Insulinoma-Anaesthetic Implications with Review of Literature
Thomas JT*, Gupta S and Kilpadi K
St John's Medical College and Hospital, India
Submission: March 07, 2017; Published: June 08, 2017
*Corresponding author: Jithumol Thankam Thomas, St John's Medical College and Hospital, India, Tel: 09632094554, Email: drjithu88@gmail.com
How to cite this article: Thomas J, Gupta S, Kilpadi K. Insulinoma-Anaesthetic Implications with Review of Literature. J Anest & Intern Care Med. 2017; 3(1): 555605. DOI: 10.19080/JAICM.2017.03.555605
Abstract
Insulinomas are rare tumours that present with recurrent hypoglycaemic episodes. They are often misdiagnosed or diagnosis is often delayed due to their bizarre clinical presentation. Diagnosis of insulinoma is made based on history (Whipple's Triad), biochemical tests and imaging modalities. Treatment includes medical and surgical management. We intend to describe the anaesthetic management of three cases of insulinoma observed over a period of one year and review the existing literature. Though rare, when they do occur, these tumours pose a great challenge to anaesthesiologists. Vigilant monitoring of blood sugars intraoperatively is a must to counteract these wide swings, hence providing a better patient outcome.
Keywords: Insulinoma; Anaesthetic management; Hypoglycaemia; Whipple's Triad; Intraoperative ultrasound (IOUS)
Introduction
Insulinomas are rare tumours with an incidence of 1-4 per million per year [1]. They are the most common functional variety of neuroendocrine tumours of pancreas. They are usually small (<2cm), solitary and benign (approximately 90%). Patients present with adrenergic or neuroglycopenic symptoms due to recurrent hypoglycaemia [1-4]. Weight gain is a common finding in 20-40% and is primarily due to overfeeding to overcome hypoglycaemia. Diagnosis of insulinoma must be suspected in otherwise normal patients who present with repeated episodes of hypoglycaemia and they warrant further investigation [1,2]. Because of the bizarre nature of the presenting symptoms, many patients will present to neurologists or psychiatrists and hence delay in early diagnosis or misdiagnosis. Surgical excision is the definite treatment.
Case Series
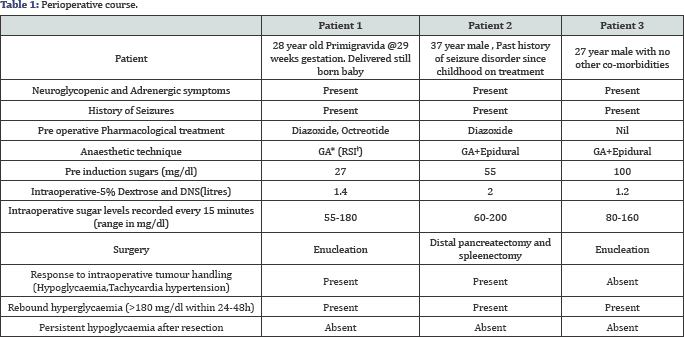
Three young patients; a 28 yr old primigravida@29 weeks gestation, a 37 year old male with past history of seizure disorder on treatment and a 29 year old male, presented with complaints of recurrent episodes of drowsiness, excessive sweating, delayed waking from sleep, seizures and altered sensorium. In view of neurological symptoms, all three were initially evaluated by neurologists. However, it was incidentally observed that they had severe hypoglycaemia during these episodes (GRBS<50mg/ dl). Diagnosis of insulinoma was then made based on history (Whipple's Triad), biochemical tests and imaging modalities (Table 1).
Discussion
The median age of presentation is approximately 47 years, with a mild female preponderance (female: male 1.4: 1) [5-7]. Diagnosis of insulinoma is clinical, biochemical and localization of the tumour. Clinical diagnosis is based on Whipple's Triad which includes repeated attacks of hypoglycaemia, serum blood glucose levels <50mg/dl during that period and relief of symptoms with glucose administration [1,2]. Biochemical diagnosis includes the 72-h fasting test which is considered as gold standard. During the fasting period, the patient is allowed to drink calorie-free fluids and physical activity is encouraged. Blood glucose should be measured 6-hourly till it reduces to 60mg/dL and then every 1 or 2 hours till it reduces to 40-45mg/dL. When symptoms of hypoglycaemia appear, blood should be sampled and diagnosis of insulinoma is made when-
- Plasma glucose <55mg/dl during episode of hypoglycaemia,
- Increased serum Insulin (>5-10microU/mL)
- Elevated C-peptide (>200micromol/L) and
- Increased proinsulin level (>25%).5
Localizing insulinomas are difficult with a failure rate of 10-27% [1,5,8]. It includes CT, MRI, Somatostatin receptor scintigraphy. Recently, the use of Endoscopic Ultrasound is shown to have increased sensitivity. Intraoperative ultrasound (IOUS) with surgical palpation has a high success rate (85-90%) [1,5,8]. Treatment includes medical and surgical management. Medical management includes dietary modification and pharmacological agents. Patient is advised frequent small meals throughout the day to avoid hypoglycaemia. Diazoxide is often started in these patients as it decreases release of insulin by stimulating alpha adrenergic receptors and thereby inhibits beta cells of islets of pancreas.
Somatostatin analogues like octreotide and lanreotide is used as they bind to somatostatin receptors on insulinomas and decrease insulin Secretion [9-11]. Steroid therapy has been considered as it inhibits insulin mediated glucose uptake and promotes release of glucose. However its value is doubtful and even considered harmful as it can cause exaggeration of the normal rebound hyperglycaemia that is seen postoperatively and there is a high risk of infection. Surgical management includes enucleation which is the treatment of choice. Some cases may require distal or partial pancreatectomy.
Anaesthetic implications-Any neurologic damage that has occurred as a result of previous hypoglycaemic episodes must be documented. Intravenous infusion of 5% Dextrose or 10% Dextrose should be started during the fasting period prior to surgery. Aim is to maintain blood glucose of more than 50mg/ dl [1,5]. Adequate NPO may not be achieved as patients may become symptomatic even after a few hours of fasting and due to poor patient compliance. Hence the risk of aspiration must be considered while inducing these patients and adequate care must be taken.
Administration of anaesthesia for removal of these tumours is challenging due to difficulty to maintain a normal blood glucose level. Wide fluctuations in blood glucose levels during tumour handling are often observed. Hypoglycaemia may be masked under general anaesthesia because signs of hypoglycaemia such as sweating, tachycardia, and hypertension and dilated pupils which can also occur due to hypovolemia, surgical stimuli, lighter surgical planes and drugs. Hence, detecting hypoglycaemia under anaesthesia is difficult. Intraoperative hypoglycaemia can cause CNS damage and such patients may often require postop ventillatory support.
It is recommended that blood glucose level must be checked before induction and every 15-30 from then on. It is imperative that glucose levels must be monitored in the recovery period also because
- Risk of rebound hyperglycaemia after resection
- Multiple adenomas may exist which can cause early postoperative hypoglycaemia which is not seen intraoperative.
Due to frequent blood sampling requirement, an arterial line is essential. Anaesthetics which decrease cerebral metabolic rate like propofol or thiopentone should be used. General anaesthesia with propofol combined with epidural is preferred choice of anaesthesia for insulinoma excision [1]. Post resection blood sugars may be high because of anti insulin hormones like GH, glucagon and glucocorticoids, which persist at high levels for a few days after removal of tumour. This hyperglycaemia is self limiting. Post op hypoglycaemia however, should raise the suspicion of either tumour not been found or multiple other insulinomas persisting.
Conclusion
Though rare, when they do occur, these tumours pose a great challenge to anaesthesiologists due to inadequate fasting, preoperative neurologic damage due to repeated hypoglycaemic episodes and wide swings in sugars during handling of tumour. Vigilant monitoring of blood sugars intraoperatively is a must to counteract these wide swings, hence providing a better patient outcome.
References
- Goswami J, Somkuwar P, Naik Y (2012) Insulinoma and anaesthetici mplications. Indian J Anaesthesia 56(2): 117-122.
- Whipple AO, Frantz VK (1935) Adenoma of islet cells with hyperinsulinism: A review. Ann Surg 101(6):1299-335.
- Chari P, Pandit SK, Kataria RN, Singh H, Baheti DK, et al. (1977) Anaesthetic management of insulinomas. Anaesthesia 32(3): 261-264.
- Jyotsna VP, Rangel N, Pal S, Seith A, Sahni P, et al. (2006) Insulinoma: Diagnosis and surgical treatment.Retrospective analysis of 31 cases. Indian J Gastroenterol 25(5): 244-247.
- Vaidakis D, Karoubalis J, Pappa T, Piaditis G, Zografos GN (2010) Pancreatic insulinomas: Current issues and trends. HepatobiliaryPancreat Dis Int 9(3): 234-241.
- Tucker ON, Crotty PL, Conlon KC (2006) The management of insulinoma. Br J Surg 93(3): 264-275.
- Abboud B, Boujaoude J (2005) Occult sporadic insulinoma: Localization and surgical strategy. World J Gastroenterol 14(5): 657-665.
- Mathur A, Gorden P, Libutti SK (2009) Insulinoma. Surg Clin North Am 89(5):1105-1121.
- Jensen RT, Berna MJ, Bingham DB, Norton JA (2008) Inherited Pancreatc Endocrine Tumor Syndromes: Advances in molecular pathogenesis, diagnosis, management and controversies. Cancer 113(Suppl 7): 1807-1843.
- Grant CS (2005) Insulinoma. Best Pract Res ClinGastroenterol 19(5): 783-798.
- Healy ML, Dawson SJ, Murray RM, Zalcberg J, Jefford M (2007) Severe hypoglycaemia after long-acting octreotide in a patient with an unrecognized malignant insulinoma. Internal Med J 37(6): 406-409.






























