Management of Intrathecal Pump Site Infection in a Patient with Metastatic Breast Cancer without the Removal of the System, a Case Report
Cyrus Yazdi* and Ryan Finn
Department of Anesthesiology and Pain Medicine, Harvard Medical School, USA
Submission: December 24, 2016; Published: January 25, 2017
*Corresponding author: Cyrus Yazdi M.D, Instructor, Anesthesiology and Pain Medicine, Harvard Medical School, Arnold-Warfield Pain Center, Beth Israel Deaconess Medical Center, One Brookline Place, Suite 105 Brookline, MA 02445, USA, Tel: (508] 793-6802; Fax: (508) 793-6819; Email: cyazdi@bidmc.harvard.edu
How to cite this article: Cyrus Y, Ryan F. Metastatic Breast Cancer without the Removal of the System, a Case Report. J Anest & Inten Care Med. 2017; 1(4) : 555568. DOI: 10.19080/JAICM.2017.01.555568
Abstract
Background: Intrathecal delivery of pain medication with an implantable infusion pump is being increasingly used for management of intractable pain in cancer patients. Infection of an intrathecal pump system is a rare but dangerous complication and usually leads to the removal of the whole system.
Case-Presentation: The author presents the case of a 69 year old woman who was diagnosed with metastatic breast cancer at presentation in 2009. She was treated systematically with chemotherapy and radiotherapy and underwent intrathecal pump implantation for targeted opioid delivery. Seven months after the implant, and a in a location of previous radiotherapy, she developed an infection at the site of the catheter insertion with exposure of the catheter itself. Clinically she presented with signs of local infection, high white blood cell count and back pain.She was treated with extensive surgical debridement of her back wound, removal the exposed intrathecal pump catheter and was started on intravenous ertapenem and later transitioned to levofloxacin and rifampicin for the duration of 3 months.
Conclusion: The author reports a case of infection following implantation of intrathecal pump in a cancer patient can be treated with intravenous antibiotics without the removal of the intrathecal pump system.
Keywords: Methecillin sensitive staphylococcus aureus; Surgical site infection; Bacteroides stercoris, Peptostreptococcus prevotii
Abbreviations: CNS: Central Nervous System; MSSA: Methecillin Sensitive Staphylococcus Aureus; SSI: Surgical Site Infection
Introduction
Intrathecal pump system implies that the medication is administered directly into the central nervous system, which potentially reduces the side effects. Previous studies comparing the efficacy of intrathecal pump in association with medical management alone showed that at 4 weeks follow-up, 84.5% of patients in the intrathecal treatment group had clinical success vs. 70.8% in the conventional medical management group. The follow-up study revealed significant improvement in 6 months survival and also reductions in fatigue. [1,2]
There are numerous possible complications documented after implantation of intrathecal pumps including: post puncture headache, dislocation or leakage of the catheter, complication regarding medication inside the pump and infection of the system. The data on the incidence and management of infections of intrathecal delivery system in cancer patient population is rather sparse. In analogy with the protocols for the management of infections of cardiovascular devices, intrathecal pump system infections can be classified as: pocket site infections including soft tissue infection around the device and the segment of the leads (i.e., not the transvenous segment), deeper infections that are associated with hematogenous spread and spinal cord infections [3].
Other types of classification include: Primary site infection, in which the pump and its pocket is the site of infection, and secondary infection due to bacteremia from a different source.
Intrathecal pump system infection diagnosis is confirmed by identification or culture of microorganisms (mostly bacteria) on sample from a suspect surgical site or implant area. Clinical signs of soft tissue infection can include local tenderness, fever, erythema, wound exudate, wound dehiscence and or skin erosion at the site. A rare but potentially fatal complication of infection is the spread of infection to intrathecal space [4].
Case Description
This is a 69 year old female who had an intrathecal pain pump placed for treatment of intractable pain secondary to metastatic breast cancer to the spine, lungs and liver who subsequently underwent palliative chemotherapy and radiation therapy, including radiation to the lumbar spine several years prior to presentation. She presented to the hospital after two months of worsening lumbar area skin infection which was unsuccessfully treated with local debridement and was being followed by infectious disease and wound care. Due to worsening pain and need of a refill of the intrathecal pump, she presented to the pain clinic. At that time, the infection had worsened and a portion of the intrathecal catheter was exposed (Figure 1). She also had some serous fluid discharge from the lumbar wound. Clinical examination revealed she was oriented and alert and had no symptoms or signs of central nervous system (CNS) infection or systemic opioid toxicity.
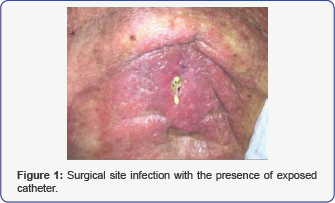
Physical exam revealed the patient to be afebrile and a lumbar back wound with exposed intrathecal pump catheter and a large area of cellulitis surrounding it. There was clear involvement of the spinous processes and the catheter tubing itself. Her blood count was: Hct=35%, WBC=12.35, (polymorpho nuclear 90%, lymphocytes 7.1%), Platelets =300,000. Cultures from the site taken during debridement showed Bacteroides stercoris, Peptostreptococcus prevotii and methecillin sensitive Staphylococcus aureus (MSSA). Spine CT Scan showed mild edema within the bones particularly the L2 spinous process and soft tissue edema. No epidural collection or cauda equina compression was noted.
Plastic surgery, infectious disease and neurosurgery were consulted. After informed consent was obtained, she was taken to the operating room under general anesthesia for extensive wound debridement, however, upon attempted removal of the catheter, the catheter was severed and a portion it was left intrathecally. The pump itself did not appear to be involved and it was left in place. She was started on intravenous Ertapenem for 8 weeks and switched to Levofloxacin and Rifampicin. The wound was treated with wound vac and left open for secondary closure. She was discharged to a rehabilitation facility in stable condition while her intrathecal pump was infusing subcutaneously. Her pain was under control with the addition of minor oral opioids.
Discussion
Significant advances were made in the management of cancer- related pain with the release of the "pain ladder" by the WHO in 1986, with an update in 1996 [5]. Using this ladder, most cancer patients quickly advance to the use of strong opioid. As such, opioids continue to be the mainstay treatment for cancer pain. Unfortunately, when the WHO pain ladder is used, effective pain control is not achieved in approximately one third of patients. In addition, approximately 14% of patients do not achieve effective pain relief at any time [6,7]. For this reason, many pain practitioners have incorporated placing an intrathecal pump in the treatment of cancer pain.
Smith et al. [1] in 2001 conducted a randomized trial study comparing comprehensive medical management to medical management plus an intrathecal pump system. After that study in a subsequent paper with extended follow-up of the same patients was published in 2005 [1]. These studies found that medical management plus an intrathecal pump system produced statistically significant reductions in reported pain scores and opioid related toxicities.
Intrathecal pumps are implanted to treat different types of pain in cancer patients. Since the intrathecal pump system releases prescribed amounts of pain medication directly to spine receptors, pain symptoms can be controlled using a smaller amount compared to the oral dose. Most people also experience fewer or more tolerable side effects, such as nausea and constipation, which can be devastating in cancer patients [8-10].
The major complications of the intrathecal drug delivery systems after pump malfunction and catheter issues are infection. The most common post-operative infection for these devices is a surgical site infection (SSI) [11]. In the context of implantable devices, SSIs are defined as infections that occur within one year after implantation if the device is not manipulated and if the infection appears to be related to the operation. Even though intrathecal pump infection is not as common as other CNS infection due to prosthetic devices (like shunts), it remains among the most disabling and feared complications and is of particular concern because of the threat it poses to CNS infection. The range of infectious complications in previous reports with intrathecal drug delivery systems is from 2% to 8% [12].
Risk of surgical site infection in other clean surgical procedures like breast surgery is elevated in cancer patients (3-15%) compared to non-cancer patients (3%) [13]. Similarly, we expect a higher risk of infection following intrathecal drug delivery implantation in cancer patients.
General risk factors for SSIs that are pertinent to cancer patients include leucopenia associated with the cancer or cancer therapy, diabetes mellitus, debilitated status, poor nutritional status, smoking and possibly corticosteroid use [14-20]. In addition, cancer patients frequently undergo treatment with chemotherapeutic agents and radiation, both of which can delay wound healing, increasing the risk of infection [16].
Infections of Intrathecal pump are dominated by bacteria of low virulence. The bacteria that are most frequently cultured in infections are the coagulate negative Staphylococci and Staphylococcus epidermidis (50-75% cases). Some of these bacteria have a unique property that appears to facilitate their colonization of tissue. The treatment of choice is usually considered to be removal of the entire infected pump system including the catheter and systemic antibiotics administration.
Conclusion
To our knowledge this was the first report of treatment of intrathecal drug delivery infection in a cancer patient without removal of an implanted intrathecal pump. We strongly believe that in cancer patients with low grade infection and mild clinical symptoms of infection, removal of intrathecal pump should not be considered as the first treatment option. We suggest initially starting conservative treatment including wound debridement and systemic antibiotics. If the treatment is started promptly, supported by our case, this may lead to avoidance of at least one surgical procedure and reduction of patient cost and risk.
References
- Smith TJ, Staats PS, Deer T, Stearns LJ, Rauck RL, et al. (2002) Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol 20(19): 4040-4049.
- Smith TJ, Coyne PJ, Staats PS, Deer T, Stearns LJ, et al. (2005) An implantable drug delivery system (IDDS) for refractory cancer pain provides sustained pain control, less drug-related toxicity, and possibly better survival compared with comprehensive medical management (CMM). Ann Oncol 16(5): 825-833.
- Baddour LM, Cha YM, Wilson WR (2012) Clinical practice. Infections of cardiovascular implantable electronic devices. N Engl J Med 367: 842-849.
- Follett KA, Boortz-Marx RL, Drake JM, DuPen S, Schneider SJ, et al. (2004) Prevention and management of intrathecal drug delivery and spinal cord stimulation system infections. Anesthesiology 100(6): 1582-1594.
- WHO(1996) Cancer Pain Relief (2nd edn). World Health Organization, Geneva, Switzerland.
- Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, et al. (2001) Symptoms during cancer pain treatment following WHO-guidelines: A longitudinal follow up study of symptom prevalence, severity and etiology. Pain 93(3): 247-257.
- Christo PJ, Mazloomdoost D (2008) Cancer pain and analgesia. Ann N Y Acad Sci 1138: 278-298.
- Ruan X (2007) Drug-related side effects of long-term intrathecal morphine therapy. Pain Physician 10(2): 357-366.
- Hamza M, Doleys D, Wells M, Weisbein J, Hoff J, et al. (2012) Prospective study of 3-year follow-up of low dose intrathecal opioids in the management of chronic nonmalignant pain. Pain Med 13(10): 13041313.
- McLaurin RL, Frame PT (1987) Treatment of infections of cerebrospinal fluid shunts. Rev Infect Dis 9(3): 595-603.
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR (1999) Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 20(4): 250-278.
- Bunn F, Jones DJ, Bell-Syer S (2012) Prophylactic antibiotics to prevent surgical site infection after breast cancer surgery. Cochrane Database Syst Rev 9(3): CD005360.
- Gea-Banacloche JC, Segal BH (2011) Infections in the cancer patient. In: DeVita VT, et al. (Eds.), Cancer: Principles and Practice of Oncology, (9th edn). Wolters Kluwer Lippincott Williams & Wilkins, Philadelphia, USA.
- Jassak P, Haeuber D (1998) Protective Mechanisms. In: Gross J & Johnson BL (Eds.), Handbook of Oncology Nursing (3rd edn). Mass: Jones & Bartlett, Boston, USA.
- King CR (1995) Out-patient management of myelosuppression. Clinical Perspectives in Oncology Nursing 1: 1-12.
- Manzano-Alonso ML, Castellano-Tortajada G (2011) Reactivation of hepatitis B virus infection after cytotoxic chemotherapy or immunosuppressive therapy. World J Gastroenterol 17(12): 1531-1537.
- National Cancer Institute (2011) Chemotherapy and You: Support for People with Cancer.
- National Cancer Institute. Managing Chemotherapy Side Effect: Infection.
- Zanussi S, Serraino D, Dolcetti R, Berretta M, De Paoli P (2013) Cancer, aging and immune reconstitution. Anticancer Agents Med Chem 13(9): 1310-1324.
- Neumayer L, Hosokawa P, Itani K, El-Tamer M, Henderson WG, et al. (2007) Multi-variable predictors of postoperative surgical site infection after general and vascular surgery: Results from the patient safety in surgery study. J Am Coll Surg 204(6): 1178-1187.






























