Therapeutic and Prognostic Factors of Upper Gastrointestinal Bleeding in the Intensive Care Unit in a Sub-Saharan African Country
Kathryn Palumbo MD1, Heather Reyes MEng MD1, Sierra Stauber PharmD2 and Jill M Cholette MD1.
1Department of Pediatrics, University of Rochester Medical Center- Golisano Children’s Hospital, Rochester New York, USA
2Department of Pharmacy. University of Rochester Medical Center- Golisano Children’s Hospital, Rochester New York, USA
Submission: June 20, 2023; Published: July 07, 2023
*Corresponding author: Jill M Cholette MD, Department of Pediatrics, University of Rochester Medical Center- Golisano Children’s Hospital, Rochester New York, USA, Email: jill_cholette@urmc.rochester.edu
How to cite this article: Kathryn Palumbo MD, Heather Reyes MEng MD, Sierra Stauber PharmD, Jill M Cholette M. Propofol “Wash-Out” Protocol: Successful Use of Propofol Infusion to Allow for Discontinuation of Multiple High-Dose, Long Duration Infusions of CNS Acting Agents in two Complex Infants.. J Anest & Inten care med. 2023; 12(5): 555848. DOI 10.19080/JAICM.2023.12.555848
Abstract
Background: The aim of our study was to determine the therapeutic and prognostic factors of upper gastrointestinal bleeding (UGB) in the ICU.
Patients and Methods: This was a retrospective descriptive study of records of patients admitted to the ICU from January 2012 to April 2017. Records of patients admitted to UGB in two intensive care units were included. The sampling was consecutive and non-exhaustive. Data were coded and analyzed using SPSS software.
Results: The sample size was 30 cases. The mean age was 55.3 ± 19.1 years. The sex-ratio was 2.3. Nineteen patients were seen more than 72 hours after the onset of bleeding (63.4%). The most frequent mode of revelation was hematemesis (50.7%). Endoscopy was performed in seven patients (23.3%). The management, in addition to resuscitation measures, consisted of parenteral administration of omeprazole. Haemostatic treatment was performed in one patient (3.4%). The mortality rate was 50%.
Conclusion: Upper gastrointestinal bleeding is an important cause of mortality in the ICU.
Keywords: Upper Gastrointestinal Bleeding; Management; Outcome; ICU
Introduction
Upper gastrointestinal bleeding (UGB) is one of the important medical emergencies worldwide, accounting for high morbidity and mortality [1-4]. It requires careful management regardless of the abundance of bleeding and the mode of revelation. Its management should be rapid and appropriate, often requiring endoscopic haemostasis. It remains, despite major advances in technology, a real vital threat. The prevalence of digestive haemorrhage varies from one country to another. UGB is defined as bleeding within the lumen of the gastrointestinal tract from any location between the upper oesophagus to the duodenum at the ligament of Treitz [5].
Critical care physicians are involved in the treatment of patients with gastrointestinal bleeding in several ways. First, patients outside the ICU suffering from gastro-intestinal tract bleeding with hypovolemic shock may need ICU treatment. Secondly, patients can develop gastrointestinal bleeding as a complication during their treatment in intensive care [6]. The global mortality ranges from 5% to 30%. According to French studies, the annual incidence of UGB is roughly estimated at about 100 to 150 episodes per 100,000 inhabitants [7]. The in-hospital mortality appeared to be around 10% in United Kingdom. In countries with limited resources and no endoscopic haemostasis, mortality from digestive haemorrhages is around 30%-40%. The aim of our study was to determine the therapeutic and prognostic factors of UGB in ICU in a low-income country.
Patients and Methods
This was a descriptive, retrospective study. It included records of patients admitted to two intensive care units from January 1st, 2012, to April 30th, 2017. It included the complete records of patients admitted to the intensive care unit for acute upper gastrointestinal bleeding in the intensive care units of the Yaoundé Central Hospital and the Yaoundé University Teaching Hospital. Data collection began after approval by the National Ethics Committee. Information was collected using a pre-established data sheet. The variables studied were socio-demographic data (age, sex), clinical data (pathological history, haemodynamic parameters, mode of bleeding, admission time, initial assessment of the bleeding, biological examinations, and endoscopic findings), therapeutic data (resuscitation measures, specific treatment) and prognostic data (prognostic scores, complications, evolution, death). Patients were stratified based on prognostic scores.
These were the Advanced Trauma Life Support Classification of the American College of Surgeons (ATLAS) and the preendoscopic Rockall score. Resuscitative measures consisted of IV fluids, oxygen therapy, intubation and mechanical ventilation and blood transfusion. Data collected was entered using CSPRO 6.3 software and analyzed using SPSS 21 software. Quantitative data was expressed as means, standard deviations for those following a nominal distribution and median and interquartile range for those with a non-nominal distribution. Categorical data were represented as numbers and percentages. The search for associations between qualitative variables was carried out using the Chi-square test or Fisher’s exact test. Medical confidentiality was respected. All information obtained was treated with utmost confidentiality.
Results
During the study period, fifty-five (55) cases of gastrointestinal bleeding were identified. A total of 32 cases with complete medical files were found from which two cases of lower gastrointestinal bleeding were excluded. This left us with a sample size of 30 patients. From 2012 to 2017, 3159 patients were admitted to the 2 intensive care units. The prevalence of upper gastrointestinal bleeding was 1.74%. The male gender was predominant, with a sex ratio of 2.3:1. The mean age was 55.3 ± 19.1 years, with extremes ranging from 16 to 82 years. The mean haemoglobin level on admission was 6.6 ± 1.9g/dl with extremes from 3.50 to 12.5g/dl. Nineteen patients were admitted to the intensive care unit more than 72 hours after the onset of bleeding (63.4%). The pre-endoscopic Rockall score was greater than 0 in 29 patients (96.7%).

UGB: Upper gastrointestinal bleeding.
HIV: Human Immunodeficiency Virus.
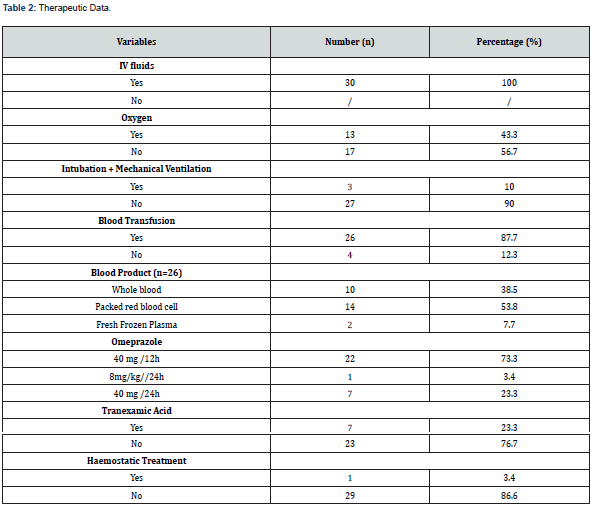
The ATLAS classification on arrival was greater than 1 in 28 patients (93.7%). Endoscopy was performed in seven patients (23.4%). It was performed more than 72 hours after the onset of bleeding. Forrest III ulcerative lesions were the most common (n=3, 42.8%). The management of UGB combined resuscitation and specific measures. IV fluids were administered to all patients. Oxygen was administered in 13 patients (43.3%). Three patients were intubated and put on mechanical ventilation (10%). A total of 26 patients (87.7%) received blood transfusions. Packed red blood cells were the most frequently administered blood product (n=14, 53.8%). Endoscopic haemostatic treatment was performed in one patient with an active Forrest Ib bleed (14.3%). It consisted of an adrenaline injection diluted at 1:10,000. All patients received parenteral omeprazole. Tranexamic acid was administered in seven patients (23.4%). The average length of hospital stay was 5.6 ± 4.9 days, with extremes ranging from 1 to 21 days. The death rate was 50% (Table 1-3).

Discussion
The main limitation of our study was the sample size, owing to the retrospective nature of the study. Some records were not found, highlighting the difficulties of archiving in our context. Another limitation was the type of study: data was collected in two different ICU wards to increase our chances of getting cases of UGB. As a result, there is bound to be a selection bias due to the divergent practices that two different ICU wards may have. The prevalence of UGB in the ICU was 1.74%. This was like findings in literature. Cook et al. in England in 1995 found a prevalence of less than 2% [8]. The incidence of upper GI haemorrhage is estimated at 143 cases per 100,000 inhabitants in France and 150 cases per 100,000 inhabitants in the USA. In Africa, several studies have reported a hospital prevalence ranging from 2 to 17.7% [9]. Our series was predominantly male with a sex-ratio of 2.3. The mean age was 55.3 ± 19.1 years. This is consistent with data from several series studied [10-11]. Our older population is because the patients referred to the ICU were mostly high-risk, elderly patients with comorbidities. The most frequent modes of revelation of UGB were haematemesis (50.7%) and melaena (29.7%). Bleeding from the gastrointestinal tract may present in five ways:
1) Haematemesis
2) Melaena
3) Haematochezia
4) Occult gastrointestinal bleeding,
5) Features of blood loss
Or anemia such as light headedness, syncope, angina, or dyspnoea. UGB typically presents with symptoms of melaena (black, malodorous feces caused by altered haemoglobin), or haematemesis (coffee-ground appearing blood-stained vomitus, caused by blood interaction with gastric acid [12]. The management of UGB combined resuscitation and specific measures. The cornerstone of the treatment of UGIB in intensive care patients is good clinical care: restore circulation, oxygenation, and haemoglobin levels. In addition, clotting disorders should be treated to enhance clot formation and haemostasis. Usually, these measures are sufficient to stop bleeding. When bleeding persists, the next step is to obtain an endoscopic examination with or without endoscopic treatment. Rapid assessment, resuscitation and correction of coagulopathy should be undertaken, and investigation or definitive management urgently arranged. For UGB, endoscopy remains the cornerstone of investigation and treatment. Initial management of haemorrhage is common to any source and involves standard resuscitative measures.
Assessment of the patient’s airway and respiratory system is performed initially, with attention made to ensure adequacy of ventilation. The patient’s heart rate and blood pressure are recorded and wide bore peripheral venous access obtained (at least two 16 or 18- gauge intravenous cannulas) [13-14]. In all cases, patients who are in a state of hypovolemic shock need appropriate shock treatment with fluids, blood- and plasma transfusions and if needed, vasopressors. Blood loss was estimated to be greater than 500ml in 28 patients (93.7%). It was class 1 ATLS. The ATLS guidelines on haemorrhage severity and class of hypovolaemic (haemorrhagic) shock are useful tools in the estimation of blood loss in patients with significant gastrointestinal bleeding. A prompt fluid bolus of 500 ml of crystalloids is recommended for initial volume replacement. It may be prudent to adopt a policy of ‘permissive hypotension’ (maintaining blood pressure at a level required to maintain tissue perfusion and cognition) until definitive control of the source of bleeding can be established.
Blood transfusion was performed in 26 patients (87.7%). The most used blood product was packed red blood cells. Whole blood was used in 38.5% of cases. Transfusion management was in accordance with the international consensus [15]. However, there is still a high use of whole blood. This may be due to the unavailability of other labile blood products in our setting. Hospitals have a transfusion policy where, for stable patients, packed red blood cell transfusion is recommended below a threshold (typically Hb ≤ 7g/dl. When used, NICE guidelines recommend a transfusion target of Hb 8.0 g/dL in patients with cardiovascular disease and 7.0 g/dL in those without [16].
Studies have quantified massive (or major) gastrointestinal haemorrhage as requiring transfusion of at least four units of packed red blood cells. The NHS transfusion service defines it as loss of one blood volume in 24 hours (70 ml/kg), 50% of total blood volume within 3 hours, or blood loss more than 150 ml/minute. A clinical aid includes systolic blood pressure less than 90 mmHg or heart rate more than 110 beats per minute. Omeprazole administration protocols were disparate, depending on the practitioner. The recommended protocols were 80mg bolus and then 40mg/12 hours (73.3%), and 8mg/hr. with an electric syringe pump for 72 hours in one patient (3.3%). Most guidelines agree that following an endoscopic diagnosis of ulcerative disease with high-risk features, high dose proton pump inhibitor is recommended.
The European Society of Gastrointestinal Endoscopy (ESGE) recommends intravenous high dose proton pump inhibitor (omeprazole 80 mg) be given as a bolus on presentation followed by continuous infusion (omeprazole 8 mg/hr.) for all patients requiring admission. Tranexamic acid was used in 7 patients (23.3%). This drug is not recommended for the intensive care management of upper GIB. Tranexamic acid has traditionally been given to patients presenting with gastrointestinal haemorrhage. However, the landmark HALT-IT trial found no difference between tranexamic acid infusion and placebo for mortality, blood transfusion and re-bleeding. With only a very small increase in venous thromboembolism, most guidelines no longer recommend tranexamic acid for GI bleeding [17]. Approximately one in four patients benefited from endoscopy. The time to perform endoscopy was > 72 hours.
This was due to the low level of technical facilities in these two hospitals and the high cost of endoscopy in our setting. This was different from the international recommendations for the management of upper GIB which suggest that endoscopy should be performed within 24 hours of the clinical manifestations of the haemorrhage in a stable patient. Patients who are haemodynamically unstable and with evidence of active bleeding should undergo immediate endoscopy after initial resuscitative measures. All patients requiring admission should receive endoscopy within 24 hours. Even after a period of stabilization, if the patient further deteriorates, immediate repeat intervention is necessary. Ulcerative lesion was the main endoscopic lesion found. This is consistent with the study by Ankouane et al, which found a peptic ulcer lesion in 38% of patients. Bagny et al. in Togo reported an ulcer lesion in 26% of cases. This high prevalence of ulcerative lesions was explained by the fact that the patients were elderly subjects with comorbidities.
Peptic ulcer disease is the most common cause for upper GI bleeding and accounts for approximately 31% to 67% of presentations. Benign peptic ulcers are best assessed endoscopically where they are typically described as having smooth, rounded edges. The Forrest classification categorises ulcers into three classes, which helps guide management and stratifies risk in patients at high risk of re-bleeding and mortality. Any ulcer other than a 2c or 3 is considered high risk. Only one patient received perendoscopic haemostatic treatment. This result could be explained by the non-availability of an endoscopy unit in these two hospitals. This highlights the lack of infrastructure for the management of UGB. The death rate was 50%. This was due to the severity of the patients admitted to the intensive care unit. The pre-endoscopic Rockall score was greater than 0 in 29 patients (96.7%), indicating a high risk of mortality. The in-hospital mortality appeared to be around 10% in United Kingdom. In countries with limited resources and no endoscopic haemostasis, mortality from digestive haemorrhages is around 30% - 40%. Mortality related to UGB was 36.6% in Brazzaville.
It remains high between 17 and 40% in other African countries [18-19]. On the other hand, in the centres that perform endoscopic haemostasis, mortality is low [20-22]. It was of 3% in Gabon in 2018, around 5% in France. The severity of bleeding is assessed by four main clinical factors and the haematocrit. The clinical factors are tachycardia greater than 110 beats per minute, systolic hypotension less than 80mmHg, tachypnoea greater than 20 cycles and the presence of skin mottling. Several scoring systems rate the severity of illness and their prognostication of patients who present with bleeding outside the ICU. A recent study showed that the AIMS65 and Glasgow- Blatchford scores performed better than the pre-endoscopic Rockall score and pre-endoscopi. Baylor score. There is an association between endoscopy performed more than 24 hours after admission and increased risk of mortality. In multivariate analysis, the risk factors for mortality were age between 30 and 60 years old, male sex and late hospitalization. The high mortality rate in our series is probably related to the lack of endoscopic haemostasis.
Conclusion
The prevalence of UGB in the ICU is low. Males predominate. The management was based on emergency measures and specific treatment. Mortality is high. The reduction of the mortality rate in ICU requires considering the identified risk factors and the acquisition of endoscopic haemostasis equipment.
References
- Gueye MN (2021) Upper Gastrointestinal Bleeding in Senegal: Preliminary Results of a Single-Centre Prospective Japanese Journal of Gastroenterology and Hepatology 6(9): 1-4.
- Bagny A, Bouglouga O, Djibril MA, Mba KB, Redah D (2012) Profil étiologique des hémorragies digestives hautes de l’adulte au CHU- Campus de Lomé (Togo). Journal Africain d'Hépato-Gastroentérologie 6(1): 38-42.
- Dicko MY, Doumbia K, Wife Samaké, Sow H Wife Coulibaly, G Soumaré et (2018) Acute Upper Digestive Bleedings Hospital in Bamako. Open Journal of Gastroenterology 8(11): 387-393.
- Ngami RS, Moulene P, Mimiesse JF, Mongo Onkouo A, Itoua Ngaporo NA et al. (2020) Risk Factors for Mortality Upper Digestive Haemorrhages at the University Hospital Centre of Brazzaville. Open Journal of Gastroenterology 10(12): 341-348.
- Loren L (2005) Gastrointestinal bleeding. In: Braunwald E, Fauci AS, Dasper DL, Hauser SL, Longo DL, Jameson JL (editors). Harrison's Principle of Internal medicine. McGraw Hill Companies Inc pp. 235, USA.
- Van der Voort PH (2017) How to prevent and treat gastrointestinal bleeding in a critically ill patient: a pathophysiological Journal of Emergency and Critical Care Medicine 1: 35.
- Hervé S (2007) Epidemiology of Upper Digestive Bleedings: New Aspects. Hepato Gastro 14: 205-210.
- Cook DJ, Fuller HD, Guyatt GH, Marshall JC, Leasa D, et al. (2002) Risk factors for gastrointestinal bleeding in critically ill patients N Engl J Med 330(6): 377-381.
- Okon AJB, Thot’o AS, Diakité M, Soro D, Ouattara A, et al. (2015) Résultats et facteurs prédictifs de mortalité des hémorragies digestives hautes en hospitalisation: étude multicentrique en Côte-d’Ivoire. Acta Endosc 45(6): 285‑290.
- Andoulo FA, Nonga BN, Noah DN, Kowo M, Babagna ID, et al. (2013) Aetiology and risk factors of acute upper gastrointestinal hemorrhage: analysis of 613 cases in Yaoundé, Cameroon. Port Harcourt Medical Journal 7(3): 175-182.
- Eloumou Bagnaka SAF, Luma Namme H, Noah Noah D, Essomba NE, Malongue A, et (2016) Risk factors associated with gastroduodenal lesions in a Douala referral hospital (Cameroon) Médecine et Sante ´ Tropicales 26(1): 104-109.
- Oakland K, Chadwick G, East JE, et al. (2019) Diagnosis and management of acute lower gastrointestinal bleeding: guidelines from the British Society of Journal of BMJ 68(5).
- Siau K, Hearnshaw S, Stanley AJ, et al. (2020) British Society of Gastroenterology (BSG) led multisociety consensus care bundle for the early clinical management of acute upper gastrointestinal bleeding. Frontline Gastroenterol 11(4): 311-323.
- Osman D, Djibre M, Da Silva D, Estcourt L, Rasheed A et al. (2012) Management by the intensivist of gastrointestinal bleeding in adults and Ann Intensive Care 2(1): 46.
- Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, et al. (2010) International consensus recommandations on the management of patients with non-variceal upper gastrointestinal bleeding. Ann Intern Med 152(2): 101-103.
- Dworzynski K, Pollit V, Kelsey A, Higgins B, Palmer K, et (2012) Management of acute upper gastrointestinal bleeding: summary of NICE guidance. BMJ 344: e3412.
- HALT-IT Trial Collaborators (2020) Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled Lancet 395(10241): 1927-1936
- Sombié R, Tiendrébéogo, Guingané A, Hagège H, Lesgourgues B, et al. (2015) Upper Digestive Haemorrhage: Epidemiological Aspects and Prognostic Factors in Burkina Faso (West Africa). Journal Africain d’Hépato Gastroentérologie 9: 154-156.
- Diarra M, Soucko Diarra A, Dolo M, H Traore, A Diallo (2007) Hémorragies digestives hautes aiguës: Expérience d’un milieu Acta Endoscopica 37: 321-326.
- Itoudi Bignoumba PE, Manganga Moussavou IF, Moussavou Kombila JB (2019) Upper Digestive Haemorrhage at the Libreville University Hospital Centre: Clinical Aspects and Actual Management: about 210 The Jour Med Health Sci 20: 20-22.
- Gonzales Gonzales JA, Vasquez Elizondo G, Garcia Compean D, Gaytan Torres JO, Flores Rendon AR, et al. (2011) Predictors of In-Hospital Mortality in Patients with Non Variceal Upper Gastrointestinal Revista Española de Enferme dades Digestivas 103(4): 196-203.
- Lim LG, Ho KY, Chan YH, Teoh PL, Khor J (2011) Urgent Endoscopy Is Associated xith Lower Mortality in High-Risk but Not Low-Risk Non-Variceal Upper Gastrointestinal Edoscopy 43(4): 300-306.






























