A Novel Approach to Determining Anesthesiologist Performance Value
Anthony J Tanella1*, Suzan Uysal2, Andrew B Leibowitz2
1Department of Anesthesiology, Yale School of Medicine, USA
2Department of Anesthesiology, Perioperative and Pain Medicine, Icahn School of Medicine at Mount Sinai, USA
Submission: August 10, 2023; Published: October 02, 2023
*Corresponding author: Anthony J Tanella, Department of Anesthesiology, Yale School of Medicine, USA
How to cite this article: Anthony J T, Suzan U, Andrew B L. A Novel Approach to Determining Anesthesiologist Performance Value. J Anest & Inten care med. 2023; 12(5): 555850. DOI 10.19080/JAICM.2023.12.555850
Abstract
Anesthesiologist reimbursements are increasingly tied to incentive programs based on healthcare quality and value. There are no standardized metrics which quantify both anesthesiologist care delivery quality and cost. The object of our study is to design an anesthesiologist specific value metric (quality/cost). We focused our study on four surgical procedures performed with high frequency and little variability at a tertiary care center: laparoscopic sleeve gastrectomy, laparoscopic appendectomy, laparoscopic robotic prostatectomy, and laparoscopic cholecystectomy. In calendar year 2018, we calculated cost of the pharmaceuticals administered by anesthesiologist and a novel quality score determined using 4 outcome measures:.
1) Total morphine equivalents administered within 6 hours postoperatively
2) Number of antiemetic medication doses administered within 6 hours postoperatively
3) Post anesthesia recovery unit length of stay
4) Time from procedure finish to tracheal extubation.
For each procedure anesthesiologists’ performance value (novel quality score / pharmaceutical cost) was compared. We identified a highly variable average cost per case-hour for each procedure and no correlation between cost and quality for any of the four procedures. The cost variability identified by our value calculations, and lack of correlation with quality, suggests there are opportunities to identify low value care and improve outcomes while reducing cost.
Keywords: Anesthesiology; Quality; Cost; Value; Benchmarking
Introduction
Healthcare insurance reimbursement has significantly changed over the past decade, shifting from the traditional fee-for-service payment model in which reimbursement is based on the services and procedures performed, to various quality-based and/or value-based payment models that incentivize desired provider behaviors to increase efficiency and effectiveness and reduce costs [1-4]. Reimbursement based on the quality of care has most commonly been applied in primary care settings; anesthesiology largely remains reimbursed by the fee-for-service model. The Centers for Medicare and Medicaid Services (CMS) Merit Based Incentive Payment program (MIPS) does reward and penalize anesthesiologist performance with positive and negative payment adjustments on several quality performance measures, such as perioperative beta blocker administration in certain patient groups (a process measure) and normothermia maintenance (an outcome measure).
While the MIPS program has had minimal financial impact on anesthesiology reimbursements, it is likely that the practice of anesthesiology will be impacted in the future given the significant market pressures to reign in expense and the shift by the healthcare insurance market toward value-based payment programs [5]. It is therefore imperative that the field of anesthesiology begin to define clinically relevant value-based metrics. Previous work sought to quantify anesthesiologist care quality [6-9] or operating room costs [10-16], yet there are no standardized metrics which quantify anesthesiologist care value (quality/cost). This study aims to identify specific, measurable, and relevant value metrics based on quality and cost of anesthesiology care. Our objective was to develop a value-based performance metric to quantify patient outcomes and anesthetic costs for several commonly performed surgeries and apply the metric to identify anesthesiologists on the low and high ends of the value spectrum to determine a value benchmark for implementing best practices at best cost.
Introduction
The Institutional Review Board/Program for Protection of Human Subjects approved this retrospective study (Human Subjects Number: IRB-19-01820). Informed consent was waived. This study adheres to the appropriate SQUIRE 2.0 guidelines. Tableau Desktop Profession Edition 2020.4.1 was used to create charts, calculate, means, standard deviations, and generalized linear regression models for Pearson correlation coefficients and p-values.
Case Identification
We queried our electronic medical record (EMR) system by Current Procedural Terminology (CPT®) codes to identify patients undergoing laparoscopic sleeve gastrectomy (CPT 43775), laparoscopic robotic prostatectomy (CPT 55866), laparoscopic appendectomy (CPT 44970), or laparoscopic cholecystectomy (CPT 47563) at our institution between January 1st, 2018, and December 31st, 2018. Cases that were converted from laparoscopic to open procedures were excluded. These four operations were chosen because they were performed frequently, and they are subject to relatively little surgical variation with respect to intraoperative management and postoperative destination. At our institution these operations are all performed with general endotracheal anesthesia, which minimizes the variation in anesthetic costs due to materials and equipment. For each procedure, we then filtered the number of cases studied by limiting them to a subset of anesthesiologists who provided anesthesia at least six times over the course of the study year. Cases in which a handoff to a second attending occurred during the procedure were excluded. Patients aged younger than 2 years old were excluded from this study as those patients are cared for by a separate group of pediatric subspecialty trained anesthesiologists.
Quality Calculation
For each case, quality was determined by two efficiency measures and two outcome measures that are easily extracted from the EMR. The two measures of efficiency were postanesthesia care unit (PACU), length of stay (LOS) and procedure finish-to-extubation time. Highly efficient anesthetics would yield low PACU LOS and low procedure finish-to-extubation times. The PACU LOS and procedure finish-to-extubation time were averaged for each anesthesiologist for each procedure. The two outcome measures were postoperative nausea and vomiting (PONV) and post-operative pain (POP), as major adverse outcomes (e.g., reintubation in PACU, cardiac arrest) are quite rare with the procedures studied [17].
These two metrics were chosen because they are specific, easily measurable, and commonly accepted ways of judging the quality of care [18-19]. More desirable anesthetics would yield low rates of PONV and low POP scores. To quantify PONV we summed the number of antiemetic medications administered per patient per CPT code within six hours postoperatively. To quantify POP, we summed the total amount of intravenous (IV) morphine equivalents administered within six hours postoperatively, based on the following conversion: Morphine 10 mg IV = Morphine 20 mg PO = Fentanyl 100 mcg IV = Hydromorphone 2mg IV = Hydromorphone 4 mg PO = Oxycodone 15 mg PO = Methadone 10 mg IV = Methadone 20 mg PO [20].
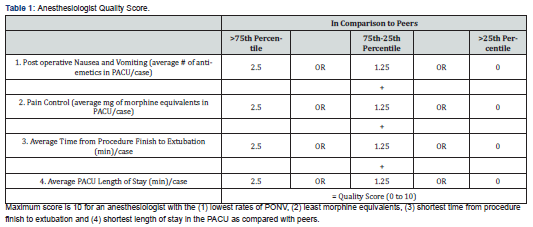
number of antiemetics administered and total IV morphine equivalents administered within six hours post operatively were averaged for each anesthesiologist for each procedure. The six-hour time frame was chosen as nearly all IV pain, nausea medications, or local anesthetics administered by the anesthesiologist during the case would be metabolized. We excluded the time beyond the six-hour limit as it would be less reflective of an anesthesiologist’s skill or influence. For each of the four procedures considered in our analysis, the four measured variables were separately ranked by lower, upper, or middle two quartiles of anesthesiologist performance and credited with “points” ranging from 0 to 2.5 (Table 1). For the four measures combined, a practitioner performing in the top quartile on all four measures would earn 10 points, while a practitioner performing in the lowest quartile on all 4 measures would earn 0 points (Table 1).
Cost Calculation
Determination of cost per case was based on pharmaceutical costs alone. Previous work identified the main cost centers in anesthesia care as staffing, equipment, and pharmaceuticals. For our cost calculation we focused on items which were at the sole discretion of the anesthesiologist. Therefore, we excluded anesthesia staffing costs as they are proportional to procedure length and procedure length is not at the discretion of the anesthesiologist. The cost of supplies (e.g., Angio catheters, tracheal tubes, laryngoscopes, syringes, etc.) was also not included as it is impossible to calculate accurately and unlikely to vary from case-to-case for the specific surgical procedures studied. The four operations chosen for this study all necessitated general anesthesia with an endotracheal tube.
Additional materials costs might be incurred if the anesthesiologist used invasive blood pressure monitoring, a flexible bronchoscope for intubation or additional intravenous access. However, these additional costs were excluded as this equipment is required for patients with certain co-morbidities and should not impact the “value” metric. All pharmaceuticals given at the sole discretion of the anesthesiologist were captured from the EMR; pharmaceuticals that were likely requested by the surgeon were excluded from the cost analysis (see Appendix). Cost data were obtained from our health system pharmacy as of October 22, 2019. While real time point of administration cost data would be preferable to account for physician cost awareness, it was not available for our analysis. Therefore, we chose cost data that corresponded to the beginning of our data aggregation.
When pharmaceuticals were available in multiple size ampules, vials, or premixed solutions, the most used medication ampule, vial, or premixed solution was used. Separate cost selections were made for bolus medications and infusion medications. To calculate the number of ampules, vials or premixed solutions used, we rounded up to the nearest whole number contained in the most used container as it is system policy and industry standard practice not to reuse an ampule, vial, or premixed solution for more than one patient. For example, if 275 mcg of fentanyl was utilized in a case, we assumed two 250 mcg vials were used. Using the costs provided by the health system pharmacy we multiplied the number of vials utilized per case by the cost per ampules, vials, or premixed solution. For each drug used during a case, we aggregated costs by the method above.
aggregated costs by the method above. For volatile anesthetics used we utilized the calculations derived by Biro and colleagues [21]. Unlike the intravenous medications, the vaporizers in our anesthesia machines can utilize a portion of the whole canister. Since our anesthesia information management system did not consistently record flow, we assumed an average standard flow rate of 2L/min to determine volatile anesthetic usage. Finally, we totaled the cost of all the anesthetics both IV and volatile. Since the length of the surgery is controlled by the surgical team and longer operations require more anesthetics, we chose to normalize pharmaceutical costs to dollars/hour. To arrive at the average cost per hour ($/hour) for each anesthesiologist, we totaled the cost for all drugs used in cases with a particular CPT code and divided by the number of hours of anesthesia care provided for those cases.
Anesthesiologist Performance Value Calculation
Anesthesiologist’s performance value was defined by the equation Value = (Quality Score Ranking)/ (Average Cost Per Hour).
Results
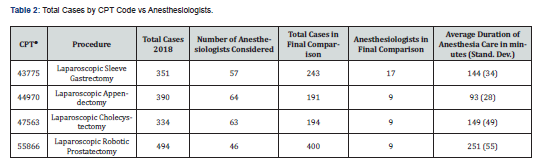
For each of the four procedures performed during the study interval, the total number of cases performed, the total number of anesthesiologists providing anesthesia care, and the total numbers of anesthesiologists and cases included in the study are presented in (Table 2). The number of anesthesiologists and cases studied for each procedure were as follows: 17 anesthesiologists provided care for 243 laparoscopic sleeve gastrectomy procedures, 9 anesthesiologists provided care for 191 laparoscopic robotic prostatectomy procedures, 9 anesthesiologists provided care for 194 laparoscopic appendectomy procedures, and 9 anesthesiologists provided care for 400 laparoscopic cholecystectomy procedures (Table 2).
For each of the four procedures the quality score per anesthesiologist, the cost per hour per anesthesiologist, and the calculated value per anesthesiologist are presented in (Figures 1-4). There was no correlation between cost and quality for any of the four procedures (Pearson correlation coefficients for CPT 43775: r = 0.137 (p=0.600), CPT 44970: r = 0.107 (p = 0.612), CPT 47563: r = 0.052 (p=0.894), CPT 55866: r = 0.421 (p=0.260), p-values > 0.05 in all cases). Notably, the cost varied by more than two-fold (highest / lowest cost) for laparoscopic sleeve gastrectomy ($59.79 / $23.86 = 251%), laparoscopic robotic prostatectomy ($30.64 / $12.65 = 242%), and laparoscopic cholecystectomy ($36.93 / $15.66 = 236%); for laparoscopic appendectomy the cost varied by 51% ($44.72 / $29.60 = 151%).
Limitations
Our anesthesiologist’s performance value calculation is imperfect and does not incorporate all valued aspects of an anesthesiologist’s care. A more perfect anesthesiologist performance value calculation would require significantly more detailed information such as patient survey information, real time cost data and materials use tracking. At present this information is not available in our EMR. Therefore, we limited the scope of this study to four specific operations to reduce the influence of surgical variability and materials cost. This anesthesiologist’s performance value calculation is most reflective of an anesthesiologist’s skill when surgical variability is limited. This study has several other limitations. First, we did not study all cases performed by all anesthesiologists for each of the four procedures.
If anesthesiologists perform cases with low frequency, value calculations are subject to unrepresentative variability from a single case cost, outcome, efficacy, or efficiency outlier. At our institution, it is common for a subgroup of anesthesiologists to work regularly with a subgroup of surgeons. As such, the value charts for laparoscopic cholecystectomy and laparoscopic robotic prostatectomy contain anesthesiologists with low anesthetic case counts for year 2018, as many of these surgical cases have their anesthetics administered by two to four anesthesiologists. Therefore, value calculations for low volume providers may be more volatile. However, we felt it beneficial to include a larger number of anesthesiologists in the value calculation analysis for illustrative purposes. Other limitations include failure to include materials cost, the PACU length of stay being determined by factors that are beyond the control of the anesthesiologist (e.g., bed availability, escort home), and possible charting errors.
Additionally, these findings may not generalize to other possible outcome measures; the outcome measures studied were specifically chosen both for their ease of extraction from the EMR and their general acceptance by anesthesiologists as reflective of their skill. Lastly anesthesiologists at our institution care for patients independently on a one physician-to-one patient basis or they may medically direct certified registered nurse anesthetists (CRNA) or resident physicians. Thus, some of the variability in the outcomes and costs may be attributable to decisions made by CRNAs or residents.
Discussion
Modern healthcare has attempted to tie physician reimbursement to the value (i.e., outcomes/cost) of the healthcare. While physician anesthesiologist reimbursement maintains many of the aspects of fee-for-service model, there is a push by CMS through their quality payment programs to quantify the variability of anesthesiologists’ care. The objective of this study was to develop a value-based performance metric to quantify patient outcomes and anesthetic costs for several commonly performed surgeries and apply the metric to determine a value benchmark for implementing best practices at best cost. To our knowledge this is the first study demonstrating that anesthesiologist performance value can be quantified, analyzed, and potentially improved.
This basic approach is a first step in determining the value of our specialty’s care on a case-by-case basis and allows for comparison amongst anesthesiologists that can be used in performance improvement initiatives and Enhanced Recovery After Surgery (ERAS) protocol development. Quantifying anesthesiologists’ performance value is practical and easily calculated, utilizing readily available data from EMRs to generate a 10-point quality score encompassing efficiency (procedure finish-to-extubation), efficacy (PACU pain and rescue anti-emetic use), and outcome (PACU LOS), adjusting for total medication cost per unit of time.
For each of the four procedures studied, costs and quality scores varied across anesthesiologists but were uncorrelated, indicating that there is an opportunity to reduce costs without compromising quality. Multiple anesthesiologists who earned higher quality scores used lower cost anesthetics (1,3 in Figure 1), (9,18 in Figure 2) and (23 in Figure 3). More specifically, the data suggest that cost may be reduced by more than 50% (lowest / highest cost) for laparoscopic cholecystectomy, laparoscopic robotic prostatectomy, and laparoscopic sleeve gastrectomy, and 33% (lowest / highest cost) for laparoscopic appendectomy, without compromising quality of care. An example of delivering higher quality care at a lower cost is utilizing sevoflurane over desflurane, a more expensive volatile anesthetic. If titrated appropriately both should yield a similar finish to extubation time, but the cost of sevoflurane is 61% the cost of desflurane.

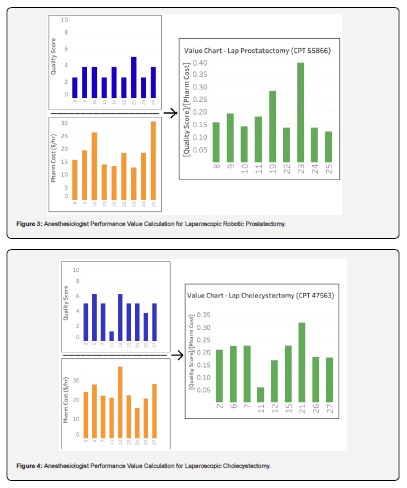


Two elements that likely influence the cost variation per procedure are duration of procedure and patient weight. The laparoscopic robotic prostatectomy, laparoscopic cholecystectomy, and laparoscopic sleeve gastrectomy procedures all had longer average anesthesia care durations than laparoscopic appendectomy (Table 2). This suggests that longer cases are subject to greater variation in cost, as more medications are likely to be administered per unit time and there is more room for practice variation amongst practitioners. Patient weight will increase cost variability given that many drugs are dosed on a milligram per kilogram basis; this was apparent in the current study data which showed overall increased average costs for laparoscopic sleeve gastrectomy compared to the other procedures.
Conclusion
The continuing rise in health costs in the United States is not sustainable, thus there is a growing move toward value-based health care in which value is derived from measuring patient health outcomes and provider performance, against the cost-of-service delivery. A key driver that governments and insurers use to reshape provider behavior toward value-based rather than quantity-based health care is financial incentives and disincentives. Top-down approaches to improving the value of healthcare imposed by government and insurers, however, may be complemented by bottom-up approaches developed by healthcare providers and administration who also recognize the importance of improving efficacy and efficiency of the healthcare that they provide and reducing waste.
Government and insurer programs that aim to influence healthcare provider behaviors by financial incentives and disincentives alone, however, may have limited power to motivate behavior change that affects quality and cost of care. Healthcare provider behavior, however, is motivated by non-financial factors as well, including social ranking [22]. Physicians are naturally ambitious and competitive and have high self-esteem. Ranking physician performance may serve as a powerful motivating force for underperforming physicians to change their practice behaviors to improve quality, reduce cost, and improve their relative rankings on specific metrics.
Implications
We present a simple means of calculating an anesthesiologist’s performance value relative to their peers providing an anesthetic for the same operation using four commonly accepted measures assessing quality. The results suggest that there is an opportunity to both reduce costs and improve quality, and thereby increase the anesthesiologist’s performance value. Future work includes the design of a real-time dashboard accessible by all anesthesiologists within our practice that allows them to review their performance value metrics (e.g., social ranking), anesthetic quality scores, and anesthetic costs on a case-by-case bases, with the goal of empowering anesthesiologists by increasing their awareness of the impact of their anesthetic regimens on their patients and cost of delivering care.
References
- Kolarczyk LM, Arora H, Manning MW, Zvara DA, Isaak RS (2018) Defining Value-Based Care in Cardiac and Vascular Anesthesiology: The Past, Present, and Future of Perioperative Cardiovascular Care. J Cardiothorac Vasc Anesth 32(1): 512-521.
- Mahajan A, Esper SA, Cole DJ, Fleisher LA (2021) Anesthesiologists’ Role in Value-based Perioperative Care and Healthcare Transformation. Anesthesiology 134(4): 526-540.
- (2020) Centers for Medicare and Medicaid Services: CMS issues new roadmap for States to accelerate adoption of value-based care to improve quality of care for Medicaid beneficiaries.
- Humana: The transition to value.
- Johnston KJ, Hockenberry JM, Joynt Maddox KE (2021) Building a Better Clinician Value-Based Payment Program in Medicare. JAMA - J Am Med Assoc 325(2): 129-130.
- Peccora CD, Gimlich R, Cornell RP, Vacanti CA, Ehrenfeld JM, et al. (2014) Anesthesia report card - A customizable tool for performance improvement. J Med Syst 38(9).
- Dutton RP (2015) Making a difference: The anesthesia quality institute. Anesth Analg 120(3): 507-509.
- McCormick PJ, Yeoh C, Vicario Feliciano RM, Ervin K, Tan KS, et al. Improved Compliance with Anesthesia Quality Measures After Implementation of Automated Monthly Feedback. J Oncol Pract 15(6): e583-e592.
- McCormick PJ, Yeoh CB, Hannum M, Tan KS, Vicario Feliciano RM, et al. (2020) Institution of Monthly Anesthesia Quality Reports Does Not Reduce Postoperative Complications despite Improved Metric Compliance. J Med Syst 44(11): 189.
- Childers CP, Maggard Gibbons M (2018) Understanding costs of care in the operating room. JAMA Surg 153(4): e176233.
- Macario A (2010) What does one minute of operating room time cost? J Clin Anesth 22(4): 233-236.
- Schuster M, Standl T, Wagner JA, Berger J, Reißmann H, et al. (2004) Effect of different cost drivers on cost per anesthesia minute in different anesthesia subspecialties. Anesthesiology 101(6): 1435-1443.
- Abouleish AE, Prough DS, Vadhera RB (2004) Influence of the type of anesthesia provider on costs of labor analgesia to the Texas Medicaid Program. Anesthesiology 101(4): 991-998.
- Schuster M, Standl T (2006) Cost drivers in anesthesia: Manpower, technique and other factors. Curr Opin Anaesthesiol 19(2): 177-184.
- French KE, Guzman AB, Rubio AC, Frenzel JC, Feeley TW (2016) Value based care and bundled payments: Anesthesia care costs for outpatient oncology surgery using time-driven activity-based costing. Healthcare 4(3): 173-180.
- Majstorovic B, Milakovic B, Markovic S, Mijajlovic M, Kastratovic D (2014) The results and methodological concerns about pharmaco-economic evaluation in anesthesia. Hosp Pharmacol J 1(2): 68-75.
- Sun EC, Dutton RP, Jena AB (2018) Comparison of Anesthesia Times and Billing Patterns by Anesthesia Practitioners. JAMA Netw Open 1(7): e184244.
- MPOG Multicenter Perioperative Outcomes Group: ASPIRE Measures.
- Anesthesia Quality Institute (2021) QCDR measure specifications 1-74.
- Shaheen PE, Walsh D, Lasheen W, Davis MP, Lagman RL (2009) Opioid Equianalgesic Tables: Are They All Equally Dangerous? J Pain Symptom Manage 38(3): 409-417.
- Biro P (2014) Calculation of volatile anaesthetics consumption from agent concentration and fresh gas flow. Acta Anaesthesiol Scand 58(8): 968-972.
- Lubarsky DA, French M, Gitlow H, Rosen L (2019) Why Money Alone Can’t (Always) “Nudge” Physicians. Anesthesiology 130(1).






























