Semaglutide; Liraglutide
Abbreviations: ASA: American Society of Anesthesiologists; BMI: Body-Mass Index; CGM: Continuous Glucose Monitoring; CSA: Cross-Sectional Area, ETT: Endotracheal Tube; GERD: Gastro-Oesophageal Reflux Disease; GLP-1RA: Glucagon-Like Peptide-1 Receptor Agonist; HFNO: High-Flow Nasal Oxygen; IVF: In-Vitro Fertilization; KPI: Key Performance Indicator; NMB: Neuromuscular Blockade; NPO: Nil per os (nothing by mouth); PACU: Post-Anesthesia Care Unit; PCOS: Polycystic Ovary Syndrome; POCGUS: Point-of-Care Gastric Ultrasound; PONV: Post-Operative Nausea and Vomiting; RCT: Randomised Controlled Trial; RSI: Rapid-Sequence Induction; SGA: Supraglottic Airway; SPAQI: Society for Perioperative Assessment and Quality Improvement
Introduction
Obesity now affects more than one-third of reproductive-aged women worldwide, and prevalence can exceed 50 % in some high-obesity regions presenting for in-vitro fertilization (IVF) have a body-mass index (BMI) > 30 kg m-2 [1]. Concomitantly, use of the new once-weekly glucagon-like peptide-1 receptor agonists (GLP-1RAs) for weight loss and dysglycaemia has exploded; almost 1 in 8 U.S. adults, and a rapidly rising number of IVF patients, now take semaglutide, tirzepatide, or related agents [2]. GLP-1RAs confer meaningful metabolic and reproductive benefits. Meta-analyses and randomised controlled trials (RCTs) in women with polycystic ovary syndrome (PCOS) show improved menstrual cyclicity, reduced androgens, and (critically for IVF programmes with BMI cut-offs) average weight-loss of 10-15 % [3,4]. A pilot RCT of liraglutide plus metformin reported an 86% clinical pregnancy-rate (PR) per embryo transfer (ET) versus 29% with metformin alone [5].
Yet the same pharmacology that slows gastric emptying and promotes satiety poses an airway threat in anaesthesia.
Case reports of refractory gastric residue and “failed NPO status” under propofol sedation prompted the American Society of Anesthesiologists (ASA) in 2023, and a broader multisociety coalition in 2024, to recommend temporary discontinuation of GLP-1RAs before elective procedures [6,7]. In Kobori’s propensity-matched endoscopy series, the prevalence of solid gastric contents rose ten-fold (0.5 % → 5.4 %) among GLP-1RA users [8], mirroring IVF recovery-room anecdotes of unexpected regurgitation. These parallel realities, fertility benefit versus aspiration risk, confront high-volume IVF centers. This mini-review integrates the anaesthesia guidelines (ASA, SPAQI) with the reproductive endocrinology literature to propose a risk-stratified, universally applicable protocol that preserves both airway safety and the metabolic gains of GLP-1RAs.
Current Gaps and Research Agenda
Despite consensus advisories, hard data remain scant: no pharmacokinetic trials have measured gastric emptying or drug-clearance half-lives in patients with obesity, and no RCT has compared different cessation intervals before oocyte retrieval. Future studies should prospectively validate point-of-care gastric ultrasound algorithms [7]. We call for:
1. Pharmacological studies stratified by BMI > 35 kg m-2 and by daily (liraglutide) versus weekly (semaglutide) dosing in late-follicular-phase patients.
2. Multicenter RCTs randomising 3-, 5-, and 7-day holds of weekly GLP-1RAs on rates of aspiration‐related events, hyperglycaemia, and live-birth.
3. Implementation research on gastric ultrasound triage in IVF recovery units.
Discussion
Anesthetic Risks in IVF Context
The use of GLP-1 receptor agonists (GLP-1RAs) in IVF patients introduces a unique anaesthetic challenge. While these agents offer substantial reproductive and metabolic benefits, their pharmacological effects, particularly delayed gastric emptying, raise the risk of regurgitation and aspiration during sedation. The American Society of Anesthesiologists (ASA) and allied societies have issued detailed guidance to mitigate these risks. However, when applied to IVF-specific procedures, the standard recommendations face new complications. This section outlines both the general ASA/SPAQI guidelines and the IVF-specific hazards that require tailored precautions.
ASA / SPAQI Guidance for GLP-1RA Use
Published ASA consensus [6] and the subsequent five-society statement [7] converge on four key pillars of perioperative management for GLP-1RA users:
Identify the agent and escalation phase: Weekly injectables, especially during dose escalation, carry the highest risk due to prolonged gastric effects.
Hold therapy before elective procedures: For daily formulations, omit the morning dose. For weekly agents, withhold for at least 7 days prior to sedation or anesthesia.
Optimize fasting protocols: Maintain standard fasting windows of 8 hours for solids and 6 hours for clear fluids, extending to 12 hours in symptomatic or high-BMI patients.
Engage in shared decision-making: Balance aspiration risk with glycaemic control, especially in patients with type 2 diabetes.
In addition, the Society for Perioperative Assessment and Quality Improvement (SPAQI) advises clinicians to continue basal insulin at 60-80% of the usual dose during the GLP-1RA hold [9], to avoid glycaemic destabilization. These overarching principles provide a strong framework for anaesthesia safety. However, they do not account for the IVF-specific procedural context, which brings added layers of complexity.
IVF/ART-Specific Anaesthetic Hazards
The routine sedation for oocyte retrieval in IVF involves deep propofol anaesthesia without airway reflexes, closely resembling general anaesthesia in its risk profile. Several factors specific to IVF patients intensify the airway risk:
• The rapid transvaginal aspiration of follicular fluid increases intra-abdominal pressure, predisposing patients to regurgitation [10].
• In obese women with PCOS, who constitute a high proportion of IVF patients, airway risk is further compounded by elevated neck circumference, higher Mallampati scores, and insulin resistance [4].
• Fasting windows are often compressed in IVF schedules, with early-morning procedures potentially coinciding with peak gastric stasis from GLP-1RAs, particularly 48-72 hours after semaglutide injection [11].
These compounded factors justify a stricter GLP-1RA cessation protocol than what is typically applied for general ambulatory surgery. Together, they support the argument that universal GLP- 1RA discontinuation may not be a sustainable or safe solution in IVF settings. Understanding these elevated risks, stemming from compressed fasting, delayed gastric clearance, and high BMI prevalence, raises a critical clinical question: Can IVF programs afford to stop GLP-1RAs indefinitely without compromising the very fertility gains they seek to harness?
Fertility Consequences of Prolonged GLP-1RA Withdrawal
The reproductive pay-off is real. Beyond the seminal liraglutide RCT [5] and Zhou’s meta-analysis in PCOS [3], observational data suggest GLP-1RAs improve endometrial receptivity markers and decrease miscarriage trends [12,13]. Prolonged discontinuation risks weight rebound and metabolic deterioration, potentially eroding reproductive gains; a 4-week cessation reversed half of prior weight-loss in Merhi’s observational cohort [14]. Section 5 therefore lays out a pragmatic, risk-stratified protocol. Thus, a short, protocolised pause, rather than blanket stopping, is essential. Section 5 therefore proposes a pragmatic, risk-stratified protocol.
A Risk-Stratified Pre-operative Protocol
Our risk-stratified pre-operative protocol (Tables 1, 2) explicitly balances airway safety with documented reproductive benefits, aligning drug-specific pharmacokinetics (Section 5.1) with established fertility outcomes (Section 5.2). Successful implementation hinges on matching the agent-specific pharmacokinetics of GLP-1RAs with the airway risk profile of each IVF patient [1,10]. Guided by ASA-2023 and SPAQI consensus, but calibrated for high-BMI populations, our protocol uses the decision nodes laid out in Table 1.
Patient & Drug Stratification
Implementing this protocol requires systematic patient and drug stratification using Tables 1 and 2. Building on the stratification matrix in Table 1, this section translates drug-specific guidance into actionable patient-level triage steps using Table 2. Effective mitigation of aspiration risk hinges on matching
(i) The airway-relevant pharmacokinetics of each GLP-1RA to
(ii) The individual clinical profile of the IVF patient.
Tables 1 and 2 work as a two-step cockpit:
• Table 1 ranks every commonly used GLP-1RA (row) across half-life, recommended pause interval, fasting target, need for point-of-care gastric ultrasound (POCGUS) and default airway plan.
• Table 2 converts those drug-specific tiers into a bedside algorithm that tells the nurse or anaesthetist exactly how long to fast, when to scan.
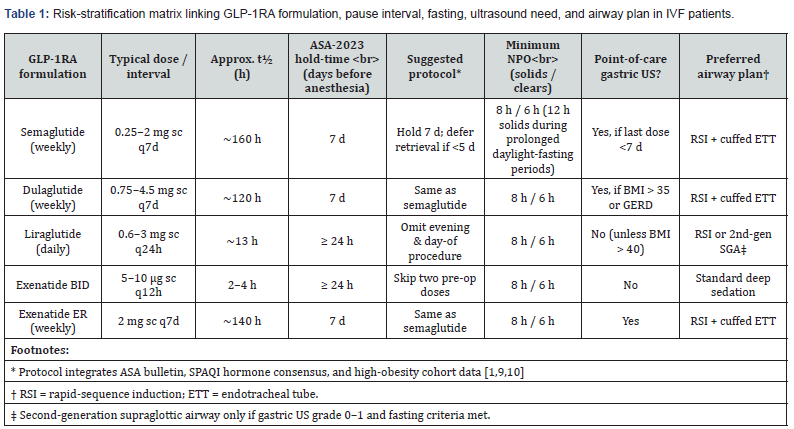
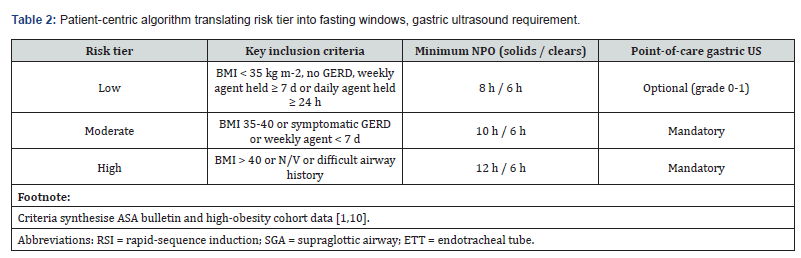
1) How to apply the matrix-plus-algorithm
Identify the Agent and Last Dose:
• Weekly injections taken < 5 days ago automatically trigger rescheduling; daily agents require the morning/evening dose to be skipped.
2) Assign the risk tier:
• BMI < 35 kg m-2 and no GERD → Low
• BMI 35-40, symptomatic GERD, or < 7-day hold → Moderate
• BMI > 40, nausea/vomiting, or difficult airway history → High
3) Link Risk Tier to Airway Plan in Table 4
• Low tier → deep sedation ± 2nd-generation SGA if POCGUS grade 0-1
• Moderate tier → POCGUS mandatory; RSI if stomach not empty
• High tier → straight RSI with cuffed ETT whatever the scan shows
4) Glycaemic Bridge:
• Continue basal insulin at 60-80 % and use CGM alerts throughout the pause
Fertility-Benefit Rationale
While GLP-1 receptor agonists (GLP-1RAs) offer promising fertility benefits, particularly in women with obesity or polycystic ovary syndrome (PCOS), the core rationale for brief, protocolized pauses stems from the need to safeguard airway safety during anaesthesia. Rather than permanent cessation, short-duration drug holds (24 hours for daily agents; 7 days for weekly formulations) are sufficient to reduce aspiration risk while preserving the metabolic stability gained from GLP-1RA therapy. This approach strikes a balance between minimising gastric content retention and avoiding metabolic rebound, which could complicate anaesthetic planning and perioperative glucose control. Therefore, the stratified pause strategy outlined in Tables 1 and 2 serves not only to protect reproductive benefits but primarily to optimize anaesthetic safety.
Point-of-Care Gastric Ultrasound & Immediate Airway Plan
For moderate- or high-risk patients, a bedside gastric ultrasound before induction is mandatory. For any moderate- or high-risk tier patient, or when the timing of the last GLP-1RA dose is uncertain, the protocol mandates a bedside gastric scan before induction, as endorsed by both the ASA consensus and the 2024 multi-society guidance.
Stepwise algorithm
1. Position & View: With the patient in RLD (right-lateral decubitus), identify the antrum between left lobe liver and pancreas.
2. Measure Cross-Sectional Area (CSA): Trace the serosa; two diameters are averaged.
3. Interpretation:
• CSA ≤ 340 mm² ≈ Grade 0-1 volume → treat as “empty”.
• CSA > 340 mm² or solid contents visualised → treat as “full stomach”.
Document & Decide: The team uploads the ultrasound image and CSA to the IVF EMR. The anaesthesiologists record the chosen airway strategy and brief the surgical team.
This threshold is supported by volunteer studies using semaglutide, where residual solids were detected on ultrasound despite ≥8 h fasting [7], and by matched-pair data showing a five-fold rise in endoscopic residue among GLP-1RA users 7. ASA guideline likewise advises treating un-held doses as a full stomach unless ultrasound proves otherwise [6]. Practical pearl If a Grade 2 stomach is found, postponement is seldom feasible in IVF cycles; instead, extend solids fast to 12 h, administer 10 mg metoclopramide, and repeat the scan after 60 min, an approach extrapolated from pro-kinetic data in dyspepsia 7.
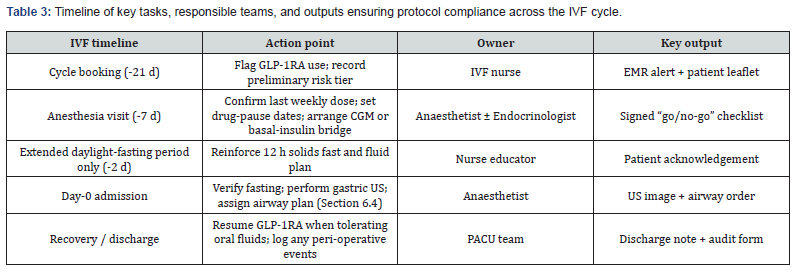
Operational Workflow & Compliance Checks
Consistent execution of this protocol relies on clearly defined operational workflows and accountability (Table 3).
Timeline & Task Owners
To ensure consistent implementation of the protocol, Table 3 provides a comprehensive, time-stamped timeline of key actions across the IVF cycle, clearly assigning responsibilities to specific team members. This “swim-lane” overview links each milestone, from initial counselling to postoperative resumption of therapy, to the appropriate stakeholder and required output, ensuring no step is overlooked.
Audit & Quality Metrics
• Primary KPI: Target same-day cancellation rate < 2 % (baseline 8%) to be verified three months after protocol rollout.
• Safety KPI: Pulmonary aspiration incidents per 1 000 oocyte retrievals (target 0).
• Process KPI: > 95 % documented drug-pause adherence and point-of-care gastric-ultrasound uploads.
Monthly dashboards are auto-generated from the EMR; any outlier triggers a root-cause huddle moderated by the SPAQI champion [6,9].
Governance
A designated peri-operative lead convenes quarterly meetings with IVF, endocrinology, and quality teams to update the protocol against emerging ASA- or multi-society statements; the most recent 2024 bulletin on “liquid-only diet for high-risk cohorts” was incorporated within two weeks [7]. With risk tiers, fasting rules, and ultrasound gates now locked, Section 7 turns to executing an airway plan that protects both patient and embryo.
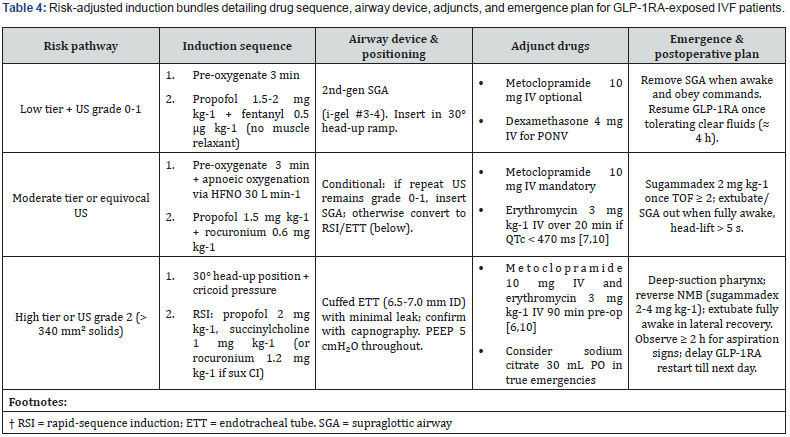
Induction and Airway Precautions
Ensuring safe induction in IVF patients receiving GLP-1RAs requires clearly defined, stepwise airway management strategies aligned with the patient’s risk tier and gastric ultrasound findings. These strategies are detailed in the risk-adjusted induction guide (Table 4). Safe induction in GLP-1RA-exposed IVF patients requires translating the predefined risk tiers and ultrasound findings from Section 6 into a clear, reproducible airway management sequence. Table 4 condenses this protocol into a risk-adjusted induction guide, and the text below elaborates each decision node. This expanded framework retains the core logic of prior sections but adds step-by-step operational clarity to support “plug-and-play” use by any anaesthetist involved in IVF care.
Key Pharmacologic Points
• Metoclopramide accelerates gastric emptying by 25-30% in GLP- 1RA volunteers [7].
• Basal-insulin bridging (Section 6.2) continues intra- and post-operatively; check capillary glucose on arrival and discharge.
Rescue & Complications
• Suspected aspiration: jaw-thrust, 100 % O₂, suction, and immediate RSI/ETT if SGA in situ. Initiate lung-protective ventilation (6 mL kg-1 IBW) and postoperative chest X-ray.
• Laryngospasm in SGA cases: is more likely after propofol-only deep sedation; keep succinylcholine 20 mg IV ready.
• Refractory nausea in recovery: ondansetron 4 mg IV; if persistent consider haloperidol 1 mg IV (avoid QT prolongation overlap).
Conclusion
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) represent a transformative advancement in reproductive medicine, enhancing weight management, metabolic control, and IVF outcomes. However, their pharmacologic effect on gastric motility introduces a measurable airway risk under anaesthesia, particularly during propofol-based oocyte retrievals. This duality demands a pragmatic, evidence-informed compromise. The risk-stratified protocol outlined in this review offers such a solution. By combining a one-week hold for weekly agents (or 24 hours for daily formulations) with enhanced fasting regimens, point-of-care gastric ultrasound, and risk-stratified airway planning, including rapid-sequence induction (RSI) where necessary, it preserves both airway safety and reproductive efficacy. These recommendations align ASA and SPAQI consensus with regional obesity profiles and IVF practices.
Crucially, this modular, risk-tiered approach is ready for immediate adoption by IVF centers in similarly high-BMI populations. Its structure, anchored in pharmacologic rationale, real-world feasibility, and patient safety, enables near-term implementation even as multicenter studies further define optimal hold durations, ultrasound cut-offs, and fertility outcomes. As such, it can serve as a replicable model for bridging metabolic innovation with perioperative vigilance in IVF care. This protocol, balancing airway safety and fertility preservation, offers a pragmatic, immediately actionable model for IVF centers in high-obesity regions, paving the way for safe metabolic innovation.
Conflict of Interest Statement
This study did not receive any external funding. B.P., serving as both the Principal Investigator and Chair of the Research Ethics Board at Al Ain Fertility Center, acknowledges a potential conflict of interest due to this dual role. This has been fully disclosed to ensure transparency and uphold research integrity. K.S.A. and D.U. declare that they have no conflicts of interest, financial or otherwise, that could have influenced the content or interpretation of this review. All authors attest that they were not involved in any concurrent research projects and that the data and content presented in this review have not been previously published, except where explicitly stated.
CRediT Authorship Contribution Statement
K.S.A. and D.U. contributed equally to this work. K.S.A. conceptualized the clinical scope, contributed to methodology framing, provided domain-specific insights for anesthesia risk management, and critically reviewed the manuscript. D.U. conceptualized the review structure, conducted literature review and synthesis, contributed to methodology framing, developed the first draft of the manuscript, prepared tables, and coordinated all revisions and correspondence. B.P. supervised the overall review, provided administrative and ethical oversight, validated the clinical framework, and reviewed and edited the final manuscript for intellectual content.
References
- Varughese MS, O’Mahony F, Varadhan L (2025) GLP‑1 receptor agonist therapy and pregnancy: Evolving and emerging evidence. Clinical Medicine 25: 100298.
- American Society of Anesthesiologists (2024) Most patients can continue diabetes, weight-loss GLP‑1 drugs before surgery; those at highest risk for GI problems should follow liquid diet before procedure, USA.
- Zhou L, Qu H, Yang L, Shou L (2023) Effects of GLP‑1 receptor agonists on pregnancy rate and menstrual cyclicity in women with polycystic ovary syndrome: A meta-analysis and systematic review. BMC Endocrine Disorders 23: 245.
- Telek SB (2024) Relevance of glucagon-like peptide-1 agonists for infertility practice. Reproductive BioMedicine Online 49:
- Salamun V, Jensterle M, Janez A, Vrtacnik‑Bokal E (2018) Liraglutide increases IVF pregnancy rates in obese women with polycystic ovary syndrome after poor response to first‑line fertility treatments: A pilot randomized study. European Journal of Endocrinology 179: 1-
- Joshi GP, Abdelmalak BB, Weigel WA, Sulpicio G. Soriano, Monica W. Harbell, et al. (2023) American Society of Anesthesiologists consensus‑based guidance on preoperative management of patients (adults and children) on glucagon‑like peptide‑1 receptor agonists, USA.
- Kindel TL, Wang AY, Wadhwa A, Allison R Schulman, Reem Z Sharaiha, et al. (2024) Multisociety clinical practice guidance for the safe use of glucagon-like peptide-1 receptor agonists in the perioperative period. Surgery for Obesity and Related Diseases 20: 1183-1186.
- Kobori T, Onishi Y, Yoshida Y, Tazu Tahara , Takako Kikuchi, et al. (2023) Association of glucagon‑like peptide‑1 receptor agonist treatment with gastric residue in esophagogastroduodenoscopy. Journal of Diabetes Investigation 14(6): 767-
- Pfeifer KJ, Selzer A, Mendez CE, Christopher M Whinney, Barbara Rogers, et al (2021) Preoperative management of endocrine, hormonal, and urologic medications: SPAQI consensus statement. Mayo Clinic Proceedings 96: 1655-1669.
- Goldberg AS, Boots CE (2024) Treating obesity and fertility in the era of glucagon‑like peptide‑1 receptor agonists. Fertility and Sterility 122(2): 211-
- Mandelbaum RS (2025) Glucagon-like peptide-1 receptor agonists: a magic bullet or a double-edged sword? F&S Reports 6(1): 10.
- Sola‑Leyva A, Pathare ADS, Apostolov A, Elina Aleksejeva, Keiu Kask, et al (2025) The hidden impact of GLP‑1 receptor agonists on endometrial receptivity and implantation. Acta Obstetricia et Gynecologica Scandinavica 104: 258-
- Varnum AA, Pozzi E, Deebel NA, Evans A, Eid N, et al. (2024) Impact of GLP‑1 agonists on male reproductive health - A narrative review. Medicina 60(1): 50.
- Merhi ZO (2025) Impact of dramatic weight loss with the new injectable medications on reproduction in healthy non-polycystic ovary syndrome obese women. F & S Reports 6(1): 4-9.






























