Abstract
Anthropogenic increases in atmospheric CO₂ concentrations, coupled with climate change-induced alterations in hydrological regimes, are increasing flood frequency across many boreal regions. Prolonged flooding creates oxygen-deprived soils that force roots to shift from aerobic respiration to anaerobic fermentation, an inefficient metabolic pathway that compromises carbohydrate utilization and leads to root dieback. We examined the interactive effects of flooding and CO₂ levels on two ecologically important boreal species: black spruce (Picea mariana [Mill.] Britton, Sterns & Poggenb.) and tamarack (Larix laricina [Du Roi] K. Koch). Seedlings were exposed to a 28-day flooding event under ambient (AC, 400μmol mol⁻¹) and elevated (EC, 800μmol mol⁻¹) CO₂ concentrations.
Flooding significantly reduced root respiration (-16% during treatment; -36% 35 days post-recovery), root biomass (-66%), root volume (-58%), and overall growth (-70%). While Photosystem II quantum efficiency declined under flooding, photosynthetic rates remained stable. Species responses diverged markedly: flooded black spruce exhibited 2.1× higher mortality under EC (59%) versus AC (28%), whereas tamarack developed adventitious roots 2.9× more frequently under EC (53%) than AC (18%) with no mortality. Contrary to our hypothesis, EC failed to mitigate flood stress or enhance recovery through carbohydrate supplementation.
These findings demonstrate that elevated CO₂ exacerbates flood vulnerability in black spruce while promoting tamarack’s adaptive capacity through morphological plasticity. Such differential responses suggest future climate conditions may drive compositional shifts in boreal forests, favoring tamarack in increasingly flood-prone landscapes.
Keywords:Black spruce; Tamarack; Climate change
Introduction
Atmospheric CO₂ concentrations have risen significantly over the past century due to anthropogenic activities [1]. Elevated CO₂ generally enhances plant photosynthetic CO₂ assimilation [2-5], water-use efficiency [6], and biomass accumulation. Additionally, it alters carbon allocation among plant organs, stimulating biomass investment in both coarse and fine roots while increasing total root length [7]. These changes are accompanied by shifts in root distribution within the soil profile, including deeper rooting depth and higher root turnover rates [7]. Elevated CO₂ also enhances root respiration due to increased carbohydrate availability [7]. While these effects are observed in both controlled environments and Free-Air CO₂ Enrichment (FACE) experiments, the magnitude of response tends to be lower in field conditions [8].
Elevated CO₂ is a major driver of global temperature increases [9] and the heightened frequency and severity of hydrological extremes such as flooding [10,11]. Flooding raises water tables, reducing soil oxygen availability and forcing tree roots to shift from aerobic respiration to anaerobic fermentation (Taiz & Zeiger, 2010) [12]. Fermentation generates only 1/18th the ATP per unit of carbohydrate compared to aerobic respiration, leading to inefficient energy production and impaired root function [12]. This inefficiency can hinder water and nutrient uptake [12] and reduce rooting depth [13]. Since roots are critical for nutrient and water absorption (Grant et al. 2012) [7], their dysfunction can suppress aboveground physiological processes and productivity [13-16]. Prolonged hypoxia or anoxia may further exacerbate these effects by inducing root mortality (Parad et al. 2013) [12]. However, elevated CO₂ may partially mitigate flooding stress by increasing photosynthetic carbon supply to roots [7], potentially compensating for inefficient carbohydrate utilization under hypoxia. Conversely, deeper rooting under elevated CO₂ could increase root exposure to flooding, complicating these interactions. Despite these possibilities, little is known about how elevated CO₂ influences tree physiological responses to flooding or alters flood resistance.
The Canadian boreal forest encompasses diverse topographies, with peatlands covering nearly 12% of the land area [17]. Two dominant peatland tree species, black spruce (Picea mariana (Mill.) Britton, Sterns & Poggenb.) and tamarack (Larix laricina (Du Roi) K. Koch) [18,19], are widely distributed across Canada. Black spruce alone constitutes ~37% of Ontario’s growing forest stock [20]. Both species endure periodic flooding, which, while detrimental to tree health, plays a vital ecological role by transporting nutrients (Benke, 2001; Mosepele et al. 2009; Junk et al. 1989; Sparks, 1995; Tockner et al. 2000). Notably, tamarack exhibits flood tolerance through adventitious root formation, facilitating oxygen transport to submerged roots [21], whereas black spruce lacks this trait. This difference raises intriguing questions about species-specific responses to elevated CO₂ under flooding.
Black spruce and tamarack thrive across diverse boreal landscapes, including nutrient-poor, waterlogged peatlands [13]. Both species have evolved flood-resistant adaptations [13,22], but it remains unclear whether elevated CO₂ further enhances their resilience or elicits divergent responses. To investigate this, we subjected two-year-old seedlings of both species to a 28-day flooding treatment under ambient and elevated CO₂, assessing ecophysiological traits and recovery dynamics. We hypothesized that elevated CO₂ would bolster flood resistance, thereby mitigating flooding- induced stress.
Materials and Methods
Seedling material and preparation Plant material
We used black spruce (Picea mariana (Mill.) BSP) and tamarack (Larix laricina (Du Roi) K. Koch) seedlings for this study. Black spruce seed cones were collected from a wetland stand at the Lakehead University Jack Haggerty Forest (48°29′2″ N, 89°29′23″ W), approximately 37km north of Thunder Bay, Ontario. Seed extraction followed protocols from The Woody Plant Seed Manual [23]. After collection, cones were rinsed, soaked in cold water for 4 hours, drained, and air-dried on metal trays for 20 hours. They were then oven-dried at 54.44°C (130ᵒF) (4-hour ramp-up, 11-hour hold) before cooling to room temperature. Seeds were extracted by shaking the dried cones. Tamarack seeds (Seed Zone 19, north of Lake Nipigon, Ontario) were obtained from the Ontario Tree Seed Centre (Angus, Ontario).
Seed stratification and germination
Seeds of both species were soaked in cold water for 24 hours, drained, and placed in moist paper towel-lined containers for cold stratification (4°C, 3 weeks). Stratified seeds were sown in 60mL Styrofoam cells (Beaver Plastics Ltd., Acheson, Alberta) filled with a peat moss: vermiculite mix (3:2 v:v), with 3-4 seeds per cell. The blocks were placed in polyethylene misting tents for 34 days under controlled greenhouse conditions (Argus Control Systems Ltd., Surrey, British Columbia): 23°C Day temperature, 17°C night temperature, 16h photoperiod (supplemented with high-pressure sodium lamps to maintain above 400μmol m⁻² s⁻¹ PAR). The blocks were misted daily during germination and subsequently watered when substrate moisture dropped to 35% (Delta-T ML2x probe & HH2 moisture meter).
Seedling establishment and fertilization
After germination, seedlings were irrigated twice weekly with starter fertilizer 11-41-8 (100.1mg/L N, 373.1mg/L P, 72.8mg/L K) for the first 2 weeks. Forestry seedling standard fertilizer (20-8-20 N-P-K) was applied thereafter. Nutrient concentrations were started at 50mg/L N, 20mg/L P, and 50mg/L K50 and gradually increased to 200mg/L N, 80mg/L P, and 200mg/L K over 2 months (Forestry Notes, 2009).
The environmental conditions were maintained at 22°C/17°C (day/night), 16-h photoperiod, and 60-70% RH.
Cold hardening
When seedlings reached ~15cm height, cold hardening was initiated according to Landis (1999): temperature was reduced by 3°C/week until reaching 7°C (day) and 4°C (night) over 5 weeks, photoperiod gradually shortened from 16h to 8h, fertilization switched to conifer finisher fertilizer (50mg/L N, 312.5mg/L P, 437.5mg/L K), and watering reduced to once weekly.
A blackout curtain ensured darkness during the night phase. Cold hardening was confirmed after 30 days by root color change (white → golden brown) and completion of terminal bud settings, and tamarack needle yellowing/shedding. Two seedlings per block were destructively sampled (roots washed and inspected) to verify hardening.
Seedling storage
Hardened seedlings were removed from containers, wrapped in plastic film, and stored in plastic-lined boxes at -3ᵒC.
Experiment design
The study employed a split-split plot design with CO₂ treatment (two levels: ambient [400 μmol mol⁻¹] and elevated [800μmol mol⁻¹]) as the whole plot (each was replicated in two separate greenhouses) and flooding treatment (flooded vs. non-flooded) as the split plot, and species (black spruce vs. tamarack) as the split-split plot. The experiment was conducted in four controlled-environment greenhouses at the Lakehead University Forest Ecology Research Complex (Thunder Bay Campus). Each CO₂ level was duplicated in two independent greenhouses.
Seedlings were removed from cold storage, thawed for 24 h at 17°C in darkness, and potted in 5×5×6-inch pots filled with a peat moss: vermiculite mix (3:2 v/v). Seedlings of each species were randomly allocated to greenhouses, then randomly assigned to flooding treatments (5 seedlings per species per treatment and per greenhouse). The seedlings (5 per species per bin) were placed in clear plastic bins (n=2 bins per greenhouse) for water inundation. Seedlings in the control treatment were placed in identical bins with drainage holes (6 × 0.5cm) to prevent waterlogging. Bins were randomly positioned on growth benches to minimize spatial bias.
Environmental conditions
The day and night temperatures in all the greenhouses were at 10ᵒC and 7ᵒC, respectively, for the initial 10 days and were then increased by 1.5ᵒC per week for 7 weeks. The photoperiod started at 8h and gradually increased to 16h at a rate of 2 hours a week. High-pressure sodium lamps were used to extend the natural photoperiod and to supplement the natural light when it fell below 400μmol m-2 s-1 PAR (on cloudy days, early morning and evening). The CO2 enrichment as achieved using CO2 generators (model GEN-2E; Custom Automated Products Inc., Riverside, California, USA).
During the seven weeks leading up to the start of the flooding treatment, the seedlings were watered when the volumetric water content of the growing medium reached about 35% as described previously. The seedlings were fertilized once a week at a concentration of 50mg/L N, 20mg/L P, and 50mg/L K in the initial two weeks of the experiment and then increased to 200mg/L N, 80mg/L P, and 200mg/L K for the remainder of the experiment.
Flooding treatment
The flooding treatment was implemented by filling the plastic bin with water up to the seedlings’ root collar position. Seedlings in the control group were watered as described previously. 0.5 cm holes were drilled in the bottom of control bins to avoid water accumulation. The flooded bins were drained completely after 28 days and the recovery process of seedling physiological processes was monitored for 36 days.
Chlorophyll fluorescence measurement
The maximum quantum yield of Photosystem II (Fv/Fm) was measured at 4-day intervals during the flooding treatment and weekly during the 35 days period after the termination of the treatment using the built-in FMS-2 portable pulse-modulated fluorometer (Hansatech Instruments Ltd. Norfolk, UK) in the PP-Systems CIRAS-3 gas exchange system (PP System Inc., Amesbury, MA, USA). The measurement was taken after at least 60 minutes dark adaptation on four randomly selected seedlings from each treatment combination. Black spruce measurements were taken on current-year foliage. Three readings were recorded on each sample and averaged for subsequent data analysis.
Foliage gas exchange measurement
We assessed the responses of foliar and root physiological traits by measuring foliar gas exchange and root respiration using a PP-Systems CIRAS-3 open gas exchange system equipped with a Parkinson conifer leaf chamber with automatic environment control (PP System Inc., Amesbury, MA, USA) and an FMS-2 portable pulse-modulated fluorometer (Hansatech Instruments Ltd. Norfolk, UK). Foliar gas exchanges were measured 17 days after initiating the flooding treatment, and 6 days and 35 days after the treatment ended. Five stable readings were recorded per sample and averaged for analysis. All measurements were performed on the same foliage of the same seedlings under identical chamber conditions. Seedlings in the ambient CO₂ treatment were measured at 400μmol mol⁻¹, while those in the elevated CO₂ treatment were measured at 800μmol mol⁻¹. All measurements were taken at 400μmol m²s¹ PAR and 25oC temperature. The sample foliage was removed from the tree after the final measurement for leaf area determination Regent WinSEEDLE (Regent Instruments, Quebec, Canada).
Root respiration measurement
Due to the destructive nature of sampling and limited seedling availability, root respiration was measured only twice: 17 days after flooding initiation and 35 days after the end of the flooding treatment. To measure respiration, roots were carefully extracted, washed, and surface-dried in the dark. Four seedlings per treatment combination were measured. The total area and length of the sample roots were measured using Regent WinRhizo system (Regent Instruments, Quebec, Canada).
Morphological and biomass measurement
Total project leaf area was determined using Regent WinSeedle system (Regent Instruments, Quebec, Canada). Seedling height and root collar diameter (RCD) were measured at the beginning and end of the experiment to determine increments and relative growth rates. The biomass of foliage, stem, and roots was measured after 16 hours of oven drying at 70°C. The root (leaf) mass ratio was calculated by dividing root (leaf) biomass by seedling biomass. Specific leaf area was calculated by dividing leaf area by leaf biomass. Specific root length was calculated by dividing total root length by total root biomass.
To assess root characteristics, the roots of four randomly selected seedlings per treatment combination (per greenhouse) were carefully extracted, washed, and analyzed using the Regent WinRhrizo system (Regent Instruments, Quebec, Canada) to quantify total root length (cm), total root surface area (cm²), and total root volume (cm³).
Adventitious roots and mortality
Adventitious root formation was determined by the emergence of white roots on the surface of the growing media after the flooding treatment. Seedling mortality was determined by the loss of foliage, branch brittleness, and absence of photosynthetic activity.
Data analysis
The data was analyzed using a split-plot ANOVA, with CO2 treatment at the whole plot, flooding treatment as the split plot, and species as the split-split plot. The assumptions of normality and homogeneity were assessed using the Shapiro tests and examining the residual plots, respectively. Since both assumptions were met for all the variables, no data transformation was necessary. When ANOVA showed a significant interaction, a Tukey’s post-hoc test was used to compare the means. All the analyses were conducted using the “R” statistics software (“R”, Geneva Switzerland).
To analyze the number of adventitious roots and percent mortality, two different models were used due to the fact that these factors were measured as a count. A logistic regression with an identity link was used for mortality analysis and a generalized linear model with a Poisson distribution and an identity link were used to analyze adventitious roots.
Results
Physiological responses
Photosynthetic rate did not differ significantly in response to CO₂ or flooding treatments in either species after 17 days of flooding or 35 days post-flooding. However, six days after flooding ceased, the photosynthetic rate was significantly lower in flooded seedlings (6μmol m⁻² s⁻¹) compared to controls (18μmol m⁻² s⁻¹) (Table 1). Elevated CO₂ significantly enhanced photosynthesis in both species, though the increase was markedly greater in L. laricina than in P. mariana (Figure 1, Figures 2A-2B, Table 1).
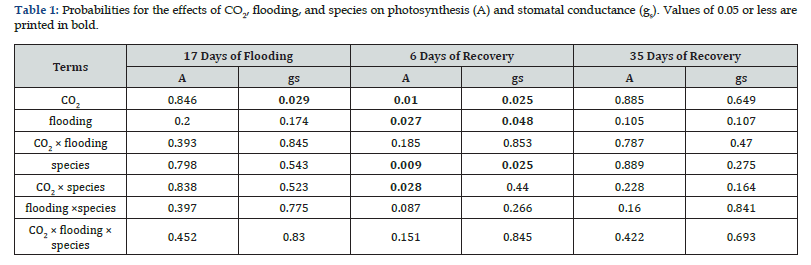
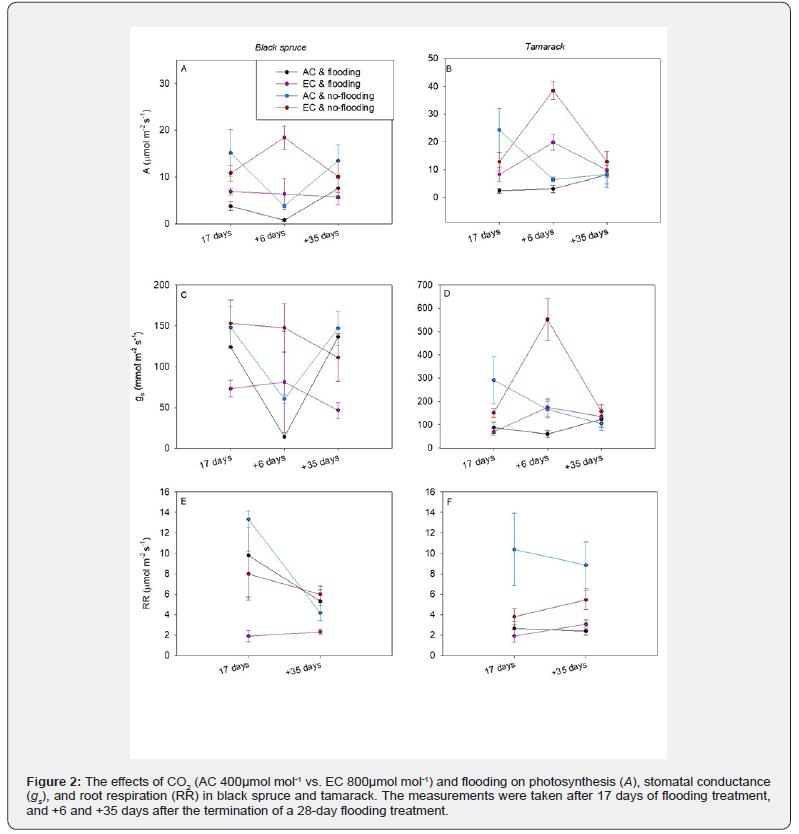
Stomatal conductance was significantly reduced under elevated CO₂ after 17 days of flooding (AC: 655mmol m⁻² s⁻¹, EC: 112mmol m⁻² s⁻¹) but increased six days post-flooding (AC: 75mmol m⁻² s⁻¹, EC: 239mmol m⁻² s⁻¹). No significant CO₂ effect was observed 35 days after flooding ended (Table 1). Flooded seedlings exhibited significantly lower stomatal conductance six days post-flooding (Flood: 83mmol m⁻² s⁻¹, Control: 231mmol m⁻² s⁻¹) (Table 1, Figures 1C-1D). Flooding had no significant effect at other measurement points. Additionally, L. laricina showed higher stomatal conductance (238 mmol m⁻² s⁻¹) than P. mariana (76mmol m⁻² s⁻¹) six days post-flooding (Figures 2C-2D, Table 1).
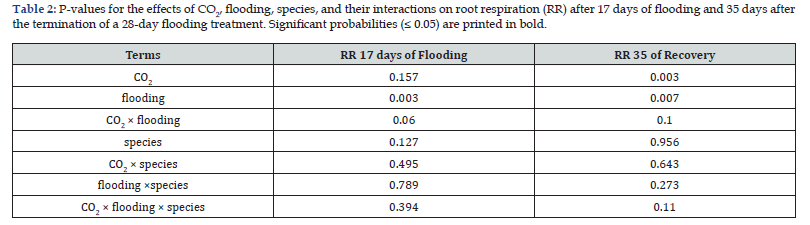
Flooding significantly suppressed root respiration during the treatment (Day 17: Flood = 7.43 vs Control = 8.87μmol m⁻² s⁻¹) and persisted 35 days post-recovery (Flood = 3.28 vs Control = 5.11μmol m⁻² s⁻¹; Figure 2E-2F, Table 2). In contrast, elevated CO₂ stimulated root respiration at 35 days post-flooding (AC = 4.18 vs EC = 4.20μmol m⁻² s⁻¹; Table 2, Figure 2E-2F).
The maximum quantum yield (Fv/Fm) showed significant depression under flooding but fully recovered by 35 days post-treatment (Figure 3, Table 4). Notably, no intermediate measurements were taken between days 17-34 to characterize this recovery trajectory. Treatment interactions and other factors exhibited minimal effects, with only isolated exceptions observed (Table 4, Figure 3).
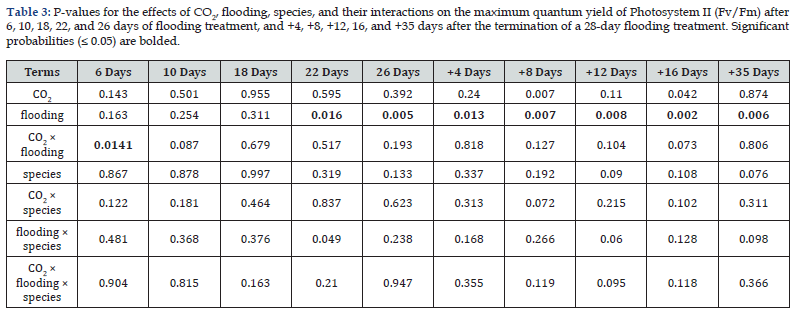
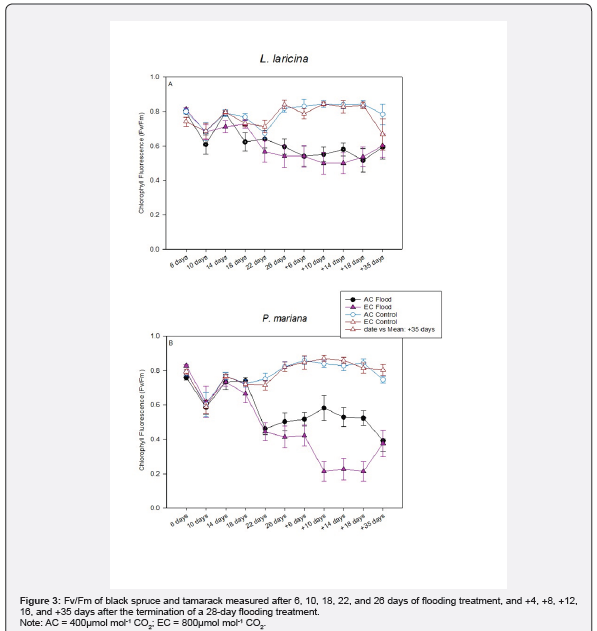
Growth and morphological responses
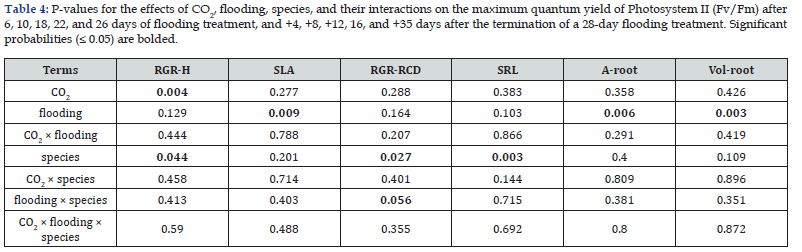
Elevated CO₂ significantly enhanced height relative growth rate (RGR) in both species (AC: 0.16cm cm⁻¹ vs. EC: 0.46cm cm⁻¹) but had no significant impact on specific leaf area (SLA), diameter growth, or root traits (Table 4). Neither flooding nor treatment interactions significantly affected height RGR (Table 2).
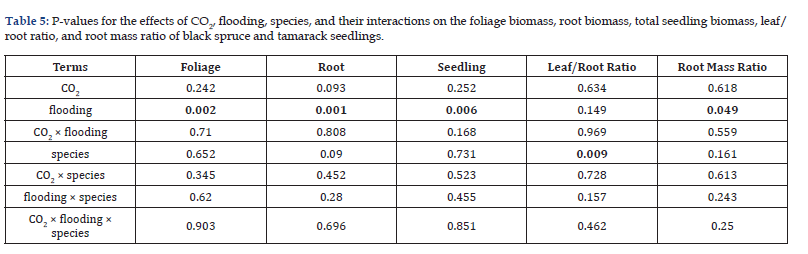
Flooding markedly increased SLA (Control: 105cm² g⁻¹ vs. Flooded: 410cm² g⁻¹) while reducing root surface area (Control: 370cm² vs. Flooded: 155cm²) and root volume (Control: 6.4cm³ vs. Flooded: 2.7cm³; Table 4). Seedling biomass was severely impacted by flooding, with significant reductions in total biomass (Flooded: 4.32g vs. Control: 14.49g), stem biomass (3.22g vs. 11.31g), root biomass (1.03g vs. 3.07g), and needle biomass (2.09g vs. 5.37g). However, root mass ratio increased under flooding (0.24 vs. 0.21; Table 5).
Species-specific responses
Tamarack (Larix laricina) exhibited superior growth performance compared to black spruce (Picea mariana), with higher height RGR (0.47 vs. 0.27cm cm⁻¹), faster diameter growth (0.32 vs. 0.14cm cm⁻¹), but lower specific root length (705 vs. 1109cm g⁻¹; Table 4).
Adventitious root formation and mortality
Flooding triggered adventitious root development in tamarack but not in black spruce. Elevated CO₂ further promoted this response, with significantly more tamarack seedlings developing adventitious roots under high CO₂ (17/32) compared to ambient CO₂ (6/32; χ² = 8.47, *p* < 0.01 for CO₂ × flooding interaction). Flooding also induced mortality in black spruce (59% under elevated CO₂ vs. 28% under ambient CO₂; χ² = 7.58, *p* < 0.01), while all tamarack seedlings survived regardless of treatment.
Discussion
Alstromeriae KRMY EMIC001 demonstrates significant potential for malathion bioremediation, achieving >85% degradation under optimized conditions. ITS-based phylogenetic analysis confirmed its identity, while enzymatic and GC-MS data elucidated a phosphatase-driven degradation pathway. This aligns with sustainable strategies for mitigating pesticide pollution, emphasizing the strain’s applicability in contaminated environments
CO₂ elevation fails to mitigate flooding effects
Contrary to our hypothesis, elevated CO₂ did not alleviate flooding-induced stress or enhance post-flooding recovery in either species. The underlying assumption that CO₂-induced increases in photosynthetic carbon gain would compensate for root carbohydrate inefficiency under flooding was not supported. While short-term CO₂ enrichment typically stimulates photosynthesis, prolonged exposure often triggers photosynthetic downregulation through reduced leaf biomass allocation and diminished photosynthetic capacity [12]. Additionally, elevated CO₂ may have exacerbated root hypoxia by increasing dissolved CO₂ in the rhizosphere, lowering pH and amplifying flooding damage- a mechanism consistent with the disproportionately higher mortality observed in flooded Picea mariana under elevated CO₂ (59% vs. 28% under ambient CO₂). This negative effect likely offsets any potential physiological benefits of CO₂ enrichment. Our findings align with the theory that flooding primarily disrupts root carbohydrate utilization rather than directly impairing photosynthesis, as evidenced by sustained photosynthetic rates despite significant biomass reductions.
Persistent flooding impacts on root function
Flooding induced long-term suppression of root respiration, with depressed rates persisting 35 days post-recovery in both species, a pattern well-documented in prior studies [13-16]. This suggests that while shoots tolerated 28-day flooding, root systems sustained severe, lasting damage. The mechanistic basis for reduced respiration likely shifted over time: during active flooding, hypoxia suppressed cytochrome respiration while inducing fermentative pathways [12], whereas post-recovery declines probably reflected preferential mortality of high-activity fine roots. This interpretation is supported by our observations of reduced root mass and altered allocation patterns following flooding.
Differential photosystem II sensitivity
Photosystem II (PSII) exhibited greater flooding sensitivity than gas-exchange parameters. While photosynthetic CO₂ assimilation and stomatal conductance remained stable, PSII quantum efficiency (Fv/Fm) declined significantly from day 22 of flooding through 35 days post-recovery. As Fv/Fm reflects the efficiency of electron transport in oxygen-evolving PSII complexes [12], its sustained depression indicates chronic photochemical impairment despite maintained carbon fixation. The delayed onset of Fv/Fm reduction suggests both species tolerate short-term flooding without photochemical damage, consistent with their natural occurrence in flood-prone habitats [13,17,24].
Species-specific adaptive strategies
The species diverged markedly in flooding responses, with CO₂ enrichment further amplifying these differences. Larix laricina’s capacity to form adventitious roots—enhanced under elevated CO₂ (17/32 vs. 6/32 seedlings; *p* < 0.01) mirrors observations by Calvo-Polanco et al. [21] and likely explains its survival across treatments. In contrast, P. mariana suffered high mortality (59% under elevated CO₂), lacking such morphological adaptations. These results suggest future atmospheric CO₂ levels may favor L. laricina dominance in floodplains while disadvantaging P. mariana, potentially altering boreal wetland species composition [25].
Author Contribution
K Richard: developed the research plan, carried out the experiment,
conducted data analysis, wrote an MScF thesis
QL Dang: initiated the research project, supervised K Richard,
commented and edited the thesis, and adopted the thesis into this
manuscript.
RZ Man: co-supervised K Ricard, commented on the thesis
and the manuscript.
Funding
The study was supported by NSERC Dicovery Grant to Q.L. Dang (203198-2013-RGPIN) and Lakehead University Graduate Assistantship to K. Richard.
Acknowledgement
We would like to thank Ms. Keri Pidgen, Greenhouse Manager at Lakehead University, for her technical support in setting up the greenhouse experiment.
References
- Graven HD, Keeling RF, Piper SC, Patra PK, Stephens BB, et al. (2013) Enhanced seasonal exchange of CO2 by northern ecosystems since 1960. Science 341(6150): 1085-1089.
- Anderson-Teixeira KJ, Miller AD, Mohan JE, Hudiburg TW, Duval BD, et al. (2013) Altered dynamics of forest recovery under a changing climate. Glob Chang Biol 19: 2001-2021.
- Bazzaz FA, Miao SL, Wayne PM (1993) CO2-induced growth enhancements of cooccurring tree species decline at different rates. Oecologia 96(4): 478-482.
- Curtis PS, Wang XZ (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113: 299-313.
- Drake BG, Gonzalez Meler MA, Long SP (1997) More efficient plants: A consequence of rising atmospheric CO2? Annual Review of Plant Physiology and Plant Molecular Biology 48: 609-639.
- Keenan TF, Hollinger DY, Bohrer G, Dragoni D, Munger JW, et al. (2013) Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 499(7458): 324-327.
- Nie M, Lu M, Bell J, Raut S, Pendall E (2013) Altered root traits due to elevated CO2: a meta-analysis. Global Ecology and Biogeography 22(10): 1095-1105.
- Leakey AD, EA Ainsworth, CJ Bernacchi, A Rogers, SP Long, et al. (2009) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot 60(10): 2859-2876.
- Pachauri RK, Allen MR, Barros VR, et al (2014) Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. IPCC.
- Huntington TG (2006) Evidence for intensification of the global water cycle: Review and synthesis. Journal of Hydrology 319(1-4): 83-95.
- Kreuzwieser J, Rennenberg H (2014) Molecular and physiological responses of trees to waterlogging stress. Plant Cell and Environment 37(10): 2245-2259.
- Lambers H, Oliveira RS (2019) Plant Physiological Ecology. (3rd edn), Springer, New York, p. 736.
- Islam MA, Macdonald SE (2004) Ecophysiological adaptations of black spruce (Picea mariana) and tamarack (Larix laricina) seedlings to flooding. Trees-Structure and Function 18: 35-42.
- Levan MA, Riha SJ (1986) Response of root systems of northern conifer transplants to flooding. Canadian Journal of Forest Research 16(1): 42-46.
- Pereira JS, Kozlowski TT (1977) Variations among woody angiosperms in response to flooding. Physiologia Plantarum 41(3): 184-192.
- Pezeshki SR, Chambers JL (1986) Variation in flood-induced stomatal and photosynthetic responses of 3 bottomland tree species. Forest Science 32(4): 914-923.
- Yang RC, Yeh FC (2007) Differential growth and rooting of upland and peatland black spruce, Picea mariana, in drained and flooded soils. Silvae Genetica 56(1-6): 73-80.
- Uchytil RJ (1991) Larix laricina. Ed. U.S. Department of Agriculture FS, Rocky Mountain Research Station, Fire Sciences Laboratory.
- Ontario Ministry of Natural Resources (2013a) Ontario's Tree Atlas: black spruce (picea mariana).
- Ontario Ministry of Natural Resources (2013b) Ontario's Tree Atlas: black spruce (picea mariana).
- Calvo-Polanco M, Senorans J, Zwiazek JJ (2012) Role of adventitious roots in water relations of tamarack (Larix laricina) seedlings exposed to flooding. BMC Plant Biology 12: 99.
- Islam MA, MacDonald SE, Zwiazek JJ (2003) Responses of black spruce (Picea mariana) and tamarack (Larix laricina) to flooding and ethylene. Tree Physiology 23(8): 545-552.
- Bonner FT, Carrfalt RP (Eds.) (2008) The Woody Plant Seed Manual. USDA Agriculture Handbook 727.
- Ballantyne DM, Hribljan JA, Pypker TG, Chimner RA (2014) Long-term water table manipulations alter peatland gaseous carbon fluxes in Northern Michigan. Wetlands Ecology and Management 22: 35-47.
- Kozlowski TT (1984) Plant-responses to flooding of soil. Bioscience 34(3): 162-167.






























