Effect of Elevated Temperature and Carbon Dioxide on Carbon Pool Dynamics in Semi-Arid Alfisol
K Sammi Reddy1*, M Vanaja2, N J Lakshmi2, P Satish2, M Eshwar2, Munna Lal2, V Girijaveni2, K Srinivas2, M S Rao2, P S Basavaraj1, and V K Singh2
1 National Institute of Abiotic Stress Management (NIASM), India
2 National Institute of Abiotic Stress Management (NIASM), India
Submission: May 15, 2023; Published: June 02, 2023
*Corresponding author: K Sammi Reddy, Director, National Institute of Abiotic Stress Management (NIASM), Malegaon, Baramati, Pune district, Maharashtra – 413115, India
How to cite this article: K Sammi Reddy, M Vanaja, N J Lakshmi, P Satish, M Eshwar, et al. Effect of Elevated Temperature and Carbon Dioxide on Carbon Pool Dynamics in Semi-Arid Alfisol. Int J Environ Sci Nat Res. 2023; 32(2): 556336. DOI 10.19080/IJESNR.2023.32.556336
Abstract
Soil responses to the projected future levels of CO2 and temperature was studied under Free Air Temperature Elevation (FATE) facility. Three levels- Ambient, elevated temperature (eT) (ambient +3oC ±0 .5oC) and its interaction with eCO2 (550ppm) (eT+eCO2) was studied in rainfed pigeonpea and maize. The results reveal that eT and eT+eCO2 reduced available water capacity in soil by 23% and 29%, respectively. Also, the eT and eT+eCO2 lowered available N, P, B and Zn in soils as compared to AC. However, there was no significant effect on soil pH, EC and CaCO3 contents due to eT and eT+eCO2. Enzymes such as dehydrogenase, urease and aryl sulphatase activities were higher under eT and eT+eCO2 over ambient control (AC). The eT showed a negative impact on SOC, MBC, POC, labile, non-labile C and carbon management index in soils. Interestingly, this negative effect was not observed in eT+eCO2 conditions. The eT showed a negative impact on yield parameters in both pigeon pea and maize. However, in maize, total and fodder biomass production increased with eT by 3.2% and 11.7% but grain yield reduced with eT by 10%. This study clearly states that the eT conditions can have a negative impact on soil properties including carbon pools. While eCO2 associated with eT (eT+eCO2) can compensate the negative effect of eT on soil properties.
Keywords: Carbon pools; Elevated temperature; Elevated CO2; FATE; Alfisol; Soil properties
Highlights:
a) Under eT and eT+eCO2, soil water retention at FC decreased by 12.4% and 14.3%, respectively over AC.
b) The soil ammonical N decreased with eT by 5-9% but increased with eT+eCO2 by 12-16% as compared to AC.
c) Under eT, Soil MBC, labile and non-labile C fractions were reduced by 28%, 14% and 5.6%, respectively over that of AC.
Introduction
The atmospheric carbon dioxide concentration has increased globally to the extent of 47.2% as compared with the pre-industrial value of 280ppm [1] which may increase to 560ppm by 2050 [2]. Increasing atmospheric CO2 concentration causes global warming as it traps long wave radiation emitted from earth’s surface. The mean annual global surface temperature was projected to increase by 1.8oC - 5.8oC by the end of this century, depending on the greenhouse emission scenario [3]. The global warming effects are not only restricted to air temperature but can also alter rainfall patterns and soil temperature [4]. Changes in soil temperature and rainfall distribution due to warmer climate may have intense effects on crop growth and soil properties in semi-arid regions.
Temperature above or below the optimum range for specific crop species often results in loss of yield due to rate-limited photosynthesis, reduced vegetative and reproductive growth [5]. Pigeonpea is the world’s sixth most important legume crop and rich source of protein to more than a billion people in the developing world and a cash crop that supports the livelihoods of millions of resource poor farmers in Asia, Africa, South America, Central America and the Caribbean. In India, it is cultivated in about 4.37 million ha mostly under rainfed conditions and contributed about 16% of the total pulse production with an average productivity of 0.66t ha-1 [6]. CROPAGRO-pigeonpea model showed that the climate change delayed anthesis by 9-20 days and maturity by 15-24 days and decreased yield by 14-66% with RCP8.5 in year 2095 in comparison to RCP2.6 in year 2010 [7]. Currently 1147.7 million t of maize is being produced jointly by over 70 countries from an area of 193.7 million ha. Earlier studies have indicated that 1°C increase in global temperature will lead to 17% reduction in maize productivity [8,9]. Vanaja et al. 2015 [10]found that the maize grown under 550ppm CO2 had higher biomass and harvest Index (HI) due to higher photosynthesis rate. In general, eCO2 positively affects crop yield but has negative effect on the nutritional quality particularly nutrient and protein contents [11]. Increase in plant growth due to eCO2 leads to increased N uptake by plants thereby promoting a large reduction in plant available N in soil. Changes in soil nutrient availability induced by eCO2 and eT during crop development can influence yield and nutritional quality. Thus, soil responses to eT and eCO2 should also be considered when developing adaptation strategy for climate change.
Drivers of climate change such as elevated temperature, eCO2, variable precipitation and atmospheric N deposition will affect soil organic matter status, C and N nutrient cycling, plant available water and hence plant productivity which in turn will affect soil pH [12]. Using elevation gradient as a surrogate for increasing temperature and decreasing precipitation under climatic change scenarios, Smith et al. [13] found that electrical conductivity (EC) decreased and soil pH increased in a semi-arid environment. The findings on soil C sequestration have been inconsistent, with some studies showing an increase [14], no change [15] and a decrease [16] in soil C contents under eCO2 conditions. Labile soil organic C was rapidly depleted as the temperature rises [17]. In addition, elevated CO2 in the future may reduce sequestration of root derived soil C, a major source of labile light fraction C [18]. However, soil microbial C (SMC) similar to labile C has been shown to be responsive to short term environmental changes with recent studies revealing significant decline in SMC during long-term simulated climatic warming experiments [19].
Elevated temperature often increases soil N mineralization and availability while the effect of eCO2 on soil N varies in direction and magnitude amongst ecosystems. Interaction of elevated temperature and elevated CO2 potentially influence the demand and supply mechanism of P in a soil under changing climate [20]. A few available studies suggest the possibilities of both increase as well as decrease in available P in soil under elevated temperature and CO2. From this brief review of literature, it is understood that much of the earlier research confined to investigate the effect of eCO2 on crop growth and soil properties under open top chambers (OTC) or in free air CO2 enrichment (FACE). However, the effect of eT and interactive effect of eT and eCO2 on soil properties and carbon pools dynamics and its processes has not been properly understood particularly in rainfed Alfisol soils. Therefore, the present study was undertaken to study the effect of elevated canopy temperature (eT) (+3oC) and its interaction with eCO2 (550ppm) (eT+eCO2) on changes in soil properties, C pool dynamics, soil moisture and nutrient availability and dry matter production of rainfed pigeonpea and maize grown in Alfisol soil.
Methods
Experimental site and design
A field experiment was conducted in monsoon season of 2017 and 2018 with pigeonpea and maize, respectively under three environmental conditions in Free Air Temperature Elevation (FATE) facility viz., (i) Ambient temperature and CO2 concentration (380ppm) (i.e. Ambient Control, AC), (ii) elevated canopy temperature (eT) of ambient + 3oC±0.5oC (and ambient CO2 concentration) and (iii) elevated temperature of ambient+3oC±0.5oC and elevated CO2 concentration of 550ppm (eT+eCO2). FATE facility is at Hayatnagar Research Farm, Central Research Institute for Dryland Agriculture (CRIDA), Hyderabad between 17.20oN latitude and 78.3oE longitude. FATE facility consisting of nine rings with 8m diameter. Among the nine rings, six were fitted with 24 arrays of 2000W capacity ceramic infrared heaters (Elstein, model FSR – 1000) above the canopy to maintain elevated canopy temperature (eT) of ambient+ 3oC±0.5oC. Three warming rings were also provided with CO2 release system at 0.3m height from the base of the ring to study the interactive effect of elevated temperature and CO2 (eT+eCO2). The polyurethane tubing perforation releases the CO2 within ring to maintain the elevated concentration of 550ppm. The CO2 release was controlled by solenoid valves which in turn regulated by the SCADA based control system linked with CO2analyser, wind direction and wind speed. The CO2 concentration at the centre of the ring was continuously monitored by IRGA based CO2 analyser (Priva, modwl-200821), the duration of CO2 release was based on the set CO2 concentration for the specified area as well as wind direction and speed.
Infrared sensor (Ray teck Fluke, model-RAYCMLT33) is fitted in each ring to monitor the canopy temperature. The duration and intensity of heating is regulated by canopy temperatures of control plots and uses as proportional integral-derivative feedback system to maintain the heating treatment [21]. Signals from each sensor are being recorded and monitored and controlled by Program Logic Control (PLC) and Supervisory Control and Data Acquisition system.
Field experiment with pigeon pea, variety PRG 176 was taken up in 2017. Pigeon pea was planted at a spacing of 0.9m between rows and 0.2m between plants within a row. Recommended dose of farmyard manure (FYM) and fertilizer were applied @ 10 t FYM ha-1, 30kg N ha-1, 30kg P2O5 ha-1 and 25kg K2O ha-1, respectively. While, maize (var DHM 117) was taken up in 2018. Maize crop received FYM, N, P2O5 and K2O at 10t ha-1, 65, 75 and 50kg ha-1, respectively. Urea, Diammonium phosphate and muriate of potash were the sources of N, P and K, respectively. Both pigeonpea and maize were grown in FATE rings as per the recommended agronomic practices. Manual weeding was done 2 to 3 times as per the need. No significant infestation of pests and diseases were observed in both pigeonpea and maize crops. In case of the pigeonpea, pod number, pod weight (g plant-1), total biomass, vegetative biomass and seed yield (g plant-1) were recorded after harvesting. Similarly in case of maize, cob weight (g plant-1), total biomass, vegetative (fodder) biomass, kernel number and seed weight (g plant-1) at harvesting were recorded. All these components of both pigeonpea and maize were air dried in sun till the constant weight was obtained and then recorded dry weights.
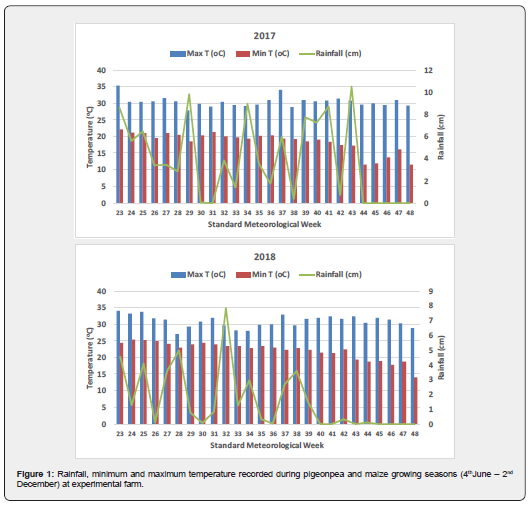
Experimental soils were sandy loam in texture, neutral in reaction (pH 7.5), Non-saline (Electrical conductivity 0.20dS m-1), low in organic C (4.1g kg-1), low in available N (156kg ha-1), high in available P (54kg ha-1), high in available K (216kg ha-1) and sufficient in available S (12mg kg-1), available Zn (0.55mg kg-1) and available B (1.4mg kg-1). The rainfall, maximum and minimum temperature data recorded at the experimental farm during the pigeonpea and maize growing seasons are furnished in Figure 1.
Soil sampling and analysis
Field-moist soil samples were collected from the plough layer (0-15cm depth) from each ring after harvesting of pigeonpea in 2017 and after harvesting maize in 2018. Soil samples were collected from 3 rings for each of the 3 treatments. Four subsamples collected from each ring were bulked together on a ring basis to get a composite soil sample per ring. Soil samples were air-dried at room temperature and passed through a 2-mm sieve for chemical analyses. Soil samples thus collected after pigeonpea and maize were analysed for NH4-N, NO3-N, available nutrients (N, P, K, Zn and B). Soil samples collected after harvesting maize were also analysed for pH, electrical conductivity, and CaCO3 content. Water retention at 1/3 bar (Field capacity, FC) and water retention at 15 bar (Permanent wilting point, PWP) were also determined in soil samples collected after harvesting maize and computed available water content. Gravimetric soil moisture content was determined during pigeonpea and maize seasons periodically.
Determination of soil properties and plant available nutrients in soils
Each soil sample was analysed in triplicate for determination of pH, electrical conductivity (EC) [22], CaCO3 and water retention [23]. Potentially available (mineralizable) N was determined by distilling the soil with alkaline potassium permanganate solution and measuring the NH4-N in the solution [24]. Soil samples were analyzed for Olsen-P by extracting with sodium bicarbonate (0.5M NaHCO3, pH 8.5, 1:20 soil to water extract ratio). Phosphate concentration in the extract was estimated colorimetrically by ascorbic acid blue colour method of Murphy & Riley [25]. Available K (1M ammonium acetate extractable) was determined according to the procedure of Hanway &Heidel [26]. Soil available Zn was extracted in 0.005M DTPA-CaCl2 at pH 7.3 [27] and its concentrations in the extracts was determined by atomic absorption spectrophotometer (Perkin Elmer Model 700AA). Hot water extractable B (available B) was determined by azomethane yellow colour method [28].
Estimation of soil organic C and its fractions and computation of Carbon management index (CMI)
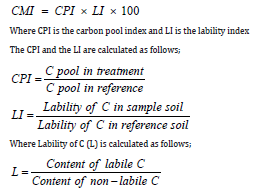
Total soil organic C (SOC) concentration was determined by dry combustion, using a CN analyser (Elementar Vario El Cube) in soil samples (0.25mm mesh) collected after harvesting maize. The microbial biomass carbon (MBC) was determined by the fumigation and extraction method of Vance et al. [29]. Particulate organic carbon (POC) was determined following Cambardella & Elliot [30] method. Soil labile C (CL) was determined by the modified CMI method of Blair et al. [31] using 333mM KMnO4 as the oxidizing agent. Non-labile C (CNL) was determined as a difference between total SOC and the labile C (CL). The relative amount of these two fractions and the total carbon in a cropped and reference soil have been used by Blair et al. [31] to calculate a CMI. Composite soil sample was collected from a neighboring cultivated fallow plot and analyzed in triplicate for carbon fractions. The fallow plot soil was considered as the reference soil.
Estimation of soil enzymes
Aryl sulphatase activity (ASA) was estimated by measuring the p-nitrophenyl released after incubation of the buffered soil with p-nitrophenyl sulphate as substrate [32]. The soil urease activity was determined by incubating soil with urea [33], and the residual urea was determined titrimetrically. Dehydrogenase activity (DHA) was determined by using 2, 3, 5, triphenyl tetrazolium chloride as an electron acceptor, and methanol was used as an extractant for the formed formazan [34].
Farmyard manure (FYM)
The FYM applied to pigeonpea contained 0.74% N, 0.24% P, 0.70% K, 15mg Zn kg-1 and 12mg B kg-1 whereas the FYM applied to maize contained 0.8%N, 0.22% P, 0.68%K, 12mg Zn kg-1 and10mg B kg-1.
Statistical analysis
The SAS (2005) [35]was used to analyse the variance and determine the significance of treatment effects at p <0.05 level of significance. The Duncan’s multiple range test was used for means comparison.
Results
Crop yield parameters
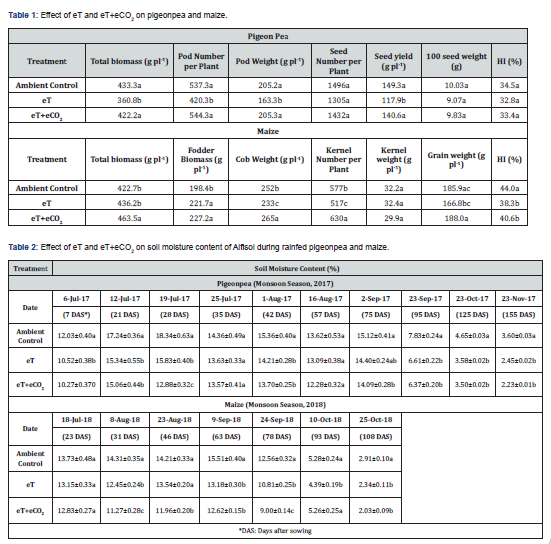
Pigeon pea and maize were studied inthe monsoon season of 2017 and 2018 respectively. In our study, eT negatively influenced the yield parameters in pigeonpea and maize to a larger extent as compared to eT+eCO2. In pigeopea, the total biomass production followed the order Ac > eT+eCO2 eT while in maize, the order was eT+eCO2 > eT > Ac. Similar is the trend with fodder biomass in maize. However, cob weight, kernel number, grain weight and HI were lower in eT as compared to Ac. Maize being a C4 plant, it could produce higher biomass under eT as compared to control. While pigeonpea being a C3 plant, eT had negative impact on total biomass as compared to AC. (Table 1). Thus, the extent of decline is more in pigeon pea as compared to maize. The eT has reduced the harvest index (HI) by 5% over the AC while eT+eCO2 has decreased the HI by 7.7% over the AC in pigeon. While in maize, eT and eT+eCO2 resulted in a decrease in the harvest index by 12.9 and 7.7%, respectively as compared to AC. The eT has resulted in the reduction of 100 seed weight by 9.57% while the combined effect (eT+eCO2) has resulted in reduction upto 1.99% in pigeon pea. This suggests that along with raised temperatures, if there is elevated CO2 (eCO2) condition, then this may have minimal impact on 100 seed weight in pigeon pea in the climate change scenario. Similarly, in maize, the extent of reduction was 10.27% under eT. A non-linear relationship was found between harvest index (Y) and the interactive effect of elevated temperature and elevated CO2 concentration (X) in pigeon pea (Y = 1.15x2 – 5.15x + 38.5, r = 0.99) and in maize (Y = 4x2 – 17.7x + 57.7, r = 0.99) indicating that both eT and also eT+eCO2 can directly impact HI.
Impact of eT and eCO2 on Soil moisture content
6.2.
The impact of eT and eT+eCO2 on volumetric soil moisture content in surface soil (0-15cm) was studied at every week during initial stages and at 15-30 days interval during the later stages (Table 2). When pigeonpea was grown, soil moisture content significantly decreased by 4-32% with eT as compared to AC throughout the growing season except on 25th July 2017 (35 DAS) and 16th Aug 2017(57 DAS). The soil moisture was further decreased by 6-38% under eT+eCO2 as compared to AC at 8 sampling stages except at 35 DAS and 57 DAS. The difference in soil moisture contents of soils subjected to eT and eT+eCO2 were not significant at all sampling stages except at 28 DAS. Soil moisture content was drastically reduced in the month of October and November 2017 due to with drawl of monsoons on 30 September 2017. More or less similar effects were obtained in the maize crop grown in 2018. Soil moisture content was decreased by 5-20% significantly with eT as compared to AC throughout the maize season except at 23 DAS and 46 DAS. Soils subjected to eT+eCO2 also maintained significantly lower moisture content by 14-30% in comparison to AC. In our study, eT+eCO2 maintained low soil moisture as compared to eT but was not significant statistically.
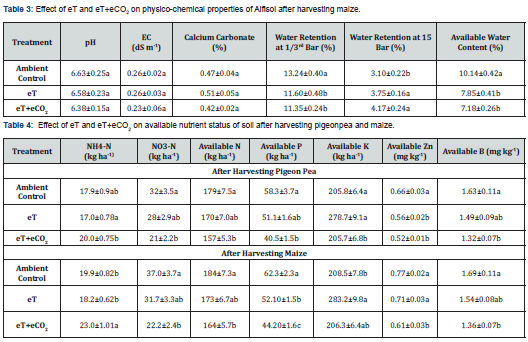
Also, water retention and available water content was studied and it was found that the eT and eT+eCO2 had significant effect on soil moisture constants such as water retention at field capacity (FC), water retention at permanent wilting point (PWP) and available water capacity of soils collected in post-harvest soil samples. Soil water retention at FC decreased with eT and eT+eCO2 by 12.4% and 14.3%, respectively over AC with corresponding increase in water content at PWP by 21% and 34%, respectively (Table 3). This influence of eT and eT+eCO2 on water retention at FC and PWP resulted in decrease in available water capacity of soil by 22.6% and 29.2%, respectively as compared to AC. The eT and eT+eCO2 were at par with each other in influencing soil moisture content.
Impact of eT and eCO2 on Soil and plant nutrient status
In our study, available N, P, K, Zn and B status of soils for both the study years are given in Table 4. The eT and eT+eCO2 decreased the available N, P, and K nutrients in soil as compared to AC. However, the decrease with eT+eCO2 was significant. The mean decline in available N during the experimental period under eT and eT+eCO2 was 5.50 and 11.58% respectively over Ac. The mean decline in available P during the experimental period under eT and eT+eCO2 was 14.36 and 29.8% respectively. While the decline in available K was 2.35 and 1.56% respectively under eT and eT+eCO2. Available Zn status in soil after harvesting pigeonpea and maize was decreased with eT and eT+eCO2 however, the decrease with eT was significant after pigeonpea. But decline in available Zn status of soils after harvesting maize with eT+eCO2 was significant as compared to AC. The eT+eCO2 maintained lower available Zn status in soil after harvesting maize as compared to eT.
Treatments had significant effect on inorganic N. The ammonical N in soils after harvesting both the crops decreased with eT by 5-9% but increased with eT+eCO2 by 12-16% as compared to AC. While nitrate N in soils after harvesting decreased with eT by 14% and by 34-40% under eT+eCO2. The eT+eCO2 maintained the lowest NO3-N content in soils after harvesting pigeonpea and maize crops.
SPAD readings
SPAD readings were taken during 43,60,90, 120 and 150 days after sowing in pigeon pea. In this study, the SPAD reading ranged from 39.33 to 54.27, 34.93-48.27 and 36.07-51.67 in aT, eT and eT+eCO2. This study clearly suggests that SPAD reading were low in eT as compared to AC and eT+eCO2.
Soil enzyme activities
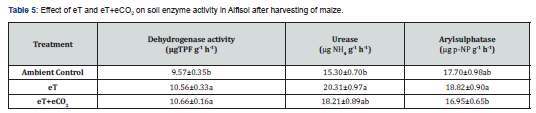
Activities of soil enzymes viz., dehydrogenase, urease and aryl sulphatase in soils exposed to eT were significantly higher as compared to ambient control (Table 5). The eT+eCO2, similar to eT, also increased the activities of dehydrogenase (10.66 ug TPF g-1 h-1) and urease (18.21ug NH4 g-1 h-1) as compared to ambient control. The effect of eT+eCO2 on aryl sulphatase (16.95ug p-NP g-1 h-1) was not significant. Activity of aryl sulphatase in soils exposed to eT+eCO2 was significantly lower by 10% as compared to eT. The activities of dehydrogenase and urease under eT and eT+eCO2 were at par with each other.
Soil carbon pools and carbon management index
After two years of cropping, the treatment plots maintained higher soil organic carbon (SOC) content as compared to fallow plot (Table 6). The eT had reduced the soil organic carbon and its fractions such as microbial biomass carbon (MBC), particulate organic carbon (POC), labile and non-labile C contents as compared to ambient control. Soil MBC, labile and non-labile C fractions were reduced with eT by 19%, 14%, 14.3% and 5.6%, respectively over that of AC. But the reduction in MBC due to the effect of eT was significant. On the other hand, eT+eCO2 increased significantly organic carbon, MBC, labile and non-labile C contents of soils as compared to ambient control by 6.4%, 20%, 28%, 19% and 5%, respectively. The soils exposed to eT+eCO2 maintained significantly higher lability of C, lability index and carbon management index over the ambient control and eT. The eT reduced the carbon pool index (CPI) while eT+eCO2 enhanced the CPI over ambient control but the affect was not significant. Soil carbon management index (CMI) was higher in soils subjected to eT+eCO2 (127.9) and lower in soils exposed to eT (88.9) as compared to ambient control (105.9). But the effect of eT was not significant. These results clearly showed that in short term the eCO2 associated with eT not only compensated the negative effect of eT on soil organic matter but also maintained at par with ambient control.
Discussion
Soil moisture content during pigeonpea and maize seasons
Understanding the effect of eT and eCO2 on soil moisture status during the cropping season under rainfed conditions is essential due to large temporal variability in rainfall and the preponderance of small precipitation events. Elevated temperature increases potential evaporation (E) and transpiration (T) loss of water from the soil which might have resulted in low soil moisture content in eT plots (Table 2). The eT+eCO2 maintained significantly lower soil moisture as compared to Ac but at par with the soil moisture content of eT despite higher biomass production in this treatment as compared to eT. Earlier studies have shown that eCO2 reduced evapotranspiration (ET) and increased soil moisture content in semi-arid shortgrass steppe of North America [36]. High evaporation demand was offset by the reduced plant transpiration on soil moisture under CO2 enrichment. The stable isotope partitioning of ET in the semi-arid shortgrass steppe shown that the evaporation component of ET was significantly lower under eCO2 [37] and resulted in percolation of more water into deeper layers of soil under eCO2 condition. But in the present investigation, association of eT with the eCO2 has changed the dimension. The association of eT offset the beneficial effect of eCO2 in reducing the evaporation component of ET. Thus, in dryland regions, simultaneous occurrence of climate change drivers (eT+eCO2) might increase the water requirement of rainfed crops. Therefore, the supplemental irrigation to rainfed crops through harvested rainwater might be crucial for meeting high ET demand of higher drymatter production with eT+eCO2 under changing climate conditions.
Soil Physico-chemical properties
Investigating the influence of climate change on soil health indicators help in developing adaptation strategies for climate change. After two cropping seasons, soil pH non-significantly reduced by eT and eT+eCO2 as compared to AC (Table 3). According to Celia et al. (2002) [38], the increase in the CO2 concentration influences soil pH which consequently influence the rate of weathering and to some extent, the availability of plant nutrients. When CO2 was introduced into the soil, Ravi et al. (2010) [39] found insignificant change in soil pH but Smith et al. (2005) [40] observed a decrease in soil pH.
Changes in electrical conductivity (EC) affects the structural condition and biological activity of soil under rainfed conditions [41]. Thus, more emphasis should be given to assess the impact of drivers of climate change on soil EC. Smith et al. (2002) [13] found a decrease in EC with increasing temperature and decreasing precipitation in a semi-arid environment. Pariente (2001) [42] investigated the dynamics of soluble salt concentration in soils from Mediterranean, semi-arid, mildly arid and arid climatic regions and found a non-linear relationship between soluble salts concentration and precipitation. In the present study, the EC was not affected by the eT and eT+eCO2 after two seasons of the cropping which might be due to very low initial EC of the Alfisol soil (Table 3).
The arid climatic adversaries favour the sequestration of inorganic C as CaCO3 with concomitant development of soil sodicity due to rise in mean annual temperature (MAT) and decrease in mean annual rainfall (MAR). In SAT environments, the water loss through evapotranspiration (ET) was considered as a primary mechanism in precipitation of pedogenic CaCO3 (PC). PC found in ferruginous Alfisols of Southern India due to the present day semi-arid climate. The formation of PC resulted in concentration of sodicity in clay rich Vertisols [43]. In present study, the CaCO3 content was very low in Alfisol and negligibly affected by eT and eT+eCO2 owing to a short period of experimentation.
Available nutrient status of soil after harvesting
Soil nutrient transformations are primarily carried out through microbial activities which are further influenced by fluctuations in temperature and CO2 concentration. Climate change causes high intensity rainfall in the short time which causes waterlogged condition in soils without proper drainage. Under changing climatic condition, soils become waterlogged, hypoxic and produce phytotoxic organic solutes which impair the root growth and function due to high intensity rainfall in short time.
Available N, P, Zn and B status decreased with eT and eT+CO2 as compared to AC in post-harvest soils (Table 4). The decrease in available N with eT may be due to increasing volatilization losses from soil. Higher temperature can increase nutrient sequestration by soil microorganisms to their increased growth and activity in soils which may induce a reduction in the amount of available nutrients in soil [44]. Microbial N immobilization was stimulated by CO2 enrichment which was responsible for transformation of large amounts of N into organic forms rendering less available to plants. The increase in temperature from 15 to 45oC increased cumulative ammonia losses from 3.3 to 19.6mg kg-1 soil [45]. They also reported a drastic decline in NO3-N in crop rhizosphere under elevated CO2 in wheat crop. Figueiredo et al. (2015) [46] observed decline in the NH4-N fixation rate in clayey soil with the co-elevation of temperature and CO2. In the present study, the increase in NH4-N status of soil might be due to reduction in NH4-N fixation under eT+eCO2.
Few of available literature on the effect of eT and eCO2 on plant available P in soils suggest the possibilities of increasing or decreasing in soil under eT and eCO2. A possible increase in the activity of P solubilizing rhizo-chemicals due to eT and eT+eCO2 may increase the available P in the soil. Contrarily, increased microbial activity in rhizosphere under eT and eCO2 due to increased deposition of organic substrates in rhizosphere may reduce the availability of P in soil owing to P immobilization by soil microorganisms [47,48]. The decrease in the available P status of post-harvest soils due to eT and eT+eCO2 (Table 4) might be ascribed to P immobilization by soil microorganisms. Further decrease in available P status of soil due to eT+eCO2 as compared to eT might be attributed to higher removal of P by crops due to higher biomass production in eT+eCO2 over eT (Table 1). Phosphorus uptake by wheat grown on Inceptisol increased with eCO2 and decreased with eT and with overall increase of 17.4% under eCO2+eT signifying higher P requirements by plants due to climate change [20]. Available K in soils increased with eT as compared to AC and eT+eCO2. Similar to available P, available Zn and B in post-harvest soils after pigeonpea and maize decreased with eT as compared to AC. eT+eCO2 resulted in further decrease in the status of available Zn and B which may be due to higher removal by higher biomass yield.
Soil enzyme activities
Soil enzymes play an important role in transformations of soil organic matter, nutrient cycling and availability of plant nutrients in soil ecosystem. The activity of dehydrogenase is considered as an indicator of the oxidative metabolism in soils and thus of the microbiological activity [49]. The conversion of organic N to inorganic N through hydrolysis of urea to ammonia and CO2 by urease is an important pathway of N transformation in soil. Thus, measurements of enzyme activity in soils were useful in examining the impacts of environmental changes. In the current study, eT and eT+eCO2 increased the activities of dehydrogenase, urease and aryl sulphatase activities in soil as compared to ambient control (Table 5). Increased activity of soil enzymes indicates the higher microbial activity with eT & eT+eCO2 which led to sequestration of available nutrients in microorganisms. This might be the reason for reduction in the status of available N, P and Zn with eT and eT+eCO2 (Table 4). Das et al. [50] reported a significant increase in the enzyme activities (dehydrogenase, B-glucosidase, urease, alkaline and acid phosphatases) at higher temperature (45oC) and elevated CO2 (600ppm) in four soil types and thereby accelerated the turnover of organic C fractions of the soil due to increase in microbially mediated processes. The activities of dehydrogenase, urease and aryl sulphatase increased in rainfed Alfisol with elevated CO2 up to 550ppm in open top chambers [51].
Soil organic carbon dynamics
How climate change impact soil organic matter is the main concern of debate. On one hand that a rise in air temperature and that of the soil would be consistent with an increase in decomposition and loss of soil organic matter in the form of CO2 to atmosphere. On the other hand, the global warming and increasing CO2 concentration in the atmosphere can favour increased plant growth, which in turn could provide more organic matter to the soil. Thus, there a lot of interest was generated to study the effect of climate change drivers viz., eT and eCO2 over a short- and long-term on transformation of SOC fractions and sequestration in soil. The balance of opinion presently is that in the absence of mitigation action, soil organic matter losses through decomposition are likely to exceed levels gained from increased plant growth thus emission of CO2 into atmosphere and greenhouse effect.
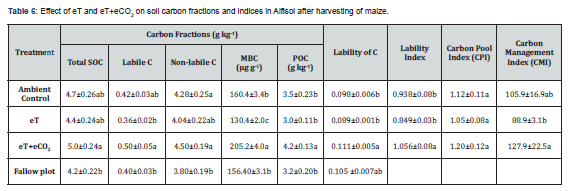
Elevated temperature resulted in a reduction in SOC by 6.4%, MBC by 28%, POC by 20%, labile C by 14%, and non-labile C by 5.6% over the ambient control soil. Except MBC, the decreases were not significant statistically (Table 6). The reduction in labile pools due to eT was more as compared to decline in non-labile C. But when the eCO2 combined with eT increased the contents of SOC by 6.4% and labile pools i.e., POC, MBC & labile C by 20%, 28% and 19%, respectively over the ambient control soil. The increase in soil organic carbon under eT+eCO2 suggests a prospective soil C sequestration. Higher root biomass produced in the presence of eCO2 might have contributed higher C input into the soil which would lead to higher C sequestration. It appears that the amount of C input into soil under eT+eCO2 exceeded the amount of SOC loss under eT. The improved photosynthesis and plant growth under elevated CO2 concentration could lead to increase C input to the soil [52]. Though soil microorganisms are often carbon limited, more C input would be expected to increase the soil microbial biomass and activity [53,54]. The increased C assimilation by plants and its subsequent sequestration in soil may counter balance to some extent the CO2 emissions. However, the enhanced C sequestration due to eT+eCO2 in soil could only sustain if increases in the C input are sustained [55] and increase in the C input exceeds the soil C mineralization over a longer period of time [56].
The soil organic C pool and the C lability directly influence soil physical, chemical and biological attributes as well as the structural capacity of soils [57]. Therefore, the integration of both soil organic C pool and C lability into the C management index (CMI) can provide a useful parameter to assess the capacity of management systems to promote soil quality [58]. In thepresent short term study, the increase in CMI with eT+eCO2 indicates that if recommended rates of fertilizers and manure are applied to crops, the reduction in SOC, C pools and CMI due to eT could be minimized in semi-arid Alfisol soils (Table 6).
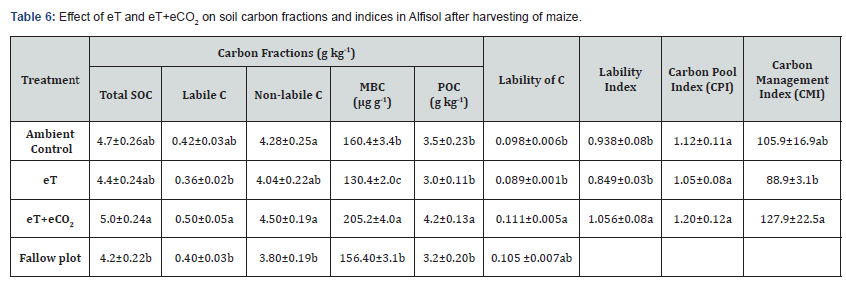
Our results showed that the increase in soil enzyme activity including dehydrogenase activity with eT caused reduction in the SOC, MBC, labile C and non-labile C contents. But eT+eCO2 resulted in higher contents of these carbon pools in soil even though soil enzyme activity increased with the eT+eCO2. In the present study, sufficient quantity of farmyard manure (10t ha-1) and recommended rates of deficient plant nutrients applied to both pigeonpea and maize. With the application of manure and deficient nutrients at recommended rates, eCO2 combined with eT resulted in higher biomass production as compared to ambient control and eT. This might have contributed more root, plant residues, and root exudates to soil exposed to eT+eCO2 and thereby maintained higher organic C and its fractions in soil over the ambient control, eT and fallow plots. These results clearly showed that the loss of soil organic matter and crop productivity due to global warming could be arrested by the application of all deficient plant nutrients and organic manures at recommended rates to different crops grown on rainfed soils.
Biomass production of pigeonpea and maize
The rising CO2 concentration in atmosphere and increase in surface temperature have a direct linkage with the growth and metabolism of plants. As CO2 is a primary raw material in the process of photosynthesis, increase in atmospheric CO2 concentration causes fertilization effect resulting in enhanced biomass and yields. This has been primarily attributed to the enhanced rate of photosynthesis upon exposure to higher CO2 levels. It is important to note that CO2 concentration and atmospheric temperature would increase concurrently under the future climate changing scenarios [59]. The CO2 fertilization effect may be modified under increased surface temperature conditions. In the present study, the increased temperature decreased the total biomass, vegetative biomass, pod number and weight, seed number and weight and harvest index of pigeonpea (Table 6). The eT+eCO2 produced higher total and vegetative biomass, seed number and seed weight and harvest index as compared to eT. But the effects of eT and eT+eCO2 on seed number and HI were statistically not significant. The effect of eT+eCO2 on all these parameters was statistically similar to that of AC except seed yield. These results clearly indicated that the elevated CO2 ameliorated negative effect of eT on biomass and grain production in pigeonpea. Lenka et al. (2017) [60] reported higher seed yield and 100 seed weight of soybean under eT+eCO2 over AC and eT. In maize, the elevated temperature increased the total and vegetative biomass but decreased the grain weight thereby reduced the HI. While the eT+eCO2 increased total and vegetative biomass and grain yield of maize (Table 6).The increased temperature reduced the reproductive biomass of maize while it improved the vegetative biomass [61]. Evaluation of climate change impact on maize yield under different N management strategies revealed that there was more reduction in yield under RCP 8.5 compared to RCP 4.5. Maize yields in sub-tropical climate of Central India in future could be improved by 3t ha-1 by providing optimum dates of sowing and good management practices [62].
Conclusion
Our results revealed that
a) The eT and eT+eCO2 maintained lower soil moisture content during most of the period of pigenpea and maize seasons as the association of eT offset the beneficial effect of eCO2 in reducing the evaporation component of ET.
b) Soil quality parameters such as pH, EC and CaCO3 contents were not significantly affected by eT and eT+eCO2. But the activities of dehydrogenase, urease and aryl sulphatase were significantly higher with eT and eT+eCO2. eT and eT+eCO2 maintained the lower status of available N, P, B and Zn in post-harvest soils as compared to AC. But the available nutrient status in soils under eT and eT+eCO2 were higher than initial nutrient status of soils which indicates buildup due to application of farmyard manure and fertilizers.
c) The eT reduced the status of SOC, MBC, labile and non-labile C in post-harvesting soils while eT+eCO2 enhanced their status as compared to AC. Soil carbon management index was higher under eT+eCO2 and lower in eT.
d) The eT and eT+eCO2 had differential effects on pigeanpea and maize. In pigeonpea, total biomass, pod weight, seed yield and harvest index were significantly reduced due to eT but eT+eCO2 produced total biomass, pod number, pod weight, seed yield, 100 seed weight and harvest index at par with that of AC. In maize, total and fodder biomass production increased with eT by 3.2% and 11.7% but grain yield reduced with eT by 10%. While the eT+eCO2 increased total and fodder biomass and grain yield of maize.
e) Thus, these results have clearly brought out that in short-term, the eCO2 associated with eT not only compensated negative effect of eT on soil organic matter but also produced higher yield of maize than AC and pigeonpea yield at par with AC.
f) In this study, recommended rates of farmyard manure and plant nutrients (N, P, and K) were applied to both pigeonpea and maize. Therefore, it can be concluded that the deleterious effect of elevated temperature on soil organic matter and crop production in semi-arid areas could be minimized by enhancing CO2 fertilization through integrated and balanced nutrient management.
Conflicts of Interest
The authors declare no conflict of interest.
Funding
The authors are highly thankful to the authorities of the Indian Council of Agricultural Research, New Delhi for funding this research work under National Innovations in Climate Resilient Agriculture (NICRA) project.
References
- WMO (2021) Greenhouse gas bulletin (GHG bulletin) No. 17, The state of greenhouse gases in the atmosphere based on global observations through 2020. World Meteorological Organization. Geneva, Switzerland.
- IPCC (2014) Summary for policy makers, climate change 2014. In: Pachauri RK, Meyer LA (Core Writing Team & Eds.), Impacts, Adaptation and vulnerability-contributions of the working group II to the Fifth Assessment Report of the International Panel on Climate Change, Inter-Governmental Panel on Climate Change, Geneva, Switzerland, p. 151.
- IPCC (2013) Summary for policy makers in Climate Change 2013. In: Stocker TF, Qin D, Platner GK, Tignor MMB, Allen SK, et al. (Eds.), The Physical Science Basis. Working Group, I, Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, pp. 1-27.
- Pramanik, P, Chakrabarti B, Bhatia A, Singh SD, Mridha N, et al. (2018) Effect of elevated carbon dioxide on soil hydrothermal regimes and growth of maize crop (Zea mays ) in semi-arid tropics of Indo-Gangetic Plains. Environmental Monitoring and Assessment 190(11): 652-661.
- Siebers MH, Yendrek CR, Drag D, Locke AM, Rios Acosta L, et al. (2015) Heat waves imposed during early pod development in soybean (Glycine max) cause significant yield loss despite a rapid recovery from oxidative stress. Global Change Biology 21(8): 3114-3125.
- FAI (2020) Fertilizer Statistics - 2019-20, Fertilizer Association of India (FAI), New Delhi, India.
- Yadav MK, Patel C, Singh RS, Singh KK, Balasubramanian R, et al. (2021) Assessment of climate change impact on different pigeonpea maturity groups in north Indian Condition. Journal of Agrometeorology 23(1): 82-92.
- Allen LH, Pan D, Boote KJ, Pickering NB, Jones JW (2003) Carbon dioxide and temperature effects on evapotranspiration and water use efficiency of soybean. Agronomy Journal 95(4): 1071-1081.
- Thomson AMRA, Brown NJ, Roseberg R, Lazuralde C, Benson N (2005) Climate change impacts for the conterminous USA: an integrated assessment Part 3. Dry land production of grain and forage Crops. Climate Change 69: 43-65.
- Vanaja M, Maheshwari M, Jyothi L, Satish P, Yadav S K, et al. (2015) Variability in growth and yield response of maize genotypes at elevated CO2 Advances in Plants and Agriculture Research 2(2): 00042.
- Taub DR, Miller B, Allen H (2008) Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Global Change Biology 14(3): 565-575.
- Reth S, Reichstein M, Falge E (2005) The effect of soil water content, soil temperature, soil pH value and root mass on soil CO2 Plant and Soil 268: 21-33.
- Smith JL, Halvoson JJ, Bolton Jr H (2002) Soil properties and microbial activity across a 500m elevation gradient in a semi-arid environment. Soil Biology and Biochemistry 34(11): 1749-1757.
- Rice CW, Garcia FO, Hampton Co Owensby CE (1994) Soil microbial response in tall grass praire to elevated CO2. Plant and Soil 165: 67-74.
- Van Kessel C, Boots B, Anne De Graaff M, Harris D, Blum H, et al. (2006) Total soil C and N sequestration in a grassland following 10 years of free air CO2 Global Change Biology 12(11): 2187-2199.
- Cardon ZG, Czaja AD, Funk JL (2002) Periodic carbon flushing to roots of Quercus rubra saplings affects soil respiration and rhizosphere microbial biomass. Oecologia 133(2): 215-223.
- Knorr W, Prentice IC, House JI, Holland EA (2005) Long-term sensitivity of soil organic carbon turnover to warming. Nature 433(7023): 298-301.
- Allen DE, Singh BP, Dalal RC (2011) Soil health indicators under climate change: A review of current knowledge. In: Singh BP, Cowie AL, Chan KY (Eds.), Soil Health and Climate Change, Springer- Verlag Berlin Heidelberg, p. 29.
- Rinnan R, Michelsen A, Baath E, Jonasson S (2007) Fifteen years of climate change manipulations after soil microbial communities in a subarctic heath ecosystem. Global Change Biology 13(1): 28-39.
- Kumar M, Swarup A, Patra AK, Purakayastha TJ, Manjaiah KM, et al. (2011a) Elevated CO2 and temperature effects on phosphorus dynamics in a rhizosphere of wheat grown in a Typic Haplustert of sub-tropical India. Agrochimica 55: 14-31.
- Kimball BA, Conley MM, Wang S, Lin X, Luo C, et al. (2008) Infrared heater arrays for warming ecosystem field plots. Global Change Biology 14(2): 309-320.
- Hendershort WH, Latande H, Duquette M (1993) Soil reaction and exchangeable acidity. In: Carter MR (Ed.), Soil sampling and methods of analysis, Lewis publishers, USA, pp. 141-149.
- Gee GW, Dani O (2002) Particle size analysis. In Methods of soil analysis. In: Dane JH, Topp GC (Eds.), Part-4: Physical methods, Soil Science Society of America Book series no. 5: 255-293. Madison, USA: SSSA.
- Subbiah BV, Asija GL (1956) A rapid procedure for the estimation of available nitrogen in soils. Current Science 25(8): 259-260.
- Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27: 31-36.
- Hanway JJ, Heidal H (1952) Soil Analysis Methods used in Iowa State College. Soil Testing Laboratory. Iowa Agriculture 57: 1-31.
- Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Science Society of America Journal 42(3): 421-428.
- Berger KC, Truog E (1939) Boron determinations in soils and plants. Industrial and engineering chemistry. Analytical Edition 11(10): 540-545.
- Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry 19(6): 703-707.
- Cambardella AA, Elliott ET (1992) Particulate soil organic matter changes across a grassland cultivation sequence. Soil Science Society of America Journal 56(3): 777-783.
- Blair GJ, Lefroy RD, Lisle L (1995) Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Australian Journal of Agricultural Research 46(7): 1459-1465.
- Tabatabai MA, Bremner JM (1970) Arylsulphatase activity of soils. Soil Science Society of America Proceedings 34(2): 225-229.
- Tabatabai MA, Bremner JM (1972) Assay of urease activity in soils. Soil Biology and Biochemistry 4(4): 479-487.
- Casida L, Klein D, Santoro T (1964) Soil dehydrogenase activity. Soil Science 98(6): 371-376.
- SAS Institute, Inc. (2005) SAS User’s guide, System – Release, Version, 8.02. SAS Institute Inc., Cary, NC, USA.
- Field CB, Jackson RB, Mooney HA (1995) Stomatal responses to increased CO2: Implications from the plant to the global scale. Plant, Cell & Environment 18(10): 1214-1225.
- Ferretti DF, Pendall E, Morgan JA, Nelson JA, Lecain DR, et al. (2003) Partitioning evapotranspiration fluxes from a Colorado grassland using stable isotopes: Seasonal variations and implication for elevated atmospheric CO2. Plant and Soil 254: 291-303.
- Celia MA, Peters CA, Bachu S (2002) Geological storage of CO2: leakage pathways and environmental risks. American Geophysical Union, Spring Meeting abstract. GC32A-03 in SAO/NASA Astrophysics Data System.
- Ravi PH, Jeremy JC, Steven MD (2010) Effects of CO2 gas as leaks from geological storage sites on agro-ecosystems. Energy 35(12): 4587-4591.
- Smith KL, Colls JJ, Steven MD (2005) A facility to investigate effects of elevated soil gas concentration on vegetation. Water, Air, and Soil pollution 161: 75-96.
- Gil SV, Meriles J, Confote C, Figoni G, Basanta M, et al. (2009) Filed assessment of soil biological and chemical quality in response to crop management practices. World Journal of Microbiology and Biotechnology 25: 439-448.
- Pariente S (2001) Soluble salts dynamics in the soil under different climatic conditions. Catena 43(4): 307-321.
- Pal DK, Dasog GS, Vadivelu S, Ahuja RL, Bhattacharya T (2000) Secondary calcium carbonate in soils of arid and semi-arid regions of India. In: Lal, R, Kimble JM, Eswaran H, Stewart BA (Eds.), Global Climate Change and Pedogenic Carbonates, Boc Raton, Florida, Lewis Publishers, pp. 149-185.
- Jonasson S, Havstrom M, Jensen M, Callaghan TV (1993) In situ mineralization of nitrogen and phosphorus of arctic soils after perturbations simulating climate change. Oecologia 95: 179-186.
- Kumar M, Patra AK, Swarup A (2011b) Impact of climate change on fertilizer demand in agriculture: concerns and imperatives for food security in India. Indian Journal of Fertilizers 7: 48-62.
- Figueiredo N, Carranca C, Goufo P, Pereira J, Trindade H, et al. (2015) Impact of agricultural practices, elevated temperature and atmospheric carbon dioxide concentration on nitrogen and pH dynamics in soil and floodwater during the seasonal rice growth in Portugal. Soil and Tillage Research 145: 198-207.
- Korner C (2000) Biosphere responses to CO2 Ecological Applications 10(6): 1590-1619.
- Sardans J, Penuelas J, Estiarte M (2006) Warming and drought alter soil phosphatase activity and soil P availability in a Mediterranean shrubland. Plant and Soil 289: 1141-1146.
- Wlodarczyk T, Stepniewski W, Brzezinska M (2002) Dehydrogenase activity, redox potential and emissions of carbon dioxide and nitrous oxide from Cambisols under flooding conditions. Biology and Fertility of Soils 36: 200-206.
- Das S, Bhattacharyya P, Adhya TK (2011) Interaction effects of elevated CO2 and temperature on microbial biomass and enzyme activities in tropical rice soils. Environmental Monitoring and Assessment 182(1-4): 555-569.
- Srinivasarao Ch, Kundu S, Shankar AK, Naik RP, Vanaja M, et al. (2016) Continuous cropping under elevated CO2: Differential effects on C4 and C3 crops, soil properties and carbon dynamics in semi-arid Alfisols. Agriculture, Ecosystems & Environment 218: 73-86.
- Cheng W, Johnson DW (1998) Elevated CO2 rhizosphere processes and soil organic matter decomposition. Plant and Soil 202: 167-174.
- Anderson TH, Domsch KH (1985) Determination of eco-physiological maintenance carbon requirements of soil microorganisms in a dormant state. Biology and Fertility of Soils 1: 81-89.
- Smith JL, Paul EA (1990) The significance of soil microbial biomass estimations. In: Bollag JM, Stotzky G (Eds.), Soil Biochemistry. Vol. 6, Marcel Dekker, Inc., New York, pp. 357-396.
- Kicklighter DW, Bruno M, Donges S, Esser G, Heimann M, et al. (1999) A first order analysis of the potential of CO2 fertilization to affect the global carbon budget: a comparison of four terrestrial biosphere models. Tellus B: Chemical and Physical Meteorology 51(2): 343-366.
- Raich JW, Schlesinger WH (1992) The Global Carbon-Dioxide Flux in Soil Respiration and Its Relationship to Vegetation and Climate. Tellus B: Chemical and Physical Meteorology 44(2): 1-99.
- Blair N, Crocker GJ (2000) Crop rotation effects on soil carbon and physical fertility of two Australian soils. Soil Research 38: 71-84.
- Blair N, Faulkner RD, Till AR, Korschens M, Schulz E (2006) Long-term management impacts on soil C, N and physical fertility. Part II. Bad Lauchstadt static and extreme FYM experiments. Soil and Tillage Research 91: 39-47.
- Sicard P, Anav A, De Marco A, Paoletti E (2017) Projected global ground-level ozone impacts on vegetation under different emission and climate scenarios. Atmospheric Chemistry and Physics 17(19): 12177-12196.
- Lenka NK, Lenka S, Thakur JK, Elanchezhian R, Aher SB, et al. (2017) Interactive effect of elevated carbon dioxide and elevated temperature on growth and yield of soybean. Current Science 113(12): 2305-2310.
- Vanaja M, Sathish P, Vijay Kumar G, Abdul Razzaq, Vagheera P, et al. (2017) Elevated temperature and moisture deficit stress impact on phenology, physiology and yield responses of hybrid maize. Journal of Agrometeorology 19(4): 107-116.
- Mohanty M, Sinha NK, Sonali P, Chaudhary RS, Sammi Reddy K, et al. (2017) Climate change impacts vis-à-vis productivity of soybean in Vertisol of Madhya Pradesh. Journal of Agrometeorology 19(1): 10-16.






























