Neuroprotective Effects of Dalteparin on ExperimentalTraumatic Brain Injury in Rats
Hasan Cakir1, Zeynep Nur Orhon2*, Cem Orhon3, E Nursen Koltka2, Serdar Yesiltas4 and Melek Celik2
1Department of Anesthesiology and Reanimation, Turkey
2Department of Anesthesiology and Reanimation,Medeniyet University, Turkey
3DepartmentofNeurosurgery,Turkey
4 Department of Anesthesiology and Reanimation, BezmiAlemVakif University, Turkey
Submission: June 07, 2018; Published: July 02, 2018
*Corresponding author: Zeynep Nur Orhon, Medeniyet University, Department of Anesthesiologyand Reanimation, Hizirbey CadCüre Apt. 221/4Göztepe, Istanbul, Turkey.
How to cite this article: Hasan Ç, Zeynep N O, Cem O, E Nursen K, Serdar Y, Melek Ç. Neuroprotective Effects of Dalteparin on ExperimentalTraumatic Brain Injury in Rats. J Anest & Inten Care Med. 2018; 7(1): 555704. DOI: 10.19080/JAICM.2018.07.555704
Abstract
The aim of this study was to investigate the effects of a low molecular weight heparin, dalteparin, on different parameters of damage following experimental Traumatic Brain Injury (TBI) in rats. A total of 30 adult male Sprague Dawley rats were randomly divided into three groups: Group 1 (control), Group 2 (trauma alone), and Group 3 (trauma+dalteparin treatment). Severe trauma was induced by the weight dropping technique in Groups 2 and 3. Group 3 received 50 IU/kg dalteparin 15 minutes after the trauma. The animals were sacrificed at 4 h after the injury. Their brain tissues were removed. Malondialdehyde (MDA), Superoxide Dismutase (SOD), Glutathione Peroxidase (GPx), and Catalase (CAT) levels and caspase-3 activity were detected.
Light microscopic findings were recorded. In the trauma group, MDA levels in the tissue increased significantly (P<0.01); SOD, GPx, and CAT activities decreased significantly (P<0.01); and caspase-3 activity increased significantly (P<0.01). In trauma+dalteparin treatment group, MDA levels decreased significantly (P<0.05); SOD, GPx, and CAT activities increased significantly (P<0.05); and caspase-3 activity decreased significantly (P<0.01). In trauma group, neurons became degenerated, and the number of apoptotic neurons significantly increased (P<0.01). In the trauma+dalteparin treatment group, the neurons were well protected, and the number of apoptotic neurons significantly decreased (P<0.01). The present study suggests that 50 IU/kg dalteparin administered as a single dose 15 minutes after the TBI improved all parameters in the study and dalteparin could possibly has neuroprotective effects in experimental TBI in rats
Keywords: Dalteparin; Neuroprotective effects; Traumatic brain injury
Abbrevations: TBI: Traumatic Brain Injury; MDA: Malondialdehyde; SOD: Superoxide Dismutase; GPx: Glutathione Peroxidase, CAT: Catalase, IC: Intravascular Coagulation
Introduction
Brain injuries cause an immeasurable amount of human misery; lifelong physical, cognitive, or psychological impairment and pose a high cost burden to the community. Traumatic Brain Injury (TBI) is a common cause of mortality and morbidity among people less than 45 years of age throughout the World [1].Acute damage in the brain occurs as a result of a mechanical trauma or subsequent interruption of the blood supply and leads a cascade of pathological events. TBI involves primary and secondary injury. Primary injury can be focal and/or diffuse and results from the mechanical forces and often leads to irreversible tissue damage. Secondary injury has different time courses, i.e., it may occur within minutes, hours, days or months following the primary insult and causes significant impairments of the brain functions and outcomes.
In TBI, edema and hemorrhage within and around the damaged tissue cause an increase in intracranial pressure that provokes compression of cerebral blood vessels, leading to reduced blood flow and ischemia [2].Formation of microthrombi has been reported to occur in head trauma patients [3], and may contribute to the secondary ischemic insult. Autopsy results of more than 90% of the patients with fatal TBI showed the evidence of cerebral ischemia. According to these results, it was concluded that most of cerebral damage was secondary and occurred after an impact [4]. In some reports on autopsies in human and animal experiments, abundant fibrin microthrombi have been noted within cerebral vessels, particularly in and around cerebral contusions [3,5,6].
The development of treatment for brain trauma has focused on re-establishing blood flow to ischemic areas as quickly as possible, mainly with antithrombotics or thrombolytics and on protecting neurons from cytotoxic events. This suggests that a therapeutic strategy with anticoagulant drugs is useful for treatment of brain injury. Anticoagulants such as heparin or Low Molecular Weight Heparin (LMWH) have been shown to be neuroprotective in focal cerebral ischemia in rats [7,8]. However, heparin possesses potent anticoagulant properties, acting on different coagulation factors like factor IIa (thrombin) or factor Xa (key component of the prothrombinase complex). Moreover, the main drawbacks of unfractionated heparin are the very short half life and high risk of bleeding, which limits its use in clinical indications.
In contrast, LMWH has six times less anti-Ila activity and half anti-Xa activity compared with heparin, which reduces the risk of hemorrhage [9,10]. In addition to the anticoagulant effects, anti-inflammatory [7] and trophic properties [11] have been attributed to unfractionated heparin. Dalteparin is a LMWH with a mean molecular weight of 5,000 and it has anti-Xa activity. There are some reports about the effects of dalteparin in preventing thrombosis in deep arterial injury, on cellular apoptosis and imflammatory process in an incisional wound healing model and in induced liver injury [12-15]. However, in literature there is no report about the use of dalteparin in TBI. Therefore, in this study, we planned to investigate the therapeutic effect of dalteparin in experimental TBI in rats.
Material and Methods
Animals and Experimental Design
The present study was approved by Marmara University Animal Ethics Committee ( Approval No: 87. 2013. mar). Thirty male Sprague-Dawley rats were enrolled in this experimental study. Ages of all rats were under 3 months. The average body weight was 250-300g. Animal care and all procedures were performed according to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication 85-23, revised 1985). Rats were maintained on a 12 h light–dark cycle and allowed free access to food and water. Temperature was kept at 20°C ±2°C and humidity was 50% ± 10% at the animal facility. The animals were randomly divided into three groups (n= 10 in each group). Group 1 (control group), Group 2 (trauma alone group),Group 3 (trauma+dalteparin treatment group).
General anesthesia was administered through an intraperitoneal injection of ketamine (from Pfizer, Turkey) 90 mg/kg and xylazine 2% (Agrar Veterinary Pharmaceuticals, Netherlands) 10 mg/kg. The animals maintained spontaneous breathing. No trauma induction or treatment approach was implemented in the control group (Group 1). In trauma group (Group 2), severe trauma was induced using the weight dropping technique described by Shapira et al.[16]. In the weight drop injury model, an anesthetized animal was placed under a ‘trauma device’ and was subjected to TBI using gravitational force of freefalling weight from 7 cm height onto the frontoparietal convexity (1-2 mm lateral to the midline) of the skull and an impact of 0.5 J was delivered to the intact skull. In trauma + treatment group (Group 3) severe trauma was induced as described for Group 2 and 15 minutes after the trauma 50IU/kg dalteparin (Fragmin; Pharmacia, Uppsala, Sweden, 25 000 IU/ml) was given intraperitoneally.
The dalteparin dose used for rats was comparable to that for human prophylaxis for the deep venous thrombosis after major surgical procedures. The animals were sacrified 4h after the injury. Their brain tissues were removed and divided into two pieces. One of them was put in 10% formalin for histopathological analysis. The other one was immediately frozen in liquid nitrogen and stored at −80°C until analyses. Analyses were performed mainly using 3 techniques; biological analysis, immunohistochemistry and light microscopy. Biological analysis were performed to detect the level of Malondialdehyde (MDA), Superoxide Dismutase (SOD), Glutathione Peroxidase (GPx)and Catalase (CAT). Immunohistochemistry was used to determine Caspase-3 activity whereas light microscopy was used to evaluate the histological appearance of the brain tissues of the rats.
Statistical Analysis
SPSS for Windows (version 11.0) was used for statistical analysis. All data were presented as the mean ±Standard Deviation (SD). The groups were compared by the Kruskal-Wallis test. A Pvalue of less than 0.05 was considered significant. When the Pvalue was less than 0.05, Mann-Whitney U Test was used for pair-wise comparisons between the groups.
Results
Biochemical Findings
The levels of MDA, SOD, GPx and CAT are shown in Table 1. Compared with control group, MDA level in the brain tissue increased significantly (P<0.01) and the amount of the antioxidant enzymes SOD, GPx ve CAT decreased significantly in trauma group (P<0.01). In trauma+dalteparin treatment group, MDA level was decreased significantly (P<0.05), and antioxidant enzyme levels (SOD, GPx and CAT) were increased significantly (P<0.05) (Figure 1).
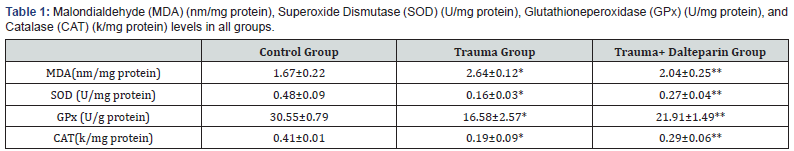
MDA: Malondialdehyde; SOD: Superoxide dismutase;GPx: Glutathione peroxidase; CAT: Catalase Kruskal-Wallis test was used for statistical analysis. All data were presented as the mean±standard deviation. For each group, n=10.*P< 0.01 compared with the control group.
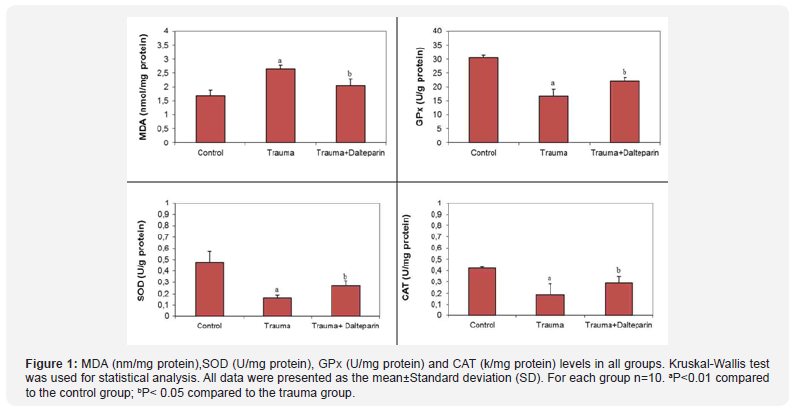
Light Microscopic Findings
Hematoxylin and eosin-stained slides containing frontal cortex of the control group showed normal ultrastructure of brain tissue and those containing frontal cortex of the trauma group showed severe degenerative changes in the neurons, puckered cytoplasm, dark stained picnotic nucleuses and vacualisation. In trauma+dalteparin group hematoxylin and eosin-stained slides containing frontal cortex showed decreased degenerative changes in neurons and a significant decrease in puckered cytoplasm, dark stained picnotic nuclei and vacuolization (Figure 2).
Immunohistochemical Findings
Caspase-3 immunohistochemical evaluation of the neurons under the light microscope revealed that caspase-3 activity was insignificant in the control group, increased significantly in trauma group, and decreased significantly in trauma+ dalteparin group (Figure 3). Table 2 shows the number of apoptotic neurons (caspase-3 immunopositive)in the groups.The amount of apoptotic neurons increased after the trauma and decreased with dalteparin treatment.
Discussion
Dalteparin treatment was found to be effective in improving all parameters evaluated in the study. Hematoxylin and eosinstained slides containing frontal cortex of trauma group showed severe degenerative changes in the neurons; puckered cytoplasm, dark stained picnotic nuclei, and vacuolization,i.e., edema formation in the tissue. In trauma+dalteparin group, hematoxylin and eosin-stained slides showed decreased degenerative changes in the neurons and a significant decrease in puckered cytoplasm, dark stained picnotic nuclei and vacuolization; MDA level was decreased significantly,antioxidant enzyme levels (SOD, GPx and CAT) were increased significantly, and caspase-3 activity was decreased significantly. This means that edema in the brain tissue, oxidative brain damage and degeneration in neurons were improved, the number of apoptotic neurons, was decreased by dalteparin treatment.
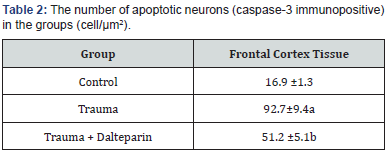
Kruskal-Wallis test was used for statistical analysis. All data were presented as the mean±standard deviation. For each group, n=10. aP< 0.001 compared with the control group. bP< 0.01 compared with the trauma group.
Some investigators found that more than 90% patients with fatal TBI had cerebral ischemia. They also found that cerebral damage was a secondary insult and it occured after trauma. Despite the improvements in the treatment of TBI, the incidence of cerebral ischemia did not decreased [4].A study was conducted to determine whether there is a relation between traumatic cerebral ischemia and intravascular thrombosis after traumatic brain injury.A strong link was found between intravascular microthrombosis and neuronal death by assessing the frontal cortex and hippocampus in humans who had a fatal TBI [17]. In another experimental study, researchers aimed to determine the frequency of cerebral Intravascular Coagulation (IC) in TBI. Moreover, they assessed the incidence of IC in different species and found microthrombi in arterioles and venules, ranging from 10 to 600 μm. They also found significant IC in mild and diffuse injuries. In addition to these findings, it has been observed that there is a significant link between the coagulopathy and IC. It was also reported that trauma leads tissue thromboplastin release, which causes IC in the brain.
Excesive release of tissue thromboplastin may cause IC. The clotting factors consumption leads to hemorrhage because of consumption coagulopathy. Prevention of IC would improve the results of TBI [18].Several mechanisms are involved in the development of secondary ischemic brain damage, including microthrombi formation, which is thought to play a prominent role. Ninety-four autopsy cases were macro and microscopically examined by specific staining for fibrin andshowed microthrombi formation and secondary ischemic insult in head trauma patients. Enhanced release of factor VIII from damaged endothelial cells might cause microthrombi and increased adhesion between platelets and endothelial cells[3]. Based on this knowledge, a group of investigators decided to use anticoagulant drugs for the treatment of brain injury. They conducted a study on the effect of enoxaparin, a LMWH, in TBI.
As a result, they concluded that LMWH improved the pathological and behavioral effects of experimental TBI and enoxaparin or other LMWH could be used for the treatment of acute neurodegenerative diseases [19].The preclinical data for enoxaparin in in vivomodels of ischemia and brain trauma in rats were studied. In addition to anticoagulant effects, enoxaparin has many other pharmacological effects (i.e. reduction of intracellular Ca2+ release; antioxidant effect; anti-imflamatory or neurotrophic effects) that could act in synergy to explain the neuroprotective activity of enoxaparin in acute neurodegenerative diseases.Using the transient middle cerebral artery occlusion, demonstrated taht in different invivomodels of acute neurodegenerative diseases, enoxaparin reduces brain edema and lesion size and improves motor and cognitive functional recovery with a largetherapeutic window of opportinity (compatible with a clinical application).
Taking into account these experimental data in models ofischemia and brain trauma, the clinical use of enoxaparin in acute neurodegenerative diseases warrants serious consideration [20].The neuroprotective effects of AT III and enoxaparin were compared after severe traumatic brain injury. AT III has been more effective than enoxaparin in reducing neuronal cell death and it was concluded that AT III and enoxaparin could be used in the treatment of traumatic brain injuries [21].According to our knowledge, dalteparin, an other LMWH, has not been used before in the treatment of experimental TBI. Thus, for this purpose, dalteparin was used for the first time by our group.The effect of dalteparin on the inflammation and cellular apoptosis was evaluated in an incisional wound-healing rat model, and it was concluded that dalteparin has an impact on suppressing early inflammatory process and leads to increase in cellular apoptosis, which impedes wound healing [12].
Based on this knowledge, we claimed that suppresion of the early inflammatory process is one of the beneficial effects of dalteparin in TBI. Another study designed to examine the effects of dalteparin on ischemia/reperfusion injury found that dalteparin could improve liver injury in rats by reducing inflammatory responses. These therapeutic effects might play a critical role in preventing intravascular coagulation in TBI [14]. In several studies, it has been revealed that LMWHs are capable of inhibiting adhesion of human polymorphonuclear leukocytes to endothelial cells, the production of reactive oxygen species and the expression of cell adhesion molecules, L- and P-selectin, on endothelial cells [15,22-24]. We claim that the beneficial effect of dalteparin on improving the results after TBI is probably due to the mechanisms mentioned above.
Insummary,we concluded that dalteparin given 15 minutes after TBI, improved brain edema, oxidative brain damage and degeneration of neurons in rats. Similar results may be obtained from studies in humans, and dalteparin could be used as a promising drug for the treatment of acute TBI in animals and humans. Further studies on this drug are needed.
Acknowledgements
The authors would like to acknowledge BAP Commitee of Istanbul Medeniyet University for the financial support.
Ethical approval
All procedures were performed in accordance with the ethical standards of our institutional research committee and with those of the 1964 Helsinki declaration and its later amendments.
References
- Kalsbeek WD, McLaurin RL, Harris BSH, Miller JD (1980) The national head, spinal cord injury survey: major findings. J Neurosurg 53: 19-31.
- Graham DI, Ford I, Adams JH, Doyle D, Teasdale GM, et al. (1989) Ischemic brain damage is still common in fatal non-missile head injury. J Neurol Neurosurg Psychiatry 52(3): 346-350.
- Lafuente JV, Cervós-Navarro J (1999) Craniocerebral trauma induces hemorheological disturbances. J Neurotrauma 16(5): 425-430.
- Graham DI, Adams JH (1971) Ischaemic brain damage in fatal head injuries. Lancet 1(7693): 265-266.
- Kaufman HH, Hui KS, Mattson JC, Borit A, Childs TL, et al. (1984) Clinicopathological correlations of disseminated intravascular coagulation in patients with head injury. Neurosurgery 15(1): 34-42.
- Van der Sande JJ, Emeis JJ, Lindeman J (1981) Intravascular coagulation: a common phenomenon in minor experimental head injury. J Neurosurg 54(1): 21-25.
- Cade JF, Buchanan MR, Boneu B, Ockelford P, Cater CJ, et al. (1984) A comparison of the antithrombotic and haemorrhagic effects of low molecular weight heparin fractions: the influence of the method of preparation. Thromb Res 35(6): 613-625.
- Yanaka K, Spellman SR, McCarthy JB, Oegema TR Jr, Low WC, et al. (1996) Reduction of brain injury using heparin to inhibit leukocyte accumulation in a rat model of transient focal cerebral ischemia. I. Protective mechanism. J Neurosurg 85(6): 1102- 1107.
- Noble S, Spencer CM (1998) Enoxaparin: a review on its clinical potential in the management of coronary artery disease. Drugs 56(2): 259- 272.
- Tyrrell DJ, Kilfeather S, Page CP (1995) Therapeutic uses of heparin beyond its traditional role as an anticoagulant. Trends Pharmacol Sci 16(6): 198-204.
- Del Zoppo GJ, Schmid Schonbein GW, Mori E, Copeland BR, Chang CM (1991) Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke 22(10): 1276-1283.
- Civelek A, Ak K, Kurtkaya O, Tekeli A, IsbirS, et al. (2007) Effect of a low molecular weight heparin molecule, dalteparin, on cellular apoptosis and inflammatory process in an incisional wound-healing model. Surg Today 37(5): 406-411.
- Emerick KS, Deschler DG (2007) The Effect of Low-Molecular-Weight Heparin on Microvenous Thrombosis in a Rat Model. Arch Facial Plast Surg 9(1): 19-21.
- Harada N, Okajima K, Uchiba M (2006) Dalteparin, a low molecular weight heparin, attenuates inflammatory responses and reduces ischemia-reperfusion–induced liver injury in rats. Crit Care Med 34(7): 1883-1891.
- Menger MD (2006) Dalteparin: Only protective in hepatic ischemia– reperfusion or also capable of preventing injury in liver hyperperfusion syndrome? Crit Care Med 34(7): 2011-2013.
- Shapira Y, Shohami E, Sidi S, Soffer D, Freeman S, et al. (1988) Experimental closed head injury in rats: mechanical, pathophysiologic and neurologic properties. Crit Care Med 16(3): 258-265.
- Stein SC, Graham DI, Chen XH, Smith DH (2004) Association between intravascular microthrombosis and cerebral ischemia in traumatic brain injury. Neurosurgery 54(3): 687-691
- Stein SC, Chen XH, Sinson GP, Smith DH (2002) Intravascular coagulation: a majör secondary insult in nonfatal traumatic brain injury. J Neurosurg 97(6): 1373-1377.
- Wahl F, Grosjean Piot O, Bareyre F, Uzan A, Stutzman JM (2000) Enoxaparin reduces brain edema, cerebral lesions, and improves motor and cognitive impairments induced by a traumatic brain injury in rats. J Neurotrauma 17(11): 1055-1065.
- Stutzmann JM, Mary V, Wahl F, Piot OG, Uzan A, et al. (2002) Neuroprotective Profile of Enoxaparin, a Low Molecular Weight Heparin, in In Vivo Models of Cerebral Ischemia or Traumatic Brain Injury in Rats: a Review. CNS Drug Rev 8(1): 1-30.
- Sen O, Sonmez E, Cekinmez M, Ozen O, Caner H (2011) Antithrombin III and enoxaparin treatment inhibit contusion-triggered cell death, inflammation, hemorrhage and apoptosis after severe traumatic brain injury in rats. Turk Neurosurg 21(2): 203-209.
- Lever R, Hoult JR, Page CP (2000) The effects of heparin and related molecules upon the adhesion of human polymorphonuclear leucocytes to vascular endothelium in vitro. Br J Pharmacol 129(3): 533-540.
- Manduteanu I, Voinea M, Capraru M, Dragomir E, Simionescu M (2002) A novel attribute of enoxaparin: inhibition of monocyte adhesion to endothelial cells by a mechanism involving cell adhesion molecules. Pharmacology 65(1): 32-37.
- Nelson RM, Cecconi O, Roberts WG, Aruffo A, Linhardt RJ, et al. (1993) Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood 82(11): 3253-3258.






























