Understanding Extinction and its Consequences: An Experimental Microcosm Model
Amabogha ON1* and Amabogha B2
1Department of Natural Science, Middlesex University, United Kingdom
2Department of Chemical Engineering, Federal University, Nigeria
Submission: March 16, 2023; Published: April 04, 2023
*Corresponding author: Amabogha ON, Department of Natural Science, School of Science and Technology, Middlesex University, The Burroughs, London NW4 4BT, United Kingdom
How to cite this article: Amabogha ON, Amabogha B. Understanding Extinction and its Consequences: An Experimental Microcosm Model. Int J Environ Sci Nat Res. 2023; 31(5): 556326. DOI 10.19080/IJESNR.2023.31.556326
Abstract
Species extinction as one of a wide range of environmental changes poses serious threats to ecological systems. Society depends on biodiversity to sustain important ecological processes necessary for providing vital services to humanity. Loss of biodiversity has a potential to disrupt some of these important ecosystem processes. In addition, species extinction could also indirectly affect ecosystems via the ecological connections they are part of; meaning the extinction of a species could potentially cause massive changes to the abundance and composition of interacting species. Understanding the mechanisms and consequences of extinctions is critical to making predictions on extinction effects and devising mitigation strategies. This study utilized aquatic microcosms as experimental models to investigate the consequences of the removal of a ciliate Blepharima japonicum on the abundance of residual ciliates Colpidium striatum and Paramecium caudatum as well as total bacteria population. At low enrichment, Blepharisma excluded Colpidium to extinction prior to its removal; the removal of Blepharisma caused Paramecium to increase proportionately, but did not have any effect on its time of extinction. Bacterial population of communities where Blepharisma was removed were higher than where they were not. In the bacteria trophic level, Serratia appeared to outcompete the other bacteria species significantly. The results suggests that at low energy levels, the extinction of a species will likely cause an increase in the abundance of its competitor even though this increase will likely not guarantee its long term persistence. A species’ extinction would also increase the abundance of its prey, which could cause a bloom, causing prey to use up available nutrients faster, which could potentially lead to rapid habitat collapse. Further research should utilize more trophic levels to determine the possibilities of detecting cascading effects of extinction across trophic levels. More in-depth protists-bacterial level research is also encouraged to provide insights on survival mechanisms, competition and co-existence and the factors affecting them; this will form the basis for more understanding on the fundamentals of matter and energy transfer across microbial trophic chains.
Keywords: Extinction; Environmental change; Removal experiments; Microcosms
Introduction
Earth is currently witnessing an era of rapid human-‐driven biological, hydrological and climatological changes with the potential to cause massive harm to ecological systems [1]. Flurries of scientific researchers have established series of case studies demonstrating diverse kinds of changes at different spatial scales [2-4]. Global climate is changing at unprecedented rates [4]; deforestation and biodiversity loss is increasing (Turner et al. 2011) [3,5,6]; changes in biogeochemical cycles are becoming more evident [2] etc. Environmental changes have several important drivers. One such driver of environmental change is extinction. Species extinction poses serious ecological/socioeconomic consequences. Diamond [7] famously described the ‘Evil Quartet’ of habitat destruction, over-‐exploitation, introduced species and cascading extinction as the major drivers of biodiversity loss. Hooper et al. [8] argued that the impacts of species extinctions could be as devastating for humans as air pollution and climate change. There are mounting evidences suggesting that extinctions may alter crucial processes necessary to maintain the sustainability and productivity of ecological systems [1]. If current rates of extinction continue to accelerate, changes in ecological processes will also likely accelerate to destructive proportions [9].
Environmental changes that accelerate extinction have intensified over the years; desertification, loss of tropical forests, pollution (MEA, 2005), but for most parts of the world and for most species, the rates of extinctions haven’t been adequately quantified or haven’t been quantified at all. Determining when the last individual of a species has died is an extremely difficult task [10] and monitoring the population trends of threatened species of small vertebrates/invertebrates is most times impossible [11].
This study seeks to improve understanding on the consequences of extinction on ecosystems.
Methods
Microcosms
The experiment utilized standard culture methods for protists [12] to set up simple microbial food chains in aquatic microcosms. Microcosms used in this experiment were covered 25ml Polystyrene Universal tubes. Tube lids were loosely covered to allow for air circulation but also to prevent any form of contamination. These tubes were filled with 10mL of supernatant from medium made from powdered freeze-dried Chlorella in mineral water at a concentration of 0.5g/L. Before use, the medium was autoclaved and inoculated with Bacillus subtilis, Pseudomonas fluorescens, Serratia marcescens and other unindentified bacteria filtered from the stock cultures of the protozoan species used in the experiment. Inoculations were done in a large sterile glass jar before distribution to the microcosm tubes.
Experimental protocol
Microcosms consist of a mix of three known bacteria species (B. subtilis, P. fluorescens and S. marcescens), unidentified bacteria species and three protist species (Blepharisma japonicum, Paramecium caudatum and Colpidium striatum – all ciliates). Blepharisma is an omnivore that feeds on bacteria and other ciliates; Paramecium and Colpidium on the other hand are bacterivores feeding solely on bacteria. In the experiment, seven different food webs were created: all three protist species combined (B+P+C), all possible two protist species combination (B+C; B+P; P+C), and all three protist species singly (B; P; C) with bacteria. All food web combinations were replicated five times with the exception of B+P+C, B+P, and B+C which were replicated ten times (prior to removal of Blepharisma). This yielded a total of 50 microcosms. Microcosms were given unique ID number 1 – 50 (See Table 1) and incubated under constant experimental conditions (25°C). Initial stocking density was 20 each for all three ciliates per microcosm.

B = Blepharisma, P = Paramecium, C = Colpidium.
Measuring abundance
The experiment lasted for 23 days. Population abundance count was done 4 times a week (Mondays, Tuesdays, Thursdays and Fridays) until the 23rd day after species introduction, corresponding to approximately 36 generations. Population densities were estimated by gently swirling microcosms to attain homogeneity, then sampling out known volumes of solution from each onto a sterile petri dish and counting the species. Sterile petri dishes used were left covered while counting, so that samples removed from microcosms can be returned with no contamination. In situations where species were too dense to count reliably, the samples were further divided into smaller portions and diluted with sterile media for a more convenient and reliable estimation. Efforts were taken to ensure microcosms were left out of experimental conditions (in this case 25°C) for as short a time as possible.
Removal experiments
Blepharisma spp. is widely regarded as photosensitive ciliates [13] and there have been widely reported cases of light-induced cell deaths at different levels of light intensity at specific durations (Takada and Matsuoka, 2009; Terazima et al., 1999). This research aims to use light to remove Blepharisma from aquatic communities, and to investigate the consequences on inherent community structure.
Removal experiment trial
With strong evidences to support its photosensitive tendencies, a trial experiment was carried out to ascertain the shortest time to completely kill Blepharisma with LED lights in a 10mL aquatic community and to investigate if LED light has any effect on Paramecium and Colpidium, The experiment was conducted using three ciliate species (Blepharisma, Paramecium and Colpidium) in combination with three known bacteria species (B. subtilis, P. fluorescens and S. marcescens) and unidentified bacteria species to form a food web in a 10mL microcosm. Microcosm experimental conditions were the same used in the main experiment setup. Food webs were subjected to LED lights at three different time treatments (10, 20 and 30 minutes) in three replicates making a total of 9 microcosms. Initial densities of ciliates were approximately Blepharisma 170, Paramecium 280, and Colpidium 900 per microcosm. Tube was held in place with a retort stand while LED lights were pointed from top and bottom of the tube. Tube was completely wrapped with foil so that light can bounce back and forth foil and remain within tube. After removal, known samples were removed from microcosms and counted before and after Blepharisma removal using standard techniques mentioned in section 4.3. Result showed that applying LED lights from underneath and above polystyrene universal tubes was effective in removing Blepharisma in 10, 20 and 30 minutes when in combination with Paramecium and Colpidium in a community.
The result also suggested that there was no effect of the light treatment on the population of Paramecium and Colpidium an hour after the removal of Blepharisma. No difference in Colpidium population after light treatment in 10 minutes, 20 minutes and 30 minutes (Paired two-‐sample t-‐test: DF = 2, t = 2.92, p > 0.05). In the same vein, there was no difference in Paramecium population after light treatment in 10 minutes, 20 minutes and 30 minutes (Paired two-‐sample t-‐test: DF = 2, t = 2.92, p > 0.05).
Main removal experiment
Blepharisma removal was carried out on day 10 of the experiment. Blepharisma was removed from communities B+P+C, B+P, and B+C. Every one of these food webs were established in ten replicates prior to Blepharisma removal. For each of these food web combinations, five microcosms were randomly selected from the available ten replicates. These were exposed to the LED light treatment, following protocol enumerated in section 4.4.1. Selected microcosms were exposed to light for 20 minutes to ensure complete removal of Blepharisma from communities. Species abundance was measured before and after LED light inducement. Results of species count showed Blepharisma removal was successful and there were no effects of LED light on Paramecium and Colpidium. After Blepharisma removal, the experiment comprised of ten treatments in five replicates (Table 2).

*B = Blepharisma, removal.
Bacteria density estimation
Bacteria density was estimated using the serial dilution and plate count method. This was done in the 22nd day of the experiment. Because bacterial densities are expected to be low after much grazing by protists, dilution was done 1/2 and 1/4. Serial dilution was carried out using a microtiter plate. 200μμl solution was removed from microcosms and put in the first well of the microplate plate. Subsequent wells were topped with 100μμl water. 100μμl of microcosm solution was removed from the first well (the whole) and diluted in the next well containing water; 100μμl of diluted solution was further transferred to the next well and mixed to achieve 1/4 dilution. This procedure was repeated for all 50 microcosms. 2μμl from each pocket was then transferred to LB agar plates; 5 microcosms samples per plate. To prepare LB agar, bactopeptone (10g/L), yeast extract (5g/L) and salt (5g/L) were measured and put into a 250ml duran flask, water was added to point 200ml and mixed. The pH was adjusted to 7.5, agar (1.5% of 250ml) was added to solution then water was topped to 250ml. The solution was autoclaved, allowed to cool then poured into 10 plates; approximately 25ml per plate.
Statistical analysis
To detect differences in population abundance of Paramecium and bacteria where Blepharisma extinction was induced and where it was not, a two-‐sample T-‐test assuming equal variances (homoscedasticity) was used, comparing the sample means at specific points in the growth curve. Also, a paired-‐sample T-‐test was used to ascertain if LED light treatments had any effects on Paramecium population by comparing abundance before and one hour after Blepharisma removal. The effect of competition on Colpidium was investigated using Analysis of variance (ANOVA) to compare Colpidium abundance across different combinations. Significant differences were tested at 95% confidence interval. All statistical analysis were carried out using MS Excel 2011 and StatPlus version 5.8.2.0.
Results
Observations before removal of Blepharisma
All three ciliates (Paramecium Colpidium and Blepharisma) increased in abundance in all microcosms for the first few days. Growth rate was noticeably faster in microcosms with single ciliate species than it was in combination. This growth trend continued until day 7 when Colpidium (in communities BPC and BC) took a sharp decline till they completely went extinct on day 9. See Figure 1B.
Of the different food web combinations involving Colpidium, Colpidium significantly faired better when grown alone (ANOVA, DF = 3, 26, p < 0.05), followed by when in combination with Blepharisma. There was no significant difference in Colpidium population between communities PC and BPC (ANOVA, DF = 1, 13, p > 0.05). However, their population decreased significantly in communities where Blepharisma was present (BC and BPC) until they completely went extinct on day 9. Paramecium also subsequently outcompeted Colpidium for resources even though Colpidium growth went up in the earlier instance (see Figure 1C). Apparently, competition had an effect on Colpidium as it performed better when grown alone than it did when in combination with other ciliates.
Blepharisma on the other hand increased in all microcosms it was present, peaked on day 8 and remained stable prior to removal. Blepharisma however performed best when in combination with Colpidium (ANOVA, DF = 3, 31, p < 0.05), adequately outcompeting Colpidium in all microcosms that contained both species. Blepharisma alone, Blepharisma with Paramecium and Blepharisma in combination with Colpidium and Paramecium had similar abundance and growth rate. There was no significant difference in Blepharisma population abundance in these three food web combinations (ANOVA, DF = 2, 22, p > 0.05). See Figure 1.
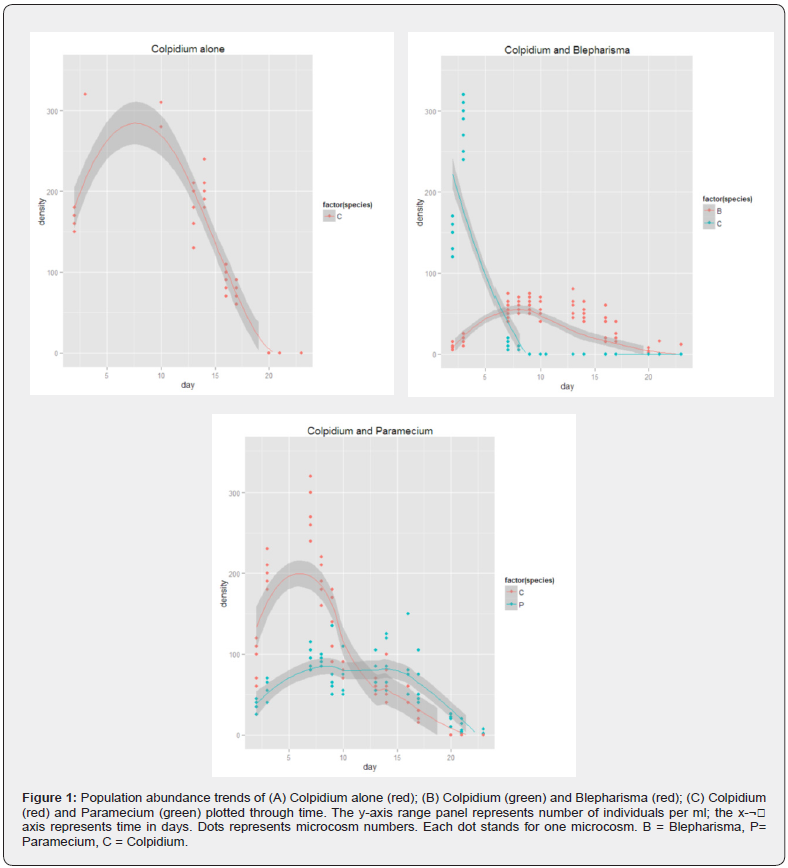
Paramecium also increased in abundance in all communities but performed best in when grown alone and when grown in combination with Colpidium (ANOVA, DF = 3, 26, p < 0.05). There was no significant difference in Paramecium abundance between communities BP and BPC (ANOVA, DF = 1, 18, p > 0.05). Generally, the growth rate of Paramecium was relatively stable. Day 10 saw Paramecium begin to decline in communities where they were in combination with Blepharisma
Observations following removal of Blepharisma
There was no effect of the 20 minutes LED light treatment (used to remove Blepharisma from the randomly selected communities) on the population of Paramecium an hour after the removal of Blepharisma. This observation was consistent for the BPC and BP communities (Paired two sample test: DF = 2, t = 2.131847, p > 0.05). Blepharisma went extinct in every community where it was exposed to LED light treatment.
Following removal of Blepharisma, a declining Paramecium population began to increase significantly in communities that had both species in the (Figure 2 & 3). They also performed significantly better than communities were Blepharisma was not removed (Two sample test: DF = 8, t = 1.86, p < 0.05). Nothing can be said of the Blepharisma+Colpidium communities because Colpidium went extinct before Blepharisma was removed from the community. Paramecium continued to decline in communities where Blepharisma was not removed but decline rates were slow and stable. See Figure 2 & 3.
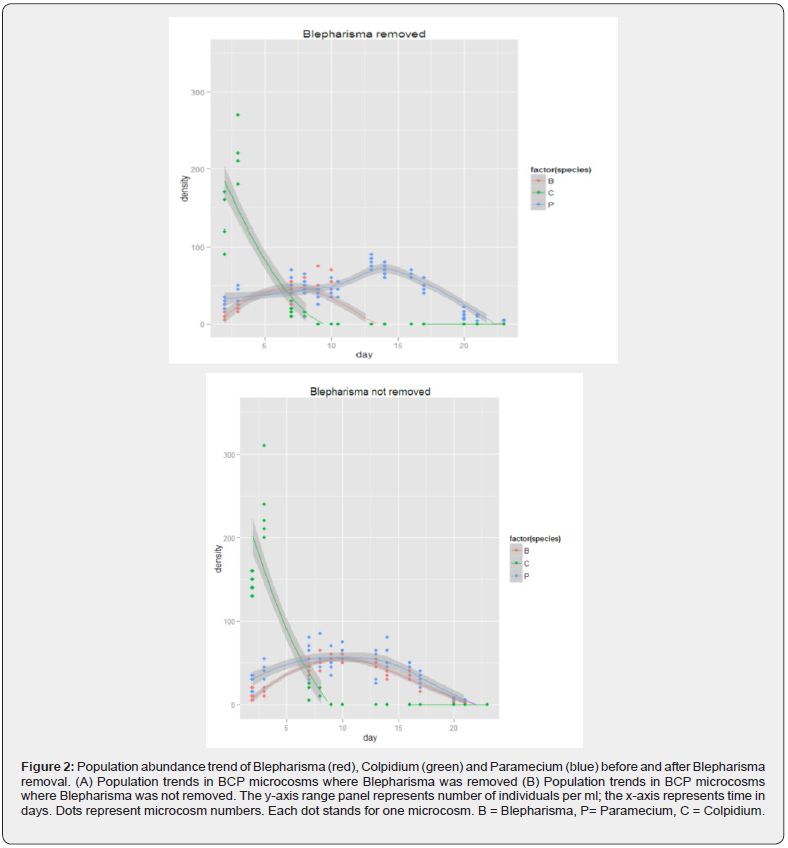
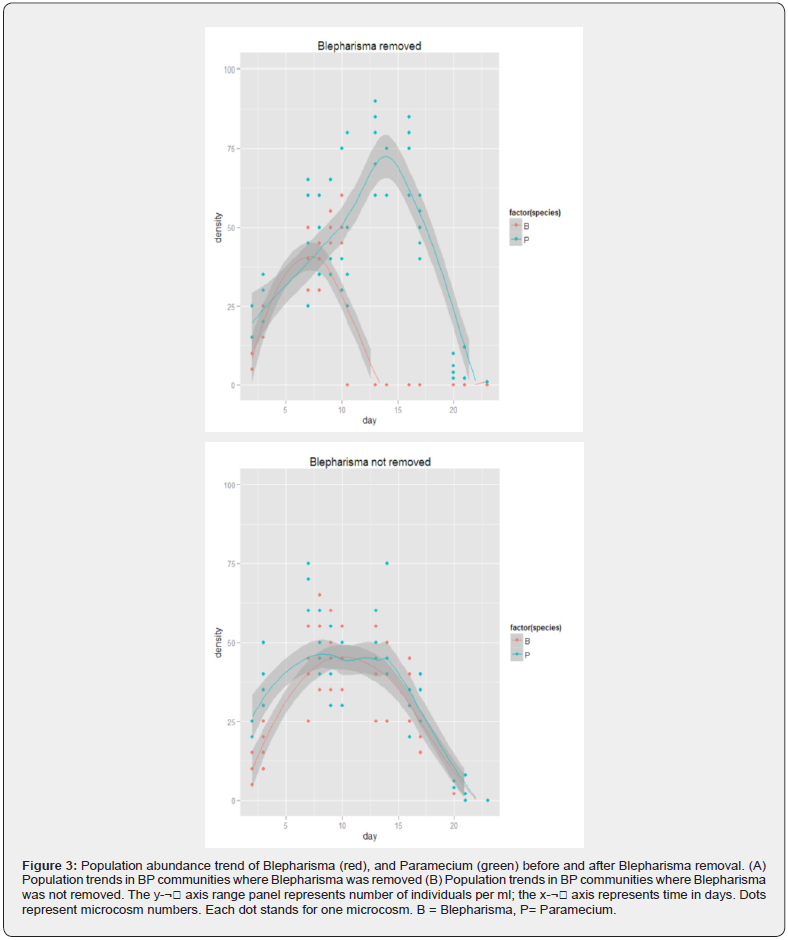
Where Blepharisma was not removed, Paramecium went completely extinct on day 23. However, time of extinction of Paramecium did not differ significantly for the both treatments (Blepharisma and Blepharisma) (Two sample test: DF = 8, t = 1.86, p > 0.05) even though Paramecium was still present in two of the five replicates in the BCP (B) community and present in one of five replicates in the BP (B) community at day 23 (last day of the experiment). See Figure 2 & 3.
Observations in combinations with no removal
In 4 combinations (20 microcosms) where Blepharisma was not removed at all, certain observations were also recorded: Colpidium continue to decline in communities where they were grown alone and in combination with Paramecium until they went completely extinct in both communities on day 20. Blepharisma when grown alone declined slowly after peaking at day 13 until it went completely extinct on day 20. Paramecium also slowly declined in population when grown alone and when in combination with Colpidium after peaking on day 13. Paramecium however persisted the longest in all communities it was part of, albeit in low numbers. Of the 50 microcosms used for the experiment, only 10 did not go extinct as at day 23 of the experiment.
Observation of bacterial density following Blepharisma removal
There was clearly an effect of Blepharisma removal on bacterial population as total bacterial densities in communities where Blepharisma was removed were significantly higher than in communities where Blepharisma was not removed. This observation was consistent for the 3 communities BPC, BP and BC (DF = 8, t = 1.85955, p < 0.05) where Blepharisma removal was induced (see Figure 3). Also, observations from plate count showed evidence of only two types of bacteria colonies present (S. marcescens and another) even though three bacterial species in addition with potentially unknown bacterial species were used to set up the communities. Plate counts showed S. marcescens significantly outcompeted the other bacteria (DF = 8, t = 1.8594, p < 0.05) in all three communities (see Figure 3) and this dominance increases with decreasing dilutions. In other microcosms, bacterial densities were highest in communities where Colpidium was grown alone, followed by when in combination with Paramecium, and then followed by Paramecium alone. Blepharisma when grown alone had the lowest bacterial density of all combinations made.
Discussion
Effect of competition on Colpidium population
The competitive exclusion principle propounded by Gause [14] states that two species competing for same resource cannot coexist stably without one having an advantage over the other till it gradually drives it to extinction in the long run. The result enumerated in section 5.1 showed how Colpidium was rapidly excluded in all microcosms in which it has a competitor, especially by Blepharisma. Competition in this scenario may have been heightened by resource availability factors given that the enrichment level of the aquatic communities in which they thrive is relatively low. It is important however to recognize that bacterial density counts suggested that under the research experimental conditions, Blepharisma in the single species microcosms reduced bacterial densities the most. Fox [15] opined that in monocultures, ciliates that reduced bacterial densities the most are the dominant competitor. Blepharisma in this scenario may have driven Colpidium to extinction via this mechanism. Grazing abilities may not be the most ideal competition gradient to judge from as competing species with varying grazing capacities have been shown to coexist in stable equilibrium [15] and competing species with analogous grazing capacities may sometimes exclude each other [16]. However, grazing capacity is a very useful and popular compass to determine a dominant competitor. It is also of utmost importance to note that Colpidium was excluded very rapidly by an omnivore (The intraguild predator Blepharisma). The mechanisms and conditions for intraguild predation are still not completely understood. A number of theories and assumptions have been propounded for these kinds of interactions: Holt & Polis [17] opined that an intraguild prey must demonstrate competitive superiority for resource to achieve stable equilibrium. The single species bacteria density results may have suggested this criterion was not met, as Blepharisma appeared to have grazed the most bacteria and so may be the dominant competitor. Diehl & Feissel [18] suggested that since the intraguild prey is both a competitor and a prey, it has the capacity to enhance or inhibit the intraguild predator. Enhancement of the intraguild predator can be attained if growth rate gain from consumption of intraguild prey is more than the growth rate loss from low density of resource where the intraguild prey is present. The opposite can be said for the inhibition criteria. This theory suggests the intraguild predator is expected to thrive at low enrichment levels and will achieve its highest densities in the presence of an intraguild prey than in its absence. The result agrees with this theory as Blepharisma thrived at low enrichment in the presence of an intraguild prey Colpidium. In direct contrast to findings of this research, Morin (1999) reported that Blepharisma was an inferior competitor for bacterial resource at low enrichment in comparison to Colpidium, but with higher bacterial density, the reverse was the case. In another report, Diehl & Feissel [19] also reported intraguild predator, Blepharisma dominance on prey following enrichment of their resources.
There is however no clear evidence of feeding trade-‐offs made by Blepharisma between intraguild prey and bacteria but the effect its presence had on Colpidium at low enrichment level is significant and could be as a result of any of the scenarios highlighted. It is also worthy of note that Paramecium also outcompeted Colpidium subsequently even though at a much slower pace.
Consequences of Blepharisma removal on Paramecium population
Among a considerable number of extinction consequences, one prominent consequence is the effect it may pose on interacting species’ population [20]. This assertion is supported strongly by the extinction cascade theory, which states that the impact of the extinction of a primary species has the potential to cause secondary extinctions by virtue of co-‐extinctions of very dependent species [21,22]. The result of this experiment however suggested that this theory is likely not applicable with competing species within the same trophic level as there is no evidence of any interdependency relationship between competitors Blepharisma and Paramecium. In fact, the population of Paramecium increased markedly following the removal of its competitor Blepharisma. With low energy levels in the aquatic community.
Blepharisma may have been exerting competitive pressure on the population of Paramecium. Its removal from the community facilitated the resurgence of Paramecium. It would have been interesting to monitor the interaction between Colpidium and Paramecium following the removal of Blepharisma but Colpidium went extinct before Blepharisma removal. Given the low energy levels, perhaps Blepharisma should have been removed two days earlier. Also, the presence of a top predator would have provided the dependence criteria needed to adequately understand the dynamics of extinction consequences across trophic levels. However, the study provided important insights on extinction and how it could have positive impacts on competitors within the same trophic levels. Alternatively, a scenario where the extinction of a species from a community will reduce the population of its competitor could also be possible depending on the dynamics of the interaction strengths/weaknesses between the two competing species and their preys [23], but this is hardly the case here in similar single trophic level interaction patterns.
Effects of Blepharisma removal on time of extinction of Paramecium
In this study, comparing time of extinction of Paramecium in microcosms where a competition interaction between Blepharisma and Paramecium was sustained with microcosms where Blepharisma extinction was induced provided insights on the veracity of this theory. The results showed that even though Paramecium increased following the removal of its competitor Blepharisma, the overall extinction time of Paramecium in both treatments did not differ significantly. This suggests that despite lack of competition for Paramecium, low energy levels of the aquatic community still couldn’t sustain their populations far long enough to go extinct a little later. This study is in conformity with the findings of Ferguson & Ponciano [24]. In summary, the research deduced that with low enrichment, the extinction of a species’ competitor would not extend the time of extinction of the species.
Effects of Blepharisma removal on bacterial prey densities
Evidence from the results of this study suggested the extinction of a bacterial predator Blepharisma from the protists trophic level might have led to a relative increase in total bacterial densities. Apparently, a combination of Blepharisma and Paramecium exerts more top-‐down pressure on the bacterial population; removal of one reduced this top-‐down influence. With a reduced protistan predator influence, bacterial prey reproduced relatively faster, thus using up scarce nutrients faster, this could be an explanation for the generally fast rate of community collapse. In conformity with findings of this research, Bell et al. [25] reported total bacterial population increase in the absence of predators. However, their study revealed that the increase in bacterial population did not lead to a corresponding increase in bacterial diversity. Diversity is often times the biodiversity gradient used to measure the stability of ecological systems [26-47].
Conclusion
As the reviews in section 1.1.3 to 1.1.5 suggest, extinctions have been predicted to pose serious threats to ecological systems both directly and indirectly. Probable cases and scenarios of direct consequences of extinction are well documented in peer-‐ reviewed journals, newspaper articles, magazines and popular literature. Indirectly, extinctions have also been predicted to disrupt ecosystems via the interacting links which species are part of. This research showed that within a simple single trophic level ecosystem involving protists and bacteria, the consequences of extinction of Blepharisma wasn’t totally disastrous for interacting species. A competing Paramecium species increased proportionately with Blepharisma extinction due to the removal of its competitor and the bacterial population where Blepharisma extinction was induced showed higher densities compared to where Blepharisma was present due to the removal of one of their predators. There was no evidence of extinction cascading effects as a consequence of Blepharisma extinction. Also, there was no evidence of any effect of Blepharisma extinction on the time of extinction of its competitor.
These findings however showed patterns that emerge within a single trophic level-primary producers community. Most real world natural processes in aquatic systems occur in very complex food web interactions, connecting multiple trophic levels (Polis and Strong 1996). The patterns of reaction to extinction may differ markedly with more trophic levels, as one would be able to monitor how dependent species would react to loss of their food source and how this will affect overall species composition, ecosystem structure and functioning. Further research should focus on detecting cascading effects of extinction across trophic levels to really grasp the concept of extinction beyond competition interactions. It may also be interesting to understand the role of temperature in these types of interaction both at the protist and the bacteria trophic levels.
Also, the patterns observed at the bacterial trophic level poses lots of questions on predation preferences, survival mechanisms, competition and co-‐existence and the factors affecting them. Understanding the patterns of these interactions will aid predictions on how protists graze on bacteria which is an important process that will form the basis of more understanding on the fundamentals of matter and energy transfer across microbial trophic chains. More research is encouraged.
Declaration
Author contribution statement
Amabogha O. developed, designed the experiments and analyzed, interpreted the data and also wrote the paper.
Amabogha B. did correction of the write up.
Funding statement
Specific grant was given to this research by funding organization NDDC.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgement
Laboratory attendants of the Department of Life Sciences, University of Bedfordshire assisted during the laboratory and in collecting the waste materials, and they are greatly appreciated.
References
- Loreau M, Naeem S, Inchausti P (2002) Biodiversity and Ecosystem Functioning: Synthesis and perspectives. (Oxford Univ Press, 2002).
- Crutzen PJ, Andreae MO (1990) Biomass burning in the tropics: Impact on atmospheric chemistry and biogeochemical cycles. Science 250(4988): 1669-1678.
- Greenpeace (2006) Eating up the Amazon. Greenpeace, Amsterdam. The Netherlands.
- IPCC (2014) Intergovernmental Panel on Climate Change; Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva.
- Fitzherbert EB, Struebig MJ, Morel A, Danielsen F, Bruhl CA, et al. (2008) How will oil palm expansion affect biodiversity? Trends in ecology and evolution 23(10): 538-545.
- Koh LP, Wilcove DS (2008) Is oil palm agriculture really destroying tropical biodiversity? Conservation letters 1(2): 60-64.
- Diamond J (1989) Overview of recent extinctions. In ‘Conservation for the Twenty-first Century’. In: D Western, MC Pearl (Eds.), Wildlife Conservation International, New York Zoological Society: New York, USA, pp. 37-41.
- Hooper DU, Adair EC, Cardinale BJ, Byrnes JE, Hungate BA, et al. (2012) A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486(7401): 105-108.
- Balvanera P, Pfisterer AB, Buchmann N, He JS, Nakashizuka T, et al. (2006) Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecology letters 9(10): 1146-1156.
- Ehrlich PR, Wilson EO (1991) Biodiversity studies: science and policy. Science 253(5021): 758-762.
- Chades I, McDonald-Madden E, McCarthy MA, Wintle B, Linkie M, et al. (2008) When to stop managing or surveying cryptic threatened species. Proceedings of the National Academy of Sciences 105(37): 13936-13940.
- Lawler SP, Morin PJ (1993) Food web architecture and population dynamics in laboratory microcosms of protists. American Naturalist 14(5): 675-686.
- Giese AC (1973) The pigment blepharismin and photosensitivity. In: Giese AC (Ed.), Blepharisma. (Stanford Univ., Stanford), pp. 266‑303.
- Gause GF (1934) The struggle for existence. Williams and Wilkins, Baltimore, p. 163.
- Fox JW (2002) Testing a simple rule for dominance in resource competition. The American Naturalist 159(3): 305‑319.
- Fox JW, Smith DC (1997) Variable outcomes of protist-‐‑rotifer competition in laboratory microcosms. Oikos, pp. 489-495.
- Holt RD, Polis GA (1997) A theoretical framework for intraguild predation. American Naturalist 149(4): 745‑764.
- Diehl S, Feissel M (2000) Effects of enrichment on three-level food chains with omnivory. The American Naturalist 155(2): 200‑218.
- Diehl S, Feissel M (2001) Intraguild prey suffer from enrichment of their resources: a microcosm experiment with ciliates. Ecology 82(11): 2977-2983.
- Dunne JA, Williams RJ (2009) Cascading extinctions and community collapse in model food webs. Philosophical Transactions of the Royal Society B: Biological Sciences 364(1524): 1711-1723.
- Koh LP, Dunn RR, Sodhi NS, Colwell RK, Proctor HC, et al. (2004) Species coextinctions and the biodiversity crisis. Science 305(5690): 1632-1634.
- Lafferty KD, Kuris AM (2009) Parasites reduce food web robustness because they are sensitive to secondary extinction as illustrated by an invasive estuarine snail. Phil Trans R Soc B 364(1524): 1659-1663.
- Estes JA, Duggins DO, Rathbun GB (1989) The ecology of extinctions in kelp forest communities. Conservation Biology 3(3): 252-264.
- Ferguson JM, Ponciano JM (2014) Predicting the process of extinction in experimental microcosms and accounting for interspecific interactions in single-‐‑species time series. Ecology letters 17(2): 251‑259.
- Bell T, Bonsall MB, Buckling A, Whiteley AS, Goodall T, et al. (2010) Protists have divergent effects on bacterial diversity along a productivity gradient. Biology letters 6(5): 639-642.
- Bascompte J, Jordano P, Olesen JM (2006) Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312(5772): 431-433.
- Borrvall C (2006) Biodiversity and Species Extinctions in Model Food Webs. Linköping Studies in Science and Technology. Dissertations. No. 1015
- Brook BW, Sodhi NS, Bradshaw CJ (2008) Synergies among extinction drivers under global change. Trends in Ecology & Evolution 23(8): 453-460.
- Chapin III FS, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, et al. (2000) Consequences of changing biodiversity. Nature 405(6783): 234-242.
- Coll M, Hargadon K (2012) Trophic and functional cascades in tropical versus temperate aquatic microcosms. Aquatic Ecology 46(1): 55‑71.
- Corlett RT (2007) The impact of hunting on the mammalian fauna of tropical Asian forests. Biotropica 39(3): 292‑303.
- Dıaz S, Symstad AJ, Chapin FS, Wardle DA, Huenneke LF (2003) Functional diversity revealed by removal experiments. Trends in Ecology & Evolution 18(3): 140-146.
- Falkowski PG, Fenchel T, Delong EF (2008) The microbial engines that drive earth's biogeochemical cycles. Science 320(5879): 1034-1039
- Fee EJ, Hecky RE (1992) Introduction to the northwest Ontario lake size series (NOLSS). Canadian Journal of Fisheries and Aquatic Sciences 49(12): 2434‑2444.
- Fritts TH, Rodda GH (1998) The role of introduced species in the degradation of island ecosystems: a case history of Guam. Annual Review of Ecology and Systematics 29: 113-140.
- Gonzalez A, Chaneton EJ (2002) Heterotroph species extinction, abundance and biomass dynamics in an experimentally fragmented microecosystem. Journal of Animal Ecology 71(4): 594-602.
- Guido A, Pillar VD (2014) Are removal experiments effective tools for assessing plant community resistance and recovery from invasion? Journal of Vegetation Science 26(3): 608-613.
- Gurevitch J, Padilla DK (2004) Are invasive species a major cause of extinctions? Trends in Ecology & Evolution 19(9): 470‑474.
- Harte J, Ostling A, Green JL, Kinzig A (2004) Biodiversity conservation: Climate change and extinction risk. Nature 430(6995): 3.
- Hogan M, Caley KJ, McGinley M (2010) Causes of extinction. Encyclopedia of Earth, Washington, DC: Environmental Information Coalition, National Council for Science and the Environment.
- Hooper DU, Chapin III FS, Ewel JJ, Hector A, Inchausti P, et al. (2005) Effects of biodiversity on ecosystem functioning: A Consensus of Current Knowledge. Ecological Monographs 75(1): 3‑35.
- IUCN (2015) International Union for conservation of Nature. Red list of threatened species.
- Ives AR, Carpenter SR (2007) Stability and diversity of ecosystems. Science 317(5834): 58-62.
- Jessup CM, Kassen R, Forde SE, Kerr B, Buckling A, et al. (2004) Big questions, small worlds: microbial model systems in ecology. Trends in Ecology and Evolution 19(4): 189‑197.
- Klein BC (1989) Effects of forest fragmentation on dung and carrion beetle communities in central Amazonia. Ecology 70(6): 1715-1725.
- Larsen TH, Williams NM, Kremen C (2005) Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecology letters 8(5): 538-547.
- MacArthur R (1955) Fluctuations of animal populations and a measure of community stability. Ecology 36(3): 533‑536.






























