Sedation Requirement in Paralyzed Patients with Covid-19 As Compared to Non-Covid-19 Patients: A Single Center Retrospective Case Control Observational Study
Chintan Ramani MD1, Jamie M Brick DNP1, Mary Lewis Griffin PharmD2, Alyssa A Burke PharmD2, Bailey E Eason PharmD MS2, Jeffrey M Sturek MD PhD1, Kyle B Enfield MD MS1, Andrew J Barros MD MS1 and Alexandra Kadl MD MS1,3*
1Department of Medicine, Division of Pulmonary and Critical Care, University of Virginia, USA
2Department of Pharmacy, University of Virginia, USA
3Department of Pharmacology, University of Virginia, USA
Submission: July 08 2022; Published: August 23, 2022
*Corresponding author: Alexandra Kadl, MD MS, UVA Division of Pulmonary & Critical Care Medicine, PO Box 800546, Clinical Department Wing., 1 Hospital Drive, Charlottesville, VA 22908-0546, USA
How to cite this article: Chintan R M, Jamie M B D, Mary L G P, Alyssa A B P, Bailey E E P M, et al. Sedation Requirement in Paralyzed Patients with Covid-19 As Compared to Non-Covid-19 Patients: A Single Center Retrospective Case Control Observational Study. J Anest & Inten care med. 2022; 12(2): 555832. DOI 10.19080/JAICM.2022.12.555832
Abstract
Severe hypoxic respiratory failure is a common complication of SARS-COV-2 infection, often requiring mechanical ventilation with deep sedation and neuromuscular blockade, higher sedation requirement had been anecdotally reported, but not been compared systematically. Sedation is a key component of critical care, linked to delirium and mortality. Sedation requirements in patients with COVID-19 have been reported to be increased, but a detailed comparative study is lacking. We conducted a retrospective cohort study in the Medical intensive care unit at the University of Virginia, a tertiary-care teaching hospital in Virginia, United States. We evaluated all adult patients with moderate to severe acute respiratory distress syndrome (ARDS) requiring neuromuscular blockade in 2018-2020. We describe 91 patients with COVID-19 and 110 patients without COVID-19, both with moderate to severe ARDS (PaO2:FIO2 of 116, IQR 76-162 in COVID-19 patients vs. of 102, IQR 72-155) in non-COVID-19 patients) and evaluate the sedation requirements to achieve a Richmond Agitation-Sedation Scale (RASS) score of -5 prior neuromuscular blockade. The patients with COVID-19 were older (58, IQR 48-67) than the non-COVID-19 patients (51, IQR 36-61) but otherwise clinically similar. Patients with COVID-19 required 4.4-fold higher mean morphine equivalent dose and 2.2-fold higher mean midazolam dose to achieve the same RASS score of -5. The opioid and benzodiazepine requirements to achieve equivalent sedation scores are significantly higher in patients with COVID-19, which does not seem to affect long term narcotic use. Confirmatory studies, as well as studies to explore the mechanism behind these results, are needed.
Keywords:COVID-19, ARDS, Mechanical ventilation, Sedation requirement, Paralysis
Abbreviations: SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; COVID-19: coronavirus disease 2019; NMBA: neuromuscular blocking agent; STROBE: Strengthening the Reporting of Observational Studies in Epidemiology; RASS: Richmond Agitation Sedation Scale; ALT: alanine transaminase, AST: aspartate transaminase, INR: international normalized ratio; PaO2: Partial pressure of oxygen in arterial blood; FiO2: fraction of inspired oxygen; MMEs: morphine milligram equivalents, IQR: Interquartile range
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is well known as the pathogen responsible for the 2019 coronavirus disease (COVID-19) and impetus of the current global pandemic. While the degree of illness severity is variable for those infected, nearly 90% of patients admitted to the ICU end up on mechanical ventilation at some point during hospitalization [1].
Focusing on the management of pain, agitation, and delirium is an essential aspect of critical care medicine and one that requires heightened attention in a disease state where clinical practices continue to rapidly evolve [2].
Prolonged need for mechanical ventilation and increased opioid and benzodiazepine requirements to target deep sedation may put patients at an increased risk for critical illness complications, including post-ICU syndrome [3], potential dependence on sedative agents, and neurocognitive impairment [4]. Critically ill patients have known alterations in their pharmacokinetic and pharmacodynamic parameters that effect medication absorption, distribution, metabolism, and elimination. These alterations typically result from hemodynamic instability, fluid resuscitation, changes in protein binding, and large increases in volume of distribution seen in critically ill patients [5]. Sedatives and analgesics commonly used in the ICU for deep sedation exhibit multi-compartmental pharmacokinetic characteristics that may result in significant drug accumulation and prolonged effects. However, anecdotal reports suggest that patients with COVID-19 have increased sedation requirements [6-8], potentially due to earlier deep sedation in COVID-19 patients [9]. To the best of the research team’s current knowledge, there are no cohort studies reported that directly compare sedation requirements in COVID and non-COVID patients.
We hypothesized that patients with moderate to severe ARDS due to COVID-19 pneumonia, compared to patients with non-COVID-19 moderate-severe ARDS, have higher opioid and benzodiazepine requirements to achieve similar sedation levels.
Material and Methods
Setting and Case Identification
We conducted this retrospective, observational study of mechanically ventilated patients at the University of Virginia. We identified all patients admitted to the medical intensive care unit (MICU) service with respiratory failure requiring a neuromuscular blocking agent (NMBA), either cisatracurium or rocuronium, between 1/1/2018 and 12/31/2020. We excluded patients who only received intermittent bolus NMBAs. The institutional review board of the University of Virginia approved this study (IRBHSR22941), and it follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline for reporting results [10].
To avoid interrater variability and minimize bias in the endpoint, we selected patients receiving continuous NMBA infusions as these infusions require deep sedation to prevent awareness. The University of Virginia defines deep sedation as a Richmond Agitation Sedation Scale (RASS) of -5 [11], and assessing sedation before NMBA infusion initiation is protocolized and managed by the nursing staff.
Data Sources
We obtained demographic information, laboratory data, and medication administration records from our institutional data warehouse. All other data was manually abstracted from the list of identified patients by an investigator with experience in the care of critically ill patients (CR, JB, MLG, and AAB).
Variables Assessed
From the data warehouse, we extracted the following variables: age; sex; weight; time of initiation and discontinuation of NMBA; medication use during paralysis; laboratory values at the initiation of NMBA; duration of mechanical ventilation; duration of ICU stay; duration of hospitalization; discharge disposition; and medications prescribed at discharge. For medication use during paralysis we queried the medication administration record for oral or intravenous administration of opioids (fentanyl, hydromorphone, morphine, oxycodone, and methadone), benzodiazepines (midazolam and lorazepam), ketamine, dexmedetomidine, and Propofol. To avoid the issues related to tachyphylaxis and receptor saturation, we have included only the first 24 hours of sedation use. Laboratory values extracted included serum levels of alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin, international normalized ratio (INR), creatinine, and receipt of renal replacement therapy in the prior 72 hours. Investigators manually reviewed records and abstracted the indication for paralysis, details of pulmonary infection, if present, and PaO2 to FiO2 ratio at the time of NMBA initiation.
Primary Outcome
The primary outcome was the average hourly administered dosed of opioids and benzodiazepines during the first 24 hours of NMBA continuous infusion. If a patient received a NMBA for less than 24 hours then the total duration of NMBA infusion was examined. Administered opioids were converted to oral morphine milligram equivalents (MMEs) by equating 3mg oral morphine to 25mcg of intravenous fentanyl, 0.2mg of intravenous hydromorphone, 2mg oral oxycodone, or 1mg intravenous morphine. For midazolam equivalents, we equated 1mg of oral or intravenous lorazepam to 3mg of intravenous midazolam.
Statistical Analysis
Mann-Whitney U test and one-way ANOVA on ranks with adjustment for multiple comparisons were used for statistical testing on continuous variables. Pearson’s chi-squared tests were used to analyze count data between groups. A two-sided p-value of 0.05 was used for statistical significance.
Results
Patient population
We identified 91 patients with COVID-19 related respiratory failure and 110 patients with non-COVID-19 related respiratory failure. Our analysis shows that patients with COVID-19 in this cohort were significantly older than non-COVID patients (58, IQR 48-67 vs. 51, IQR 36-61, p= 0.001) and more likely to be male (31.9% vs. 47.3%, p=0.027). Indication for paralysis in COVID-19 was almost always ARDS (98.9%), whereas, in non- COVID-19 patients, only 71.8% were paralyzed for ARDS. Of the non-COVID-19 patients, 22.7% were paralyzed for ventilator dyssynchrony, 8.2% for other reasons (mostly airway bleeding), and 2.7% for severe obstructive disease (indications were not mutually exclusive). The degree of hypoxia was similar between the two groups. The median PaO2:FiO2 ratio was 116 (IQR 76- 162) in those with COVID-19 vs. 102 (IQR 72-155), p=0.321. After starting mechanical ventilation, the time to initial paralysis was comparable in COVID and non-COVID patients (7, IQR 2-22 vs. 5, IQR1-25, p=0.5478). There was also no significant difference in the number of patients requiring renal replacement therapy, mean creatinine and mean ALT, AST, INR (Table 1). There was a statistically significant difference in median total Bilirubin (0.5mg/dL, IQR 0.4-0.8 vs. 0.8, IQR, 0.4-1.5 in COVID and non- COVID patients respectively) at the time of initiation of paralysis, though the relevance of this is unclear.
# 103 out of 110 available
$84 out of 91 available
$$104 out of 110 available
@81 out of 91 available
@@ 102 out of 110 available
^84 out of 91 available
^^86 out of 110 available
## 65 out of 91 available
### 8 out of 110 available
‡ numbers may exceed total number as multiple indications can co-exist.
Pearson’s Chi-Square test was used to calculate significant differences in frequencies, Mann-Whitney test to compare continuous variables.
COVID-19 patients require higher amounts of opioids and benzodiazepines
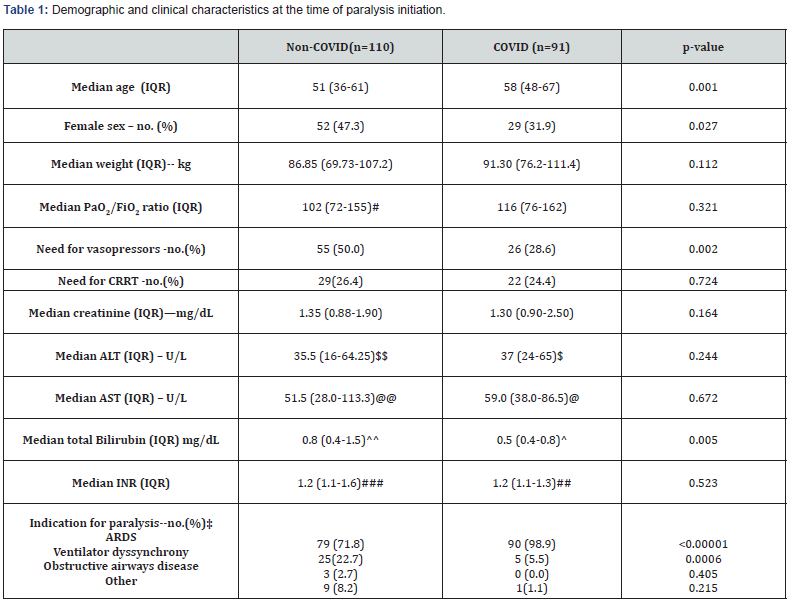
To achieve the same RASS score of -5, COVID-19 patients required 2.2-fold more midazolam, and 4.4-fold more MME than non-COVID-19 patients. Figure 1 shows that the increased use of sedation was specific to COVID-19 patients, as non-COVID paralyzed patients in 2020 did not differ from patients in 2018 and 2019. The adjunctive use of ketamine was higher in COVID-19 patients, whereas propofol was less often used (Table 2).
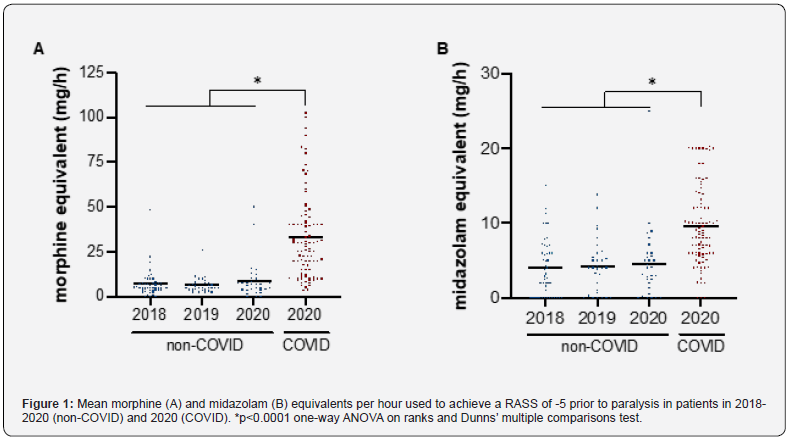
#corrected to number of patients receiving drug.
Pearson’s Chi-Square test was used to calculate significant differences in frequencies, one-way ANOVA on ranks test to compare continuous variables.
Paralysis duration, duration of mechanical ventilation, hospital and ICU length of stay were significantly longer in COVID-19 patients than in non-COVID patients (Table 3). Regardless, allcause hospital mortality and ICU mortality were not different. The higher use of opioids and benzodiazepines during paralysis did not increase the number of patients discharged on benzodiazepines (5 out of 48 discharged COVID-19 patients and 13 out of 54 discharged non-COVID patients) or opioids (16 out of 48 and 22 out of 54, respectively).
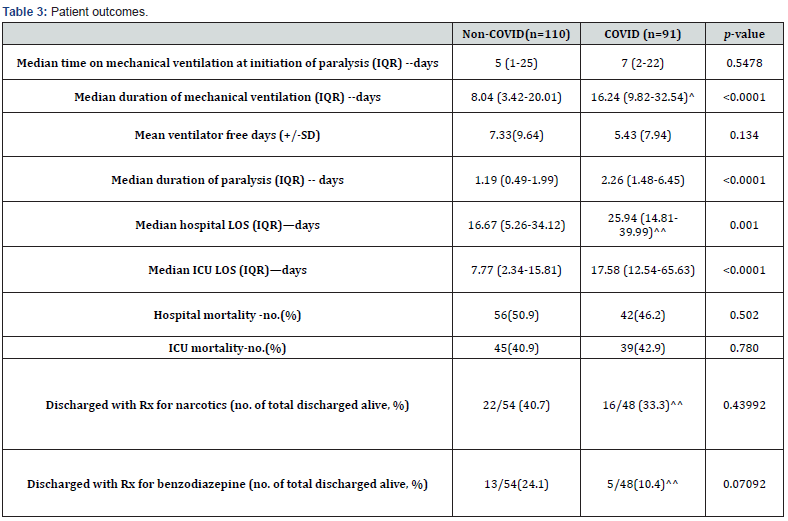
^1 patient remains admitted and intubated (on ventilation day 50 at the time of analysis)
^^3 patients are still admitted at the time of analysis, 2 awaiting placement at acute rehab
Pearson’s Chi-Square test was used to calculate significant differences in frequencies, Mann-Whitney test to compare continuous variables.
Discussion
This single-center retrospective study supports prior observations that patients with COVID-19 require a higher MME doses and higher doses of benzodiazepines and opiates to achieve similar sedation targets compared to patients without COVID-19. Our study focused on patients requiring extended paralysis to avoid potential bias in the measurement of the sedation target. This is both a strength and limitation of this study. The lack of change in sedation needs in the non-COVID-19 group from 2018- 2020 suggests that practice change did not influence these results; however. Trials evaluating the effect of paralytics on outcomes in ARDS have been mixed, with recent results from the ROSE trial showing an association with higher sedation use and hemodynamic instability [12]. The Society of Critical Care Medicine guidelines have a weak recommendation regarding paralytic use in COVID-19 ARDS for refractory hypoxia and ventilator dyssynchrony [13]. The use of continuous NMBAs is also used to facilitate the prone positioning [14]. The latter indication may explain the change as the utilization of prone positioning for severe ARDS [15] has also increased or simply show the increased burden of severe ARDS patients in 2020 due to COVID-19. A recently published international survey [16] showed no significant practice change in management of sedation, analgesia, and delirium before COVID-19 and during the pandemic.
Our study does not support the severity of illness as an explanation for this change in requirements. Comparison of baseline characteristics shows similarities in PaO2:FIO2, renal and liver function. Age was the only statistically significant difference between groups. Duration of mechanical ventilation is also unlikely to be a factor as the difference in sedation dose is based on the first 24 hours of NMBA infusion for both groups.
One possible explanation for this result is the direct neurologic effects of COVID-19. Drozdzal et al. [17] described the inflammatory reaction from COVID-19 causing increased pain above that anticipated from mechanical ventilation, procedural related pain, or prone positioning. Neural invasion of SARS-CoV-2 has been recently demonstrated [18], raising the possibility that there is a virus-specific central nervous system effect. Inflammation or direct SARS-COV-2 involvement of the respiratory center could increase the respiratory rate or change the sensitivity of target receptors for sedatives and analgesics, leading to higher requirements for the same sedative agent effects [19].
This study lacks long term outcomes data for both groups, so understanding potential long-term effects is not possible. A recently published study [1] of 2088 critically ill COVID-19 patients showed a 82% prevalence of delirium in sedated patients, whereas studies prior to COVID report a lower rate of delirium at ~50% [20]; this increased rate of delirium was potentially attributable to benzodiazepam use and lesser family involvement. We did find that discharge prescriptions for opioids and benzodiazepines are similar between the two groups despite the COVID-19 patients having significantly more sedation requirements while paralyzed. We have previously demonstrated that in the COVID-19 ICU population, at early discharge follow-up, most of our patients have zero to mild cognitive dysfunction [21].
Notably, the increased sedation needs of COVID-19 does correlate with pharmacy supply chain and operational changes made by the study institution to accommodate the requirements observed in COVID-19 patients. These changes included increased use of 503b compounded products, an increase in pharmacy department batched products, and the creation of higher concentration/volume products to support the heightened doses required in COVID-19 patients (hydromorphone and ketamine) and maintain adequate inventory to accommodate the increased use. Of these changes, increased batching requirements of pharmacy prepared medication drips and increased acquisition of outsourced products can be connected to increased drug spend in select sedative agents at the institutional level to meet the requirements of COVID-19 patients.
Conclusion
In summary this single center study reveals significant differences in sedation and analgesic requirements in patients with COVID-19 ARDS compared to non-COVID-19 ARDS. Our data suggest that this observation is not merely due to practice change but rather is specific to SARS-COV-2 infection. Larger, multicenter studies are needed to validate these findings. Additionally, subsequent mechanistic studies should focus on understanding the effects of SARS-COV-2 infection on both the pharmacodynamics and pharmacokinetics of commonly used sedative and analgesic medicines in the ICU, as well as potential direct viral effects on the CNS and respiratory control centers.
References
- Pun BT, Badenes R, Heras La Calle G, Orun OM, Chen W, et al. (2021) Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med 9(3): 239-250.
- Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, et al. (2018) Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med 46: e825-e73.
- Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, et al. (2012) The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med 185: 1307-1315.
- Pandharipande PP, Girard TD, Jackson JC, A Morandi, J L Thompson, et al. (2013) Long-term cognitive impairment after critical illness. N Engl J Med 369(14): 1306-1316.
- Tse AHW, Ling L, Lee A, Joynt GM (2018) Altered Pharmacokinetics in Prolonged Infusions of Sedatives and Analgesics Among Adult Critically Ill Patients: A Systematic Review. Clin Ther 40(9): 1598-1615.e2.
- Hanidziar D, Bittner EA (2020) Sedation of Mechanically Ventilated COVID-19 Patients: Challenges and Special Considerations. Anesth Analg 131(1): e40-e41.
- Kapp CM, Zaeh S, Niedermeyer S, Punjabi NM, Siddharthan T, et al. (2020) The Use of Analgesia and Sedation in Mechanically Ventilated Patients With COVID-19 Acute Respiratory Distress Syndrome. Anesth Analg 131(4): e198-e200.
- Flinspach AN, Booke H, Zacharowski K, Balaban U, Herrmann E, et al. (2021) High sedation needs of critically ill COVID-19 ARDS patients-A monocentric observational study. PLoS One 16(7): e0253778.
- Stephens RJ, Evans EM, Pajor MJ, Pappal RD, Egan HM, et al. (2022) A dual-center cohort study on the association between early deep sedation and clinical outcomes in mechanically ventilated patients during the COVID-19 pandemic: The COVID-SED study. Crit Care 26(1): 179.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, et al. (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335(7624): 806-808.
- Ely EW, Truman B, Shintani A, Thomason JWW, Wheeler AP, et al. (2003) Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 289(22): 2983-2991.
- National Heart L, Blood Institute PCTN, Moss M, Huang DT, Brower RG, et al. (2019) Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N Engl J Med 380(21): 1997-2008.
- Alhazzani W, Moller MH, Arabi YM, Loeb M, Gong MN, et al. (2020) Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit Care Med 48(6): e440-e469.
- Murray MJ, DeBlock H, Erstad B, Gray A, Jacobi J, et al. (2016) Clinical Practice Guidelines for Sustained Neuromuscular Blockade in the Adult Critically Ill Patient. Crit Care Med 44(11): 2079-2103.
- Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, et al. (2013) Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368(23): 2159-2168.
- Luz M, Brandao Barreto B, de Castro REV, Salluh J, Dal-Pizzol F, et al. (2022) Practices in sedation, analgesia, mobilization, delirium, and sleep deprivation in adult intensive care units (SAMDS-ICU): an international survey before and during the COVID-19 pandemic. Ann Intensive Care 12(1): 9.
- Drozdzal S, Rosik J, Lechowicz K, Machaj F, Szostak B, et al. (2020) COVID-19: Pain Management in Patients with SARS-CoV-2 Infection-Molecular Mechanisms, Challenges, and Perspectives. Brain Sci 10(7): 465.
- Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, et al. (2021) Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med 218(3): e20202135.
- Pezzini A, Padovani A (2020) Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol 16(11): 636-644.
- Girard TD, Exline MC, Carson SS, Hough CL, Rock P, et al. (2018) Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. N Engl J Med 379(26): 2506-2516.
- Ramani C, Davis EM, Kim JS, Provencio JJ, Enfield KB, et al. (2021) Post-ICU COVID-19 Outcomes: A Case Series. Chest 159(1): 215-218.






























