Peroxy-Electrocoagulation for Organic Matter and Phenolic Compounds Removal from Olive Pomace Oil Wastewater
R Martins1,2,3*, T T Grabowski3 and M V Santos3,4
1Laboratory for Separation and Reaction Engineering-Laboratory of Catalysis and Materials, Faculty of Engineering University of Porto (FEUP), Portugal
2Associate Laboratory in Chemical Engineering (ALiCE), FEUP, Portugal
3Technology and Management School, Polytechnic Institute of Bragança, Campus de Santa Apolónia, Portugal
4Federal University of Fronteira Sul, Cerro Largo Campus, Brazil
Submission: February 22, 2023; Published: May 15, 2023
*Corresponding author: R Martins, Polytechnic Institute of Bragança, Campus de Santa Apolónia, 5300-253 Bragança, Portugal
How to cite this article: R Martins, T T Grabowski, M V Santos. Peroxy-Electrocoagulation for Organic Matter and Phenolic Compounds Removal from Olive Pomace Oil Wastewater. Int J Environ Sci Nat Res. 2023; 32(2): 556333. DOI 10.19080/IJESNR.2023.32.556333
Abstract
This study reports the optimization of a peroxy-electrocoagulation process for the treatment of an olive mill wastewater (OMW), targeting the removal of organic matter (in terms of chemical oxygen demand-COD and total phenolic compounds-TPh). The electrochemical cell is constituted by two rectangular electrodes made of aluminum (40×40×2mm), with an active surface of 12cm2, placed vertically in a cylindrical vessel at a distance of 5 mm. The peroxy-electrocoagulation trials were planned through Box-Behnken design varying initial pH (2.5, 3.5 and 4.5), current density (10, 20 and 30mA/cm2) and hydrogen peroxide dosage (10, 20 and 30g/L). The best conditions were obtained at pH 3.5, 20mA/cm2 and 20g/L of H2O2, with removal efficiencies of 22% for COD and 90% for TPh (Figure 1).
Keywords: Peroxy-electrocoagulation process; Olive oil; Electrochemical treatments; Biological demands; Wastewater; Biochemical oxygen demand, Box-behnken design
Introduction
Nowadays the Mediterranean Basin is the main responsible for the world production of olive oil, about 97% [1]. In general, the steps involved in the manufacture of olive oil include leaf removal, olive washing, pressing, and oil separation [2]. One of the by-products of the manufacture of olive oil is pomace, which is made up of water, oil, pulp, skins, and olive stones [2]. The wastewater generated in the production of olive pomace oil is complex and with great variability associated with the different characteristics of cultivation and processing, has a high toxic organic load, low pH and high chemical and biological demands. Different processes have been proposed to treat this effluent, such as direct watering in fields, co-composting, physico-chemical methods (flocculation, coagulation, filtration, evaporation ponds and incineration), ultrafiltration/reverse osmosis, chemical and electrochemical treatments [3].
The purpose of this work was to optimise the removal of organic matter and phenolic compounds from an olive mill wastewater (OMW) using a peroxy-electrocoagulation (PEC) process, through the response surface methodology (RSM) with Box-Behnken Design (BBD). The effect of different parameters on the PEC process was investigated: initial pH, hydrogen peroxide’s (H2O2) dosage and current density.
Material and Methods
The OMW was collected from a plant located in Mirandela (northeastern Portugal). This industrial plant receives olive pomace coming mostly from two-stage olive oil extraction units. The water samples were sieved (0.5mm) and stored at room temperature in 25-liter containers in the laboratory. The main characteristics of the OMW are pH of 4.7, 3945mg/L of total phenolic compounds (TPh), 70g/L of chemical oxygen demand (COD), 5818mg/L of biochemical oxygen demand (BOD5), and 46g/Kg of total solids.
PEC experiments were conducted in a 600mL reactor fed with 300mL of OMW and stirred continuously. Both the cathode and anode electrodes consisted of two rectangular sheets of aluminum (40×40×2mm). Before each test, the electrodes were sanded, washed with a 5% hydrochloric acid solution and distilled water. The electrodes were placed vertically at a distance of 0.5cm between them. The active surface of the electrodes was also kept constant at 12cm2. Both electrodes were connected to a power supply (Topward 6302D) and a direct current was applied. The reaction time was 30min. The electrical current densities tested were 10, 20 and 30mA/cm2, H2O2 dosage of 10, 20 and 30g/L and initial pH of 2.5, 3.5 and 4.5. Immediately after the PEC process, the pH of a 25mL aliquot was raised to 10, to promote the decomposition of H2O2 into water and oxygen, stopping the reaction. From the aliquot, COD and TPh were determined.
Box-Behnken Design (BBD) was employed to design the experiments. Response surface methodology (RSM) was used to analyze the data and determine the optimum PEC conditions. In total there were 15 points with 3 central points. Analysis of variance (ANOVA) was used to investigate the model response and a second order polynomial model.
The required outcomes were attained using the RStudio software. ANOVA was used to assess the significance of the variables, their interactions, and the accuracy of the model fits. The model factors were evaluated with a confidence level of 95%. Contour plots were drawn to evaluate the studied properties.
Results and Discussion
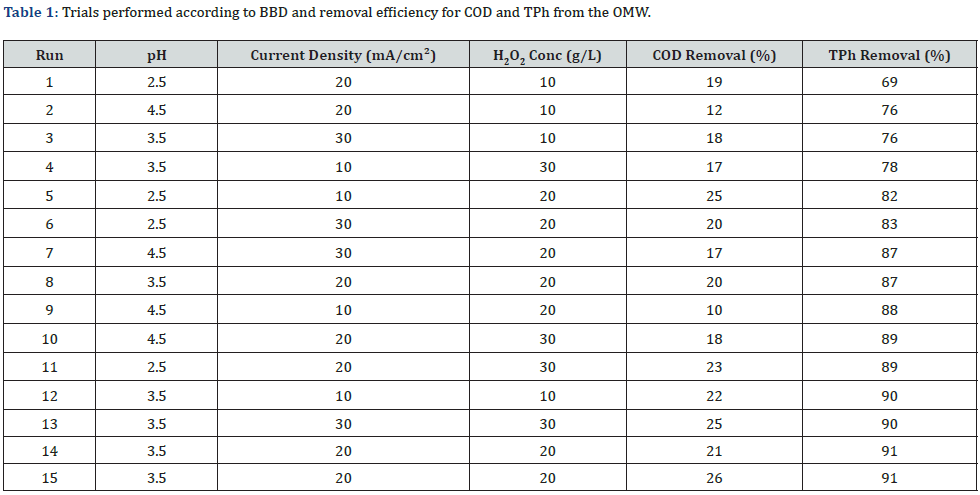
Table 1 shows the experiments generated by the BBD and the values of COD and TPh removal. According to Table 1, PCE proved to be more efficient in the removal of TPh than COD. The removal was best at the central point (pH 3.5, 20g/L of H2O2 and 20mA/ cm2) presenting a removal efficiency of 90% for TPh and only 22% for COD (Table 2).
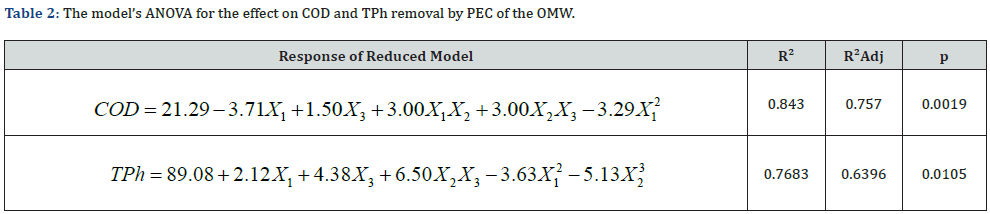
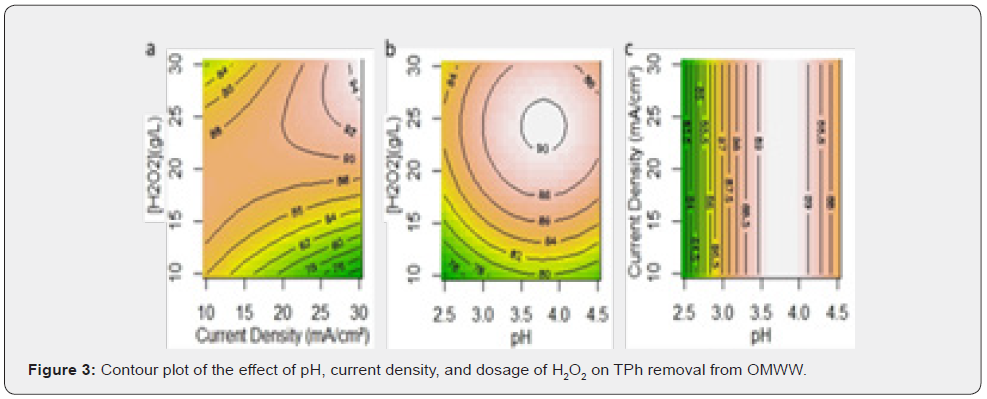
The variables pH, current density and peroxide dosage are represented by X1, X2 and X3, respectively. The equations presented are the ones that best fits the independent variables, with the smallest p-value. The R2 values and the reasonably good agreement between R2 and R2Adj show that the model fits well the experimental data and that the estimate response is accurate. The contour plot makes it simple to understand the interactions of the three independent variables on the removal of COD (Figure 2) and TPh (Figure 3) from the OMW. Each plot is made with the third independent variable at the BBD center point.
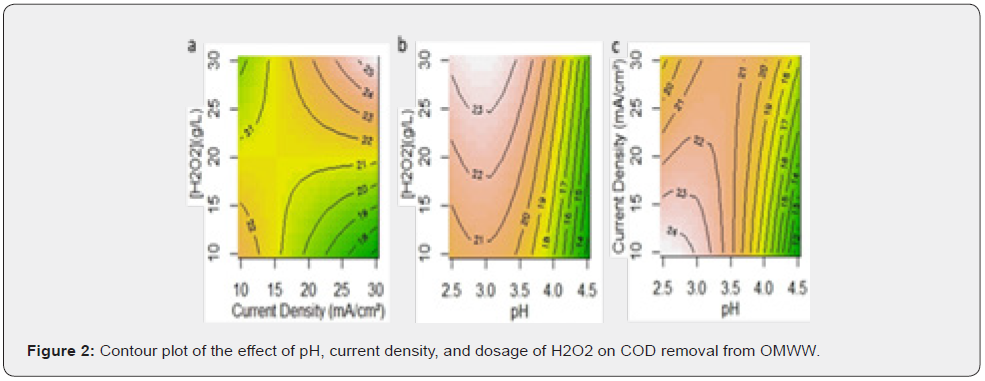
In the region with current density of 25 to 30mA/cm2 and pH 2.5 to 3.5, increasing the peroxide dosage increases COD removal (Figure 2a & 2b). pH less than 3.5 showed more advantageous for COD removal (Figure 2b & 2c). A current density lower than 20mA/cm2 and a more acidic environment (between 2.5 and 3.0) present a better COD removal efficiency with 20g/L H2O2 (Figure 2c). For pH 3.5, it is necessary higher current densities (between 25 and 30mA/cm2) and peroxide dosage above 20g/L to achieve higher values of COD removal (Figure 2a).
Regarding TPh removal, for H2O2 doses between 10 and 20g/L of H2O2, an increment on the current density, decreases the process efficiency; however, in the higher range, it is the opposite (Figure 3a). For an H2O2 dose of 20g/L, TPh removal efficiency is maximized for pH values between 3.5 to 4.0 (Figure 3b), while current density has a negligible effect (Figure 3c).
Conclusion
The peroxy-electrocoagulation process showed to be an efficient technique for TPh removal from the OMW matrix. The best operation conditions were initial pH of 3.5, current density of 20mA/cm2 and H2O2 dose of 20g/L.
Acknowledgment
This work was financially supported by (i) LA/P/0045/2020 (ALiCE), UIDB/50020/2020 and UIDP/50020/2020 (LSRE-LCM), funded by national funds through FCT/MCTES (PIDDAC), and (ii) Project NORTE-01-0247-FEDER-072124, BagaÇo+Valor - Tecnologia Limpa para a Valorização dos Subprodutos do Bagaço na Indústria Extratora de Azeite, funded by the European Regional Development Fund (ERDF).
References
- GPP (Gabinete de Planejeamento, Políticas e Administração Geral) (2020) SIAZ - Sistema de Informação do Azeite e Azeitona de Mesa.
- PS Moral, MVR Méndez (2006) Production of pomace olive oil. Grasas y Aceites 57(1): 47-55.
- Y Esfandyari, Mahdavi Y, Seyedsalehi M, Hoseini M, Safari GH, et al. (2015) Degradation and biodegradability improvement of the olive mill wastewater by peroxi-electrocoagulation/electrooxidation-electroflotation process with bipolar aluminum electrodes. Environ Sci Pollut Res 22(8): 6288-6297.






























