Determination of Lidocaine hydrochloride in Pharmaceutical Preparations and Industrial Wastewater Samples
Nief Rahman Ahmed1*, Ghfran Naif Rahman2, Zaid Naif Rahman3
1Department of Environmental Technology, University of Mosul-Iraq
2Student at Medical College, University of Mosul, Mosul-Iraq
3Student at Anesthesia Techniques, al hadba, College university, Mosul-Iraq
Submission: March 27, 2024; Published: May 10, 2024
*Corresponding author: Nief Rahman Ahmed, Department of Environmental Technology, University of Mosul, Iraq
How to cite this article: Nief Rahman A, Ghfran Naif R, Zaid Naif R. Determination of Lidocaine hydrochloride in Pharmaceutical Preparations and Industrial Wastewater Samples. J Anest & Inten care med. 2024; 13(4): 555868.DOI 10.19080/JAICM.2024.13.555868
Abstract
A simple, rapid, accurate and sensitive spectrophotometric method for determination of Lidocaine hydrochloride has been developed. The proposed method is based on the reaction between chloride ion and mercuric thiocyanate, formation of a colored complex by the reaction between released thiocyanate and ferric ions to form red soluble product with maximum absorption at 460 nm. Beer’s law is obeyed over the concentration range of 2-28μg/ml, with molar absorptivity of 0.659×104 l/mol.cm. The present method is simple because it does not need either heating or hydrolysis or solvent extraction steps. The method has been successfully applied for the determination of Lidocaine hydrochloride in pure form, pharmaceutical preparations (Gels and Ampules) and environmental wastewater sample.
Key words: Lidocaine Hydrochloride; Mercuric Thiocyanate; Pharmaceutical Preparations
Background
Lignocaine hydrochloride [NN-Diethyl (2,6-dimethylphenyl) carbamoyl] methyl-ammonium chloride monohydrate (Figure 1). The other name Lidocainr Hydrochloride, xylocard and xylotox [1]. Lidocaine hydrochloride is the local anesthetic agent. Lidocaine is used topically to relieve itching, burning and pain from skin inflammations. Lidocaine, the first amino amide-type local anesthetic, was first synthesized under the name Xylocaine [2,3]. Lidocaine was official in British Pharmacopoeia which recommends potentiometric non-aqueous method for its analysis [4]. Literature survey reveals that numerous methods have been published for quantitative analysis of Lidocaine hydrochloride alone and in combination with other drugs such as high-performance liquid chromatography HPLC [5-8], gas chromatography [9], capillary electrophoresis [10], selective membrane electrode [11] and spectrophotometric methods [12-15]. The present work describes a new, simple spectrophotometric method for the determination of Lidocaine hydrochloride in pure form, pharmaceutical formulations and in industrial wastewater samples. The method is based on reaction between chloride ion and mercuric thiocyanate, formation of a colored complex by the reaction between released thiocyanate and ferric ion.
Experimental
Apparatus
Shimadzu UV- 1700 pharma spec (Double Beam) spectrophotometer with 1.0 cm quartz cells was used for absorption measurements.
Reagents
All chemical used were of analytical or pharmaceutical grade and Lidocaine hydrochloride standard material was provided from AL-Hokamaa company for pharmaceutical industries (HPI) Mosul-Iraq.
Lidocaine Hydrochloride Standard Solution :0.01% (100μg/ml)
This solution was prepared by dissolving 0.01 gm of Lidocaine hydrochloride in 100 mL of distilled water in a volumetric flask.
Ferric Ammonium Sulphate Solution: 5%
5g of ferric ammonium sulphate [FeNH4(SO4)2.12H2O] was dissolved in 50 ml double distilled water and 20ml of concentrated nitric acid was added and diluted with double distilled water to 100ml.
Mercuric Thiocyanate Solution: 0.5%
0.5g of mercuric thiocyanate was dissolved and diluted to 100 ml with ethanol. Mixed and filtered through filter paper.
General Procedure
Different aliquots of standard Lidocaine hydrochloride solution equivalent 50-700 μg (0.5-7 mL) were transferred into a series of 25ml volumetric flasks, and 2mL of ferric ammonium sulfate solution were added and 2ml of saturated solution of mercuric thiocyanate were added to each flask and mixed well with occasional shaking. This was diluted to 25ml with double distilled water. and mixed well. Let stand for 5min, the absorbance of each solution was measured at 460 nm against a reagent blank.
Procedures For Pharmaceutical Preparations (Ampules 2%)
To minimize a possible variation in the composition of the ampules (containing 2% of Lidocaine hydrochloride/tablet were provided from AL-Hokamaa company for pharmaceutical industries (HPI) Mosul-Iraq).). The mixed content of 5 ampules was transferred into 100mL volumetric flask and diluted up to the mark with distilled water, The determination of Lidocaine hydrochloride was treated as described above under general procedure. and the concentration was calculated by using the calibration curve of this method.
Procedures For Pharmaceutical Preparations (Gels)
The content of 5 bottle) [1%] provided from AL-Hokamaa company for pharmaceutical industries (HPI) Mosul-Iraq) [1%] were mixed well in 1L dried beaker. Aliquots equivalent to 100 mg of Lidocaine hydrochloride were transferred into 1L volumetric flasks and diluted with distilled water to the volume. The determination of Lidocaine hydrochloride proceeded as described under recommended procedure and the concentration was calculated by using the calibration curve of this method.
Procedure for industrial wastewater samples
To demonstrate the practical applicability of the proposed method, real industrial wastewater samples from Al-Hokamaa company for drug industries (HPI) Mosul-Iraq were analyzed by spiked with the concentrations ranging from 5-25 μg /ml of Lidocaine hydrochloride and aliquot of this solution was treated as described above under general procedure and the concentration was calculated by using the calibration curve of this method.
Results and Discussion
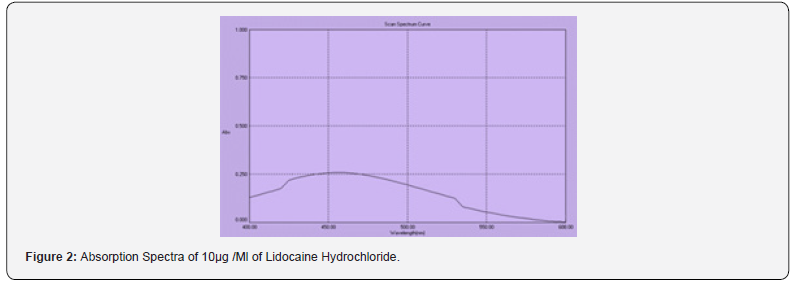
The method depends upon the displacement of thiocyanate ion from mercury (I1) thiocyanate by chloride ion in the Lidocaine hydrochloride. The released thiocyanate was found to react with Fe III at room temperature resulting in formation of red colored complex which absorbed at 460nm, and the intensity of its color is proportional to the original chloride ion [16-18].

(Figure 2) The various experimental affecting the development and stability of the reaction product were optimized by changing each variable in turn while keeping all other variables constant.
Effect of Ferric Ammonium Sulfate Solution
The amount of ferric ammonium sulfate solution (5%) for maximal color intensity was examined. The maximum constant intensity was reached at 1 ml of reagent solution and remained constant up to 5ml. However, 2 ml of the reagent solution was selected for the subsequent work.
Effect of Mercuric Thiocyanate Solution
The amount of mercuric thiocyanate solution (0.5%) for maximal color intensity was examined. The maximum constant intensity was reached at 1 ml of reagent solution and remained constant up to 5ml. However, 2 ml of the reagent solution was selected for the subsequent work.
Effect of Temperature and Time
The results obtained indicated that complete color formation occurred immediately and was not affected by temperature therefore, room temperature was selected as suitable temperature. The absorbance remained constant for 6 hours at least, and 5 min was selected as a suitable time.
Effect of Order of Addition
To test the effect of the order of the addition of the reagents on the absorbance of the product, different orders were tested. The selected order was sample solution, ferric ammonium sulphate followed by mercuric thiocyanate solution which was gave high absorbance value.
Calibration Graph
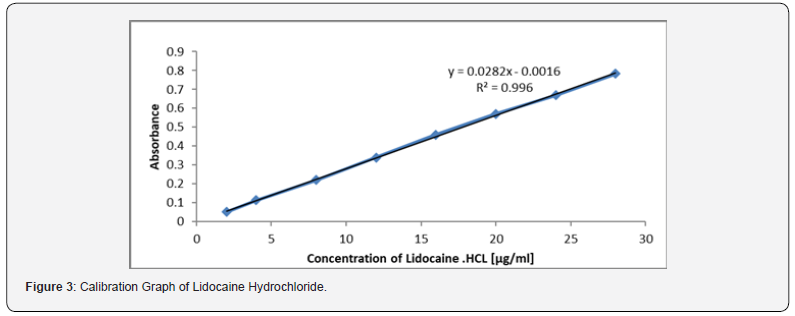
Employing the conditions described in the general procedure a linear calibration graph of Lidocaine hydrochloride which obeys Beer’s law in the concentration range of 2-28 μg/ml. Linear regression equation: Y= 0.0282X- 0.0016 (r=0.996). Where Y is the absorbance and X is concentration in μg/ml. The apparent molar absorptivity was 0.659×104 l.mol-1.cm-1 and sand ell’s sensitivity were 0. 0357μg.cm2. The limit of detection and quantification were evaluated as [19]. Where b is the stop and S_0 is the standard deviation of the regression line. The limit of detection was 0.567 μg .ml-1 and the limit of quantification as the lowest standard concentration which could be determine with acceptable accuracy, and precision was 1.9 μg.ml-1. The applied method can be used routinely for the estimation of pure drug salts through their chloride concentration (Figure 3).
Accuracy and Precision
The accuracy and precision of the method were established by analyzing the pure drug solution at three different levels. The average recovery which is a measure of accuracy is 100 ±0.69% revealing high accuracy of the method. The relative standard deviation (RSD), which is an indicator of precision, is less than 1.5%, the result is compiled in (Table 1).

Application of the Proposed Method
The proposed method was successfully applied to the analysis of Lidocaine hydrochloride in Gels, Ampules, and industrial wastewater samples. The result of analysis for pharmaceutical formulations reveals that there is close agreement between the results obtained by the proposed method and the label claim, and the results of water samples show that the recovery values obtained were close to 100%, (Table 2), (Table 3).

*Mean of ten Determinations.

*Mean of ten Determinations.
Acknowledgments
The author wishes to express gratitude to Al-Hokama company for pharmaceutical industry (HPI) (Ninevah – Iraq.) for providing gift samples of Lidocaine. HCL standard material and for permission and facilities to carry out the research work.
Conclusions
The applied method was simple, rapid, accurate, precise, sensitive, and low economical cost. Furthermore, the proposed method doesn’t require elaboration of procedures, which are usually associated with chromatographic methods. The proposed method could be applied successfully for determination of Lidocaine Hydrochloride in environmental water samples, pure form as well as in different dosage forms.
References
- Pharmaceutical Society of Great Britain (1979) The pharmaceutical codex: Incorporating the British pharmaceutical codex. 11th Edn, Pharmaceutical Press, London, pp. 493.
- British National Formulary (BNF) Royal Pharmaceutical Society (2016) pp. 91.
- The Japanese Pharmacopoeia, (17th Edition), English Version, The Ministry of Health, Labor, and Welfare (2016) 1181-1182.
- British Pharmacopoeia, H.M. Stationery office, London, UK (2014) II-109.
- Chen L (2007) Simultaneous determination of nikethamide and lidocaine in human blood and cerebrospinal fluid by high performance liquid chromatography. J Pharma Biomed Analysis 43(5): 1757-1762.
- United States Pharmacopeia and National Formulary (2018) USP 41, NF 36. 2413.
- Abdelwahab NS, Nouruddin W, Ali M, Abdelkawy M, Emam AA (2016) Validated RP-HPLC and TLC-Densitometric Methods for Analysis of Ternary Mixture of Cetylpyridinium Chloride, Chlorocresol and Lidocaine in Oral Antiseptic Formulation. J Chromatogr Sci 54(3): 318-325.
- Gandla K, Kumar DS, Praveen J, Suman E (2017) RP-HPLC Method Development and Validation for Simultaneous Estimation of Lignocaine Hydrochloride and Clotrimazole Hydrochloride in Ear Drops. Asian J Pharm Anal 7(3): 163.
- Baniceru M, Croitoru O, Popescu SM (2004) Determination of some local anesthetics in human serum by gas chromatography with solid-phase extraction. J Pharm Biomed Anal 35(3): 593-598.
- Chik Z, Johnston A, Tucker A, Burn R, Perrett D (2007) Validation and application of capillary electrophoresis for the analysis of lidocaine in a skin tape stripping study. Biomed. Chromatogr 21(8): 775-779.
- Giahi M, Pournaghdy M, Rakhshaee R (2009) A new lidocaine-selective membrane electrode based on its sulfathiazole ion-pair. J Anal Chem 64: 195-200.
- Lazeeza Sattar O, Rasul Jameel A (2017) Extraction-spectrophotometric Determination of Lidocaine. HCL in Pharmaceuticals Int J Chem 9(4): 49-61.
- Ingle S, Tegeli V, Birajdar A, Matole V, Adlinge S, et al. (2021) UV Spectrophotometric Method Development and Validation of Lignocaine Hydrochloride in Bulk and Semisolid Dosage Form Res J Pharm Technol 14: 5280-5282.
- Ismaiel SA, Yassa DA, Gad-El-Rub LN (1975) Spectrophotometric determination of lidocaine in some pharmaceutical preparations using bromocresol green. Pharmazie 30(6): 408.
- Karthik KB (2012) Analytical method development and validation of lidocaine in ointment Formulation by UV Spectrophotometric method. Int J Pharma Sci 4(2): 610-614.
- (1989) Vogel Textbook of quantitative chemical analysis, (5th Edition), John Wiley & Sons Inc p. 700.
- Nief A, Husam WY, Mohammed NA (2018) Novel estimation of triprolidine hydrochloride in pharmaceutical preparations and environmental wastewater samples. World J Pharmacy & Pharma Sci 7(11): 151-160.
- Nief RA, Nadia CS, Amenah IA (2020) Estimation of Metoclopramide. HCL in pharmaceutical preparation and environmental water. World J Pharmacy & Pharma Sci 9(1): 151-159.
- Nief RA, Najlaa SS, Mohammad JE (2020) A sensitive estimation of Tamoxifen in pharmaceutical preparations and environmental wastewater samples, Eur J Pharma Med Res 7(4): 104-107.






























