Microplastics Pollution: An Intending Threat for Aquatic Ecosystem Sustenance
Odeyemi DF*
Department of Science Laboratory Technology, Biotechnology Option, Ekiti State University, Nigeria
Submission: December 14, 2022; Published: February 17, 2023
*Corresponding author: Odeyemi Dolapo Funmi, Department of Science Laboratory Technology, Biotechnology Option, Faculty of Science, Ekiti State University, Ado Ekiti State, Nigeria
How to cite this article: Odeyemi DF. Microplastics Pollution: An Intending Threat for Aquatic Ecosystem Sustenance. Int J Environ Sci Nat Res. 2023; 31(5): 556322. DOI 10.19080/IJESNR.2023.31.556322
Abstract
Microplastics are regarded as another group of pollutants ravaging the aquatic habitat because plastics have become an increasingly important packaging option which slowly degrades into microplastics. Also, most domestic items used on a daily basis are known to contain microplastics which are ultimately released as wastes into oceans and rivers. Anthropogenic activity has led to microplastic contamination throughout the marine environment. Characteristics such as low density, good mechanical properties and low cost enables successful use of plastics in industries and everyday life but the high durability leads to its persistence in the marine environment where they cause harm to a great variety of organisms As a result of widespread contamination, microplastics are ingested by animals including fish and shellfish. Because microplastics are associated with chemicals from manufacturing and that sorbs from the surrounding environment, there is concern regarding physical and chemical toxicity However, there are primary as well as secondary sources of microplastics in the environment in which both sources poses threat to aquatic lives. This has become a major cause of concern because these microplastics are consumed by aquatic organism because they mostly appear to them as food. Evidence implicating plastics in ecotoxicity and epidemiology is emerging, Therefore this review focuses on sources, assessment, impacts and bioaccumulation of microplastics using reports from several studies in different countries, Nigeria inclusive. Studies shows humans can ingest microplastics by consuming fish polluted with microplastics, however research is needed to establish there is a route of human exposure to microplastics via fish consumption.
Keywords: Aquatic microplastics pollution; Bioaccumulation; Marine fish; Ingestion water, Environment particle
Introduction
Plastics are emerging pollutants that are posing enormous form of risk to the environment mostly on aquatic and consequently human. The presence of emerging environmental pollutants (EEPs) is gradually becoming overwhelming thereby affecting its quality to support life across all levels. Global production of plastic is constantly on the rise due to its cheapness and versatility necessary for the lifestyle of people [1]. A greater percentage of plastics being produced are non-biodegradable thereby accumulating in the marine environment. It was estimated that 10% of plastic produced worldwide ended up as waste in the marine environment due to poor recycling with only 3% recycled in 2016 [2]. Macroplastics while in the environment undergo degradation to form microplastics which is now ubiquitous in the global ecosystem [1,2].
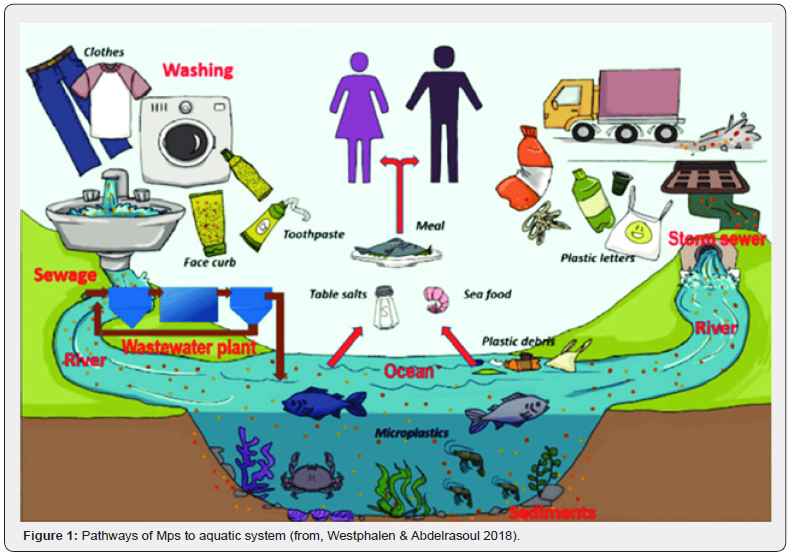
In Nigeria, plastic waste is poorly recycled, the majority ends up in landfill where it may take centuries for such material to breakdown and decompose (Figure 1). Despite plastics being an internationally recognized pollutant with legislation in place aimed to curb the amount of plastic debris entering the marine environment [1,3,4], the problem still persists (Figure 1). The National Environmental Regulations Enforcement Agency (NESREA) prohibiting persons from dropping litter (polyethene bags inclusive) on roads, public space, drainages or other undesignated places, set in 2009, is poorly implemented. The pathways of microplastics into aquatic habitat is illustrated in Figure 1.
Microplastic particles can be categorized into primary and secondary particles. Primary microplastic particles (MPPs) are those which have been specifically produced for industrial use, for instance as peeling particles in cosmetic products. Primary microplastics are plastic fragments or particles that are already 5.0mm in size or less before entering the environment. They include microfibers from clothing, microbeads, and plastic pellets [5,6]. Secondary MPPs are formed by physical, biological and chemical degradation of macroscopic plastic parts and are the main source of micro particles released into the environment. They are produced from the breakdown of larger plastic products through natural weathering processes after entering the environment. Such sources of secondary microplastics include water and soda bottles, fishing nets, plastic bags, microwave containers, tire wear and tea bags [7,8] They are mainly formed by the degradation of improperly disposed plastic waste, tire abrasion and washing of synthetic textiles Plastics of all sizes have become the most dominant form of marine litter and it has been estimated that at least 5.25 trillion plastic particles weighing above 268,000 tons have been discarded into the Oceans [9]. Moreover, according to the 2017 United Nations Environment Assembly (UNEP) an estimate of 4.8-12.7 million metric tons of plastic is introduced to the oceans annually [10]. The use of plastics is quite common all over the world, their use ranges from packaging, and other daily use products. The low cost, lightweight, strength and durability of plastics are properties that make them suitable for manufacture on a wide range of daily use products. Virtually everything is made of plastic nowadays. However, the high demand and inappropriate disposal of plastic materials have led to their dispersion and accumulation into the environment. For example, during the current COVID-19 pandemic, the worldwide production and disposal of face masks as well as other plastic laboratory and medical materials have drastically increased, adding to the vast plastic and microplastic waste in the environment [10]. Several studies on plastic size abundance and distribution have shown a permanent fragmentation of microplastic from larger to smaller, to nanoplastics (< 25μm), occurring continuously in the oceans [11,12]. One of the main concerns about the smaller fraction of plastic particles is the risk potential for filter feeders, which tend to confuse it for plankton and end up consuming plastic debris [13-15].
Sources of microplastics
Generally, microplastics (MPs) in the environment come from two main types of source, which lead to different sizes of plastic particles: one is the primary source, and another is the secondary source. However, it is not easy or even impossible to identify the exact source of MPs detected in the environment. Primary sources of environmental MPs include plastic pellets, personal care products containing microbeads, paint, washing wastewater, sewage sludge, plastic running tracks in schools, artificial turf, rubber road in cities, and vehicle tire wear.
Many personal care and cosmetic products contain a type of engineered microplastic known as microbeads. The products include scrubbing agents, shower gels and creams. The U.S. government banned its manufacture and sale, but producers still make and sell these products globally. Microbeads are manufactured polyethylene plastic. It acts as an exfoliant, delivers active ingredients, and controls viscosity in health and beauty products. Up to 10 percent of some personal care product’s weight is plastics. That’s more than the packaging material. Some items have several thousand microbeads per gram of product. Once the personal care item is used, it ends up in wastewater. These tiny particles easily pass through water filtration systems and end up in our waterways. Personal care products and cosmetics represent 2 percent of all primary microplastics in the oceans. Tires erode through heat and friction from contact with the road. The wind and rain spread the tire dust and wash it off the road. It enters tributaries, lakes and eventually the oceans. Meanwhile, secondary sources include municipal debris such as plastic bags and bottles, fishing wastes, farming film, and other large size plastic wastes. Among these sources, vehicle tire wear is regarded as one of the most important sources of environmental microplastic due to the rapid global increase in the number of vehicles. However, available studies about the presence of rubber particles in the environment are very scarce. It is estimated that secondary sources of MPs currently account for the dominant of MPs in the environment although large plastic wastes need hundreds of years to break down into MPs under natural conditions. The appropriate management of plastic wastes and wastewater is the crucial step to prevent and control microplastic pollution in the environment in the future [16].
Plastic production and consumption are on the increase in annually with 10.3% and 6.5% respectively [17]. Production and consumption stood at 436 kilotons and 1,090 kilotons in 2018 respectively [18] causing increase in the abundance of plastics in the inland freshwater system. The in-land freshwater system was estimated to be about 283,293.47 hectares, of which 70% has been degraded due to the pollution [19].
Many marine environment worldwide have been studied for the occurrence of microplastics such as in the South Pacific and North Atlantic [9,20] Kaliningrad region, Russia [21], Norderney [22], Indian coast [23], South Africa, Mozambique, and Ghana [24] with few studies in Nigeria. Enyo et al. [25] reported the abundance, distribution and composition of macrodebris and microplastic pollutants from five rivers in the south-eastern part of Nigeria [25]. Another study focused on method development rather than quantifying the occurrence of microplastics in Elechi Creek, Rivers State, Nigeria [26], In another report, gastropods collected from Osun River, Nigeria were used as a bioindicator for microplastic pollution [27]. Babayemi et al. [28] reported that the primary source of plastic in Nigeria is importation, covering the different polymer resins for production of plastic materials, products and components of imported goods [28]. In Nigeria, water sachets (500mL plastic bags) and shopping bags are the major constituent of plastic waste [29]. A single plastic bag takes 1000 years to degrade once disposed of into the environment [30]. In Nigeria, educational institutions are among the major sources of plastics. Adeniran et al. [31] characterized the waste generated within the University of Lagos and found that polyethylene bags were the largest stream amounting to 24% of the total wastes while plastic amounted to 9% of the total wastes generated in the institution. A similar study by Okeniyi & Udonwan [32] at Covenant University, Ogun State revealed that plastic represents the highest percentage of waste generated within the university consisting of plastic food packs, polyethylene bags and plastic bottles (Figure 2).

Materials and Methods for the Data Collection
Online study sites
Data used for this review were collected from many countries around the world, Nigeria inclusive. Results from different publications from several researchers are presented.
Data collection
The first phase entailed the identification of related studies, the search database used are google scholar, elsevier, reekseek, scopus.
Searching strings
Keywords used in the search are: ‘microplastics”, ‘Assessment of microplastics in water samples’, ‘sources of microplastics’ “types of microplastic” bioaccumulation of microplastics’,”Trophic transfer of microplastics” microplastics impact on human”
Assessment of Microplastics in Water
Currently, studies on microplastic pollution suffers from insufficient reliable data on its concentrations in the marine environment and on the composition of involved polymers because standard operation protocols (SOP) for microplastic sampling and detection are not available [33-36]. Although first steps towards a standardization have been made, e.g., in the European Union by TSG-ML [37], the comparability of data on microplastics is still hampered by a huge variety of different methods that lead to the generation of data of extremely different quality and resolution.
Sampling for microplastics
Presently, synthetic polymers are ubiquitously present everywhere and daily life without a plastic is inconceivable. Consequently, even microplastic sampling, preparation and analysis procedures themselves are affected by the presence of synthetic polymers in the environment. Hence, a multitude of contamination sources from sampling equipment through clothes or airborne particles can compromise the analysis of microplastics in the environment. These contaminants result in a great overestimation of concentrations of microplastics in samples. Because of their ability to hover in air, especially fibres have a high contamination potential and can cause problems during microplastic analysis [33,36,38]. Thus, a special focus should be laid on the prevention of contamination [33]. Potential sources of contamination should be avoided by replacing plastic devices or laboratory ware by non-plastic material and the strict use of control samples is highly recommended. Analysis of control samples facilitates the identification of the source in case a contamination has occurred. Because of their relatively low concentrations in the environment, sampling of microplastic particles generally requires large sample volumes. Thus, samples from the open water are usually taken with plankton nets of different mesh sizes. The sea surface is sampled for floating microplastics by manta trawls [39,40] or neuston nets [41-43]. While neuston catamarans can be operated even in higher waves, a manta trawl is best used in calm waters to prevent hopping on waves and damage to the device. The volume filtered by a net is usually recorded by a flow meter mounted at the net opening, enabling the normalization to the filtered water volume and thus a calculation of concentrations of microplastics (items/grams) per unit water volume. Relating concentrations to sampled area is also possible by multiplying trawl distance by the horizontal width of the net opening. It is important to ensure that no residual sample is left in the net, which would lead to a carryover of microplastics to the next sample. The content of the codend is finally transferred to a sample container and fixed with plastic friendly fixatives (e.g., formalin) or stored frozen. If the particles are directly sorted they should be dried and kept in the dark until further analysis [33]. The size of the particles retained and also the filterable volume is a direct consequence of the mesh size used. The mesh sizes used for sampling in previous studies varied between 50 and 3000μm [33]. Another factor influencing the filtered volume is the net size, i.e., the area, which acts as filter.
Depending on the seston concentration in the water, a few thousand litres to several hundred cubic metres can be filtered until a net becomes clogged. Seasons with red tides or plankton and jellyfish blooms are generally unfavorable for sampling large volumes of water. Nets are usually 3-4.5m long and a mesh size of around 300μm is most commonly used. These nets do not sample microplastic particles <300μm quantitatively but allow for sampling of larger volumes of water. In order to avoid the risk of clogging nets at small mesh sizes, only few studies used mesh sizes <300μm. The non-standardized use of different nets and mesh sizes seriously impedes the comparability of data sets on pelagic microplastic concentrations. Besides common net sampling, other techniques are occasionally used for assessing microplastic concentrations in the water column: bulk sampling with subsequent Filtration [44,45], screening Continuous Plankton Recorder (CPR) samples [46] or using direct in situ filtration [38]. A highly promising technique, currently under development, is the use of direct fractionated pressure filtering of large (>1m3) volumes of water through a filter cascade (developed by -4H-JENA engineering GmbH). This approach theoretically allows for the simultaneous sampling of different size fractions of microplastics down to <10μm and thus enables a more comprehensive resolution of the size spectrum of microplastics.
Size fractionation
Water samples can be fractionated by sieving. If large amounts of biological matrix (e.g., gut contents, tissue, large plankton) clog the sieve a purification step prior to sieving can be helpful. Microplastics from sediment samples are easily size-fractionated after extraction. If the sediment sample matrix consists mainly of smaller grains (<500μm) it can be sieved after drying (or wet) to reduce the volume for later extraction. In this case, the sample must be handled with care during sieving to avoid the mechanical generation of additional microplastic particles from larger, brittle plastic material. A 500μm sieve, ideally made of steel, can be used for size separation. The use of a sieve cascade of different mesh sizes allows for size separation and quantification of different size classes of microplastics [47,48]. Microplastic particles >500μm can be sorted out manually under a stereomicroscope using forceps and subsequently analyzed (visually, spectroscopically, other techniques). The effort involved in the manual sorting of particles increases for the fraction <500μm owing to difficulties in handling small particles. The suggested size separation (>500μm; <500μm) is accounted for by the techniques that can be used for later identification. Additionally, the standardized application of size fractionation enables an inter-comparison between different studies, at least for the larger fraction, even if the smaller fraction is not of interest for the study [33].
Sample purification
In preparation of microplastic samples for instrumental analysis (FTIR/ Raman Spectroscopy, pyrolysis –GC/MS), purification of microplastic samples is necessary to minimize non plastic filter on filter on which the microplastic fraction <500μm is concentrated. Also, biofilms and other organic and inorganic substances have to be removed from microplastic particles in order to enhance proper identification [49]. The most gentle way to clean plastic samples is stirring and rinsing with freshwater [48]. The use of ultrasonic cleaning [50] should be carefully considered because aged and brittle plastic material might break during treatment resulting in the artificial generation of secondary microplastics [49]. A treatment with 30% hydrogen peroxide of the dried sediment sample [51], the sample filter [35,36] or the microplastic particles themselves removes large amounts of natural organic debris. Andrady [52] suggests the use of mineral acids to disintegrate organic impurities in samples. For the digestion of the soft tissue of biotic samples Claessens et al. [34] used either acid, base and oxidizer (hydrogen peroxide) or a specific mixture thereof. Hot acid digestion with HNO3 resulted in the best purification results [34]. However, several plastic polymers (e.g., polyamide, polyoxymethylene, polycarbonate) react to strong acidic or alkaline solutions [34,51], which limits the applicability of these reagents. More promising is the use of a sequential enzymatic digestion as a plastic friendly purification step. A first attempt of enzymatic purification of samples has been made by Cole et al. [53] who used only a single enzymatic step (proteinase- K). Sample purification with different technical enzymes (lipase, amylase, proteinase, chitinase, cellulase) prior to micro-FTIR spectroscopy has successfully been applied by our group. This approach reduces the biological matrix of plankton and sediment samples (including chitin, which is present especially in marine samples) as well as the matrix of biological tissue samples to a minimum and thus proved to be a very valuable technique to minimize matrix artifacts during FTIR measurements.
Microplastics identification
Visual identification
According to Hidalgo-Ruz et al. [33] visual sorting to separate potential microplastics from other organic or inorganic material in the sample residues is an obligatory step for the identification of microplastics. If large microplastics are the target of a study this can be done by visual inspection [41] whereas smaller microplastic particles should generally be sorted out under a dissection microscope [40]. Sorting of aqueous samples can be facilitated by the use of sorting chambers (e.g., Bogorov counting chamber).
Generally, if no more accurate methods (e.g., FTIR or Raman spectroscopy) are used to verify synthetic polymer origin of potential microplastic particles the visual identification should not be applied to particles <500μm as the probability of a misidentification is very high. Hidalgo-Ruz et al. [33] thus suggest an even higher size limit of 1mm for visual identification. According to Norén [53], selection of particles according to standardized criteria in connection with a strict and conservative examination reduces the possibility of misidentification. He suggested the following criteria: (i) no structures of organic origin should be visible in the plastic particle or fibre, (ii) fibres should be equally thick and have a three dimensional bending to exclude a biological origin, (iii) particles should be clear and homogeneously colored, (iv) transparent or whitish particles must be examined under high magnification and with the help of fluorescence microscopy to exclude a biological origin [53]. General aspects that are used to describe visually sorted microplastics are source, type, shape, degradation stage, and color of the particles [33].
Identification of microplastics by their chemical composition
The repetitive fingerprint-like molecular composition of plastic polymers allows for a clear assignment of a sample to a certain polymer origin. Below are some methods applied for polymer identification with a focus on the frequently used FTIR and Raman analyses of microplastics.
Density separation with subsequent C:H:N Analysis
Law et al. [41] used the specific densities of particles to identify the polymer origin of visually sorted microplastics. For this purpose, the sample was placed in distilled water and, depending on the density of the sample, either ethanol or concentrated solutions of calcium or strontium chloride were added until the sample was neutrally buoyant. The density of the particle was indirectly assessed by weighing a certain volume of the solution. This facilitated the determination of the density with high precision. Different groups of polymers possess a characteristic elemental composition, which was used to identify the plastic origin of a particle by a subsequent C:H:N analysis. By comparison with the densities and C:H:N ratios of virgin-polymer samples the particle could be assessed as either plastic or not and assigned to a group of potential polymers [41]. This approach represents an approximation to the identification of microplastic particles by narrowing the search for the potential polymer type but not a rigorous chemical analysis. Further drawbacks are the relatively high time effort, which hampers a high sample throughput and that this technique is not applicable to smaller particles.
Pyrolysis-GC/MS
Pyrolysis-gaschromatography (GC) in combination with mass spectrometry (MS) can be used to assess the chemical composition of potential microplastic particles by analyzing their thermal degradation products [55]. The pyrolysis of plastic polymers results in characteristic pyrograms, which facilitate an identification of the polymer type. This analytical approach is already used after extraction and visual sorting of microplastics from sediments. The polymer origin of particles is then identified by comparing their characteristic combustion products with reference pyrograms of known virgin-polymer samples [36,55]. If a thermal desorption step precedes the final pyrolysis organic plastic additives can be analyzed simultaneously during pyrolysis-GC/MS runs [55]. Although the pyrolysis-GC/MS approach allows for a relatively good assignment of potential microplastics to polymer type it has the disadvantage that particles have to be manually placed into the pyrolysis tube. Since only particles of a certain minimum size can be manipulated manually this results in a lower size limitation of particles that can be analyzed. Furthermore, the technique allows only for the analysis of one particle per run and is thus not suitable for processing large sample quantities, which are collected during sampling campaigns or routine monitoring programs. However, currently promising pyrolysis-GC/MS approaches for the qualitative/ quantitative analysis of microplastics on whole environmental sample filters are being developed (Scholz-Böttcher, personal communication).
Raman spectroscopy
Raman spectroscopy is a straightforward technique that has been successfully used to identify microplastic particles in different environmental samples with high reliability [5,35,56-58]. During the analysis with Raman spectroscopy the sample is irradiated with a monochromatic laser source. The laser depends on the system used: available laser wavelengths usually range between 500 and 800nm. The interaction of the laser light with the molecules and atoms of the sample (vibrational, rotational, and other low-frequency interactions) results in differences in the frequency of the backscattered light when compared to the irradiating laser frequency. This so-called Raman shift can be detected and leads to substance-specific Raman spectra. Since plastic polymers possess characteristic Raman spectra the technique can be applied to identify plastic polymers within minutes by comparison with reference spectra. Raman spectroscopy is a “surface technique”, thus large, visually sorted microplastic particles can be analyzed and the technique can also be coupled with microscopy. Accordingly, micro-Raman spectroscopy allows for the identification of a broad range of size classes down to very small plastic particles of sizes below 1μm [5]. If Raman microscopy is combined with Raman spectral imaging it is possible to generate spatial chemical images based on the Raman spectra of a sample. Micro-Raman imaging theoretically allows for the spectral analysis of whole membrane filters at a spatial resolution below 1μm. This would facilitate the detection of even the smallest microplastic particles in environmental samples, but the applicability for microplastic research has yet to be demonstrated. Raman spectroscopy can also be coupled with confocal laser-scanning microscopy to locate polymer particles within biological tissues with subcellular precision [5].
IR spectroscopy
Similar to Raman spectroscopy, infrared (IR) or Fourier-transform infrared (FTIR) spectroscopy offers the possibility of accurate identification of plastic polymer particles according to their characteristic IR spectra [44,46,59-62]. FTIR and Raman spectroscopy are complementary techniques. Molecular vibrations, which are Raman inactive are IR active and vice versa and can thus provide complementary information on microplastic samples. IR spectroscopy takes advantage of the fact that infrared radiation excites molecular vibrations when interacting with a sample. The excitable vibrations depend on the composition and molecular structure of a substance and are wave-length specific. The energy of the IR radiation that excites a specific vibration will depending on the wave-length be absorbed to a certain amount, which enables the measurement of characteristic IR spectra. Plastic polymers possess highly specific IR spectra with distinct band patterns making IR spectroscopy an optimal technique for the identification of microplastics [33]. FTIR spectroscopy can provide further information on physico-chemical weathering of sampled plastic particles by detecting the intensity of oxidation [63]. In a case study where FPA based micro FTIR imaging for microplastic particles measurement in environmental samples following all the standard protocols stated previously in the review. The results obtained had 29 and 64 particles per samples analyzed using FTIR microscopy. Only 4% of the total number of investigated particles differed in their shape from the abundant granular material. Figure 2 showed the comparison of the spectrum of laboratory quartz (p.a grade Merck) and a spectrum obtained from the measurement of a typical granular particle by FPA –based micro FTIR spectroscopy [64].
On the other hand, in a study done in south east Nigeria [1] superficial water were collected against water current using grab sampling technique at a depth of 0-3cm and homogenized to form a composite sample for each sampling point of the water body. The water samples were collected in clean quart glass bottles, capped tightly, shielded from light and stored at 40 C to prevent evaporation. The water samples were filtered sequentially through a cellu ¬lose filter paper with a nominal pore size of 11μm (Whatman No. 1, Catalog No. 1001 110, UK) with the aid of a glass funnel. The filter paper was placed in a desiccator, under room temperature away from light and stored in Petri dishes. To isolate and count microplastics, the dried filter papers were examined under a light microscope (AmScope M150C-PS25). The microplastics was isolated using the hot needle test as de¬scribed by De Witte et al. [65] while the filter was read from left to right, then move down one row, and read from right to left to ensure pieces are not double counted. Pictures of the isolated microplastic particles were taken and classified into three dominant shapes; fiber, film, and fragment, and five types; polypropylene (PP), polyethylene (PE), polyvinyl chloride (PVC), polyethylene terephthalate (PET), and others, based on their physical characteristics throughout the entire analysis, the filter papers were covered to prevent contamination from airborne fibers when they were not under microscope [1] and rubber gloves were used to minimize sample contamination plastic materials.
In another case study in which the method stated above was used presented the total number of microplastics based on location and stations as presented in Figure 2 the quantity of particles ranges from 73 particles/l at upstream of location III to 680 particles/l at downstream of locations II. This showed that microplastics particles varies with locations; Also, distribution showed significant difference (p<0.05) with general high load of particles in down¬stream probably due to different flow pattern and topography [1]. The distribution of microplastics based on shapes was also measured.
Microplastics Impact on Aquatic organisms
Changing views of the scottish highlands
Fish is an important origin of human protein which is necessary for body growth. Contamination of fish by MPs is a major hazard that requires special focus. Exposure of fish to MPs alone or in combination with other pollutants may lead to oxidative stress, tissue damage, changes in immune-related gene expression in fish. Seafood constitutes about 17% of global population intake of protein and 6.7% of all protein consumed [64]. As microplastics interact with plankton and sediment particles, both suspension and deposit feeders may be at risk of accidentally or selectively ingesting marine debris. However, the relative impacts are likely to vary across the size spectrum of microplastic in relation to the organisms affected, which is dependent on the size of the microplastic particles encountered [65]. Microplastics in the upper end of the size spectrum (1-5mm) may compromise feeding and digestion. In some cases, organisms feeding mechanisms do not allow for discrimination between prey and anthropogenic items [47]. Secondly, organisms might feed directly on micro-plastics, mistaking them for prey or selectively feed on microplastics in place of food [67]. If there is a predominance of microplastic particles associated with planktonic prey items, organisms could be unable to differentiate or prevent ingestion. A number of studies have reported microplastics from the stomachs and intestines of marine organisms, including fish and invertebrates [68]. Adeogun et al. [68] studies on Eleyele Lake, Ibadan, Oyo state, Nigeria showed a microplastic prevalence of 69.7% in about 109 sampled fish and observed 7 out of 8 species. This reflects the extent, magnitude and abundance of plastic contamination in the lake. Watts et al. [69] showed that shore crabs (Carcinus maenas) will not only ingest microplastics along with food (evidence in the foregut) but also draw plastics into the gill cavity because of their ventilation mechanism: this highlights that it is important to consider all sorts of routes of exposure to microplastics. If organisms ingest micro-plastics they could have adverse effects on individuals by disrupting feeding and digestion [70].
Mps can enter the very base of the marine food web via absorption such was observed when charged nano-polystyrene beads were absorbed into the cellulose of a marine alga (Scenedesmus spp), which inhibited photosynthesis and caused oxidative stress [71]. Microplastics can also affect the function and health of marine zooplankton [72]. Decreased feeding was observed following ingestion of polystyrene beads by zooplankton [5].
Studies of microplastic ingestion by benthic invertebrates in the field are less common than laboratory studies. Murray & Cowie [57] identified fibres of monofilament plastics that could be sourced to fibres of trawls and fragments of plastic bags in the intestines of the commercially valuable Norway lobster (Nephrops norvegicus). These results indicated that normal digestive processes do not eliminate some of the filaments as they cannot pass through the gastric mill system. Norway lobsters have various feeding modes, including scavenging and predation, and are not adapted to cut flexible filamentous materials [57]. The identification of microplastics in organisms that are caught for commercial purposes and subsequently consumed whole (including guts) highlights the potential human health implications. For example, field-caught brown shrimps (Crangon crangon) [73] and farmed and store-brought bivalves [66,74] had microplastics in their digestive system.
Invertebrates could be used as indicator species for environmental contamination. Species such as Nephrops are able to integrate seasonal variation in microplastic abundance, providing an accurate measure of environmental contamination. Additional studies are required to understand the flux of microplastic within benthic sediments and the interaction between different species of benthic in fauna feeding in/or manipulating the sediment, such as bivalves and worms. Benthic infauna could ingest and/or excrete microplastics, the individuals or their faecal pellets may in turn be ingested by secondary consumers, thus affecting higher trophic levels.
Trophic transfer of Mps
Absorption and ingestion of microplastics by organisms from the primary trophic level, e.g., phytoplankton and zooplankton, could be a pathway into the food chain [71]. Many species of zooplankton undergo a diurnal migration. Migrating zooplankton could be considered a vector of microplastic contamination to greater depths of the water column and its inhabitants, either through predation or the production of faecal pellets sinking to the seafloor [66]. Only a few studies deal with the potential for microplastics to be transferred between trophic levels following ingestion. Field observation highlighted the presence of microplastics in the scat of fur seals (Arctocephalus spp.) and Eriksson & Burton [75] suggested that microplastics had initially been ingested by the fur seals’ prey, the plankton feeding Mycophiids. In feeding experiments, Farrell & Nelson [76] identified microplastic in the gut and haemolymph of the shore crab (Carcinus maenas), which had previously been ingested by blue mussels (Mytilus edulis). There was large variability in the number of microspheres in tissues samples, and the results have to be treated with caution as the number of individuals was low and the exposure levels used exceeded those from the field. Similarly, Nephrops-fed fish, which had been seeded with microplastic strands of polypropylene rope were found to ingest but not to excrete the strands [57], again implying potential trophic transfer. As mentioned above, microplastics were also detected in cod, whiting, haddock, bivalves and brown shrimp, which are consumed by humans and raises concerns about trophic transfer to humans and human exposure [77]. Investigations on the bioaccumulation of Mps in fish species (Penaeus semisculatus Epiinepelus coicoides) across trophic level showed dilutions of Mps level rather than magnifying in the tissues of fish [78]. However, MPs were found in planktivorous fish according Boerger et al. [79] that biomagnified to bigger predatory feeding on the fish. Biomagnifications have been observed in bluefin tuna, albacore tuna and swordfish in the Mediterranean sea [80]. Mps were found to be transferred tropically from Scombrus scombrus to Halichoerus gypus reported by [81]. Low dendity Mps can be expelled as pseudofeces by fish though mst of the Mps remain in gastrointestinal tract of fish [82-84]. Recent studies in the coastal ecosystem of Bangladesh showed that Mps abundance increases with the increase of trophic level which indicates that transfer of Mps across different trophic levels and also showed the evidence of biomagnifications of Mps in successive trophic levels [85]. Environmental monitoring of MPs occurrence in aquatic ecosystems has received increased attention in recent times because of reports identifying MPs as vectors that may enhance the transfer of several pathogens and contaminants of emerging concern (including polycyclic aromatic hydrocarbons, bisphenol-A and polychlorinated biphenyls) in aquatic environment and biota [86-92]. Several reports have focused on identification and characterization of MPs in different aquatic environment and biota matrices in order to determine the extent and severity of plastic pollution. These legitimate concerns and the toxic impacts of MPs for aquatic organisms and human health is supported by the fact that adsorbed pollutants may leach out upon ingestion, providing routes for secondary toxicity due to bioaccumulation and biomagnification along the food web [90,93,94]. Also, as demonstrated by Adeogun et al. [68] there is a relatively high prevalence (69.7%) of Mps ingestion in fish species in a lwater body in southwest, Nigeria compared to the studies of Boerger et al. [79] who reported 35% MPs prevalence in planktivorous fishes rom the North Pacific Gyre of California, 33%prevalence in fish from the Goiana estuary in Brazil [95], while 18.2% prevalence was reported from the Mediterranean Sea, Messina Italy [80]. These variations could be attributed to the different features of the aquatic ecosystem being studied. Most of these reports have used marine and brackish water fish species with characteristic high-water volume, flow rate, wave action, and serial dilution of materials, while our study was on a freshwater lentic lake characterized by low dilution and flow rate, highlighting the possibility of the observed MPs prevalence differences. However, high MPs observed in fish stomach in Adeogun et al. [68] studies compared with the above-mentioned reports could be related to the anthropogenic pressure faced by the different ecosystems; for example, large surrounding land-areas characteristic of lacustrine ecosystems may increase the possibility of anthropogenic inputs from adjacent terrestrial environments, compared with oceans and estuaries. This argument is supported by the fact that 80% of plastic wastes into the aquatic environment originate from littering on land [52].
Possible health impacts on humans
Microplastics are regarded as a global issue due to their toxicity effects on fish and humans. A number of studies have proposed that consumption of fish and other sea foods that has prior ingestion of microplastics can pose danger to humans. One potential consequence of this accumulation is the bioaccumulation of microplastics in fish. When consumed by humans, these fish can transfer the plastics to our bodies. A growing body of evidence suggests that ingesting microplastics can have harmful effects on both human health and the environment. Microplastics are found in many species intended for human consumption including invertebrates, crustaceans and fish [74]. Plastic particles are often found concentrated in an organism’s digestive tracts such that bivalves and small fish consumed whole are more likely to expose microplastics to the human diet [9]. Swelling and blockage are caused due to the buildup of MPs and nanoplastics in tissues [66]. In vitro tests that MPs concentrate in the gills, stomachs and metabolic systems of crabs and cause unfavorable cellular alterations in fish [96]. Microorganisms and pollutants have also been shown to be transported by them [97]. Negative consequences of MPs were mostly determined by the individual’s level of exposure and sensitivity [98]. MPs are long-lasting in the ecosystem and biological organisms, therefore, the animals are exposed to MPs for an extended period, potentially leading to chronic discomfort, welling, cell growth and death as well as immune cell impairment [99]. Inflammatory bowel disease was significantly higher in patients with MPs than the healthy people [100].
Knowledge Gaps/Recommendation
A larger percentage of the studies reported in this review were carried out in developed countries like Asia and Europe. There is paucity of published articles on assessment and impact of microplastics in aquatic environment in Nigeria. Also, there is need for more studies to establish biomagnification or bioaccumulation of MPs across trophic level and the possibility of MPs being transferred into humans via ingestion of fish or other aquatic fauna polluted with MPs. It is therefore recommended that more studies be done to assess presence of MPs in aquatic ecosystem, its impacts on fish health and human as a consumer of fish polluted with MPs.
Conclusion
Aquatic environment is naturally at the receiving end of pollution that occur in other environment which includes plastic pollutants. The disruption of water bodies by microplastics is of great concern due to its ecological and health impacts. Plastics makes up bulk of items domestically used on a daily basis and its mismanagement, improper handling as well as its abuse has resulted in MPs pollution in aquatic environment. MPs are enormous in aquatic environment which gives fish species easy access to them. A growing body of research demonstrates that MPs are toxic to a wide range of fish and can cause a number of health damages to fish and humans that consume plastic polluted fish are predisposed to health challenges that may result from microplastics pollution. Efficient waste management should be employed as well as reducing human dependence on the use of plastics will go a long way in curbing Mps pollution in aquatic ecosystems. In addition, awareness and implementation of ways to reduce pollution of the aquatic environments with microplastics is highly recommended. More research is needed to determine the health effects of consuming fish with microplastics in their tissues. However, as the amount of plastic in our environment continues to grow, it is important to be aware of the potential risks associated with eating seafood. Human exposure to microplastics can be reduced by buying sustainable seafood and avoiding processed foods that may contain plastics additives.
References
- Enyoh CE, Verla AW, Verla EN, Ibe FC, Amaobi CE (2019a) Airborne Micro-plastics: a Review Study on Method for Analysis, Occurrence, Movement and Risks. Environ Monit Assess 191(11): 668.
- Verla AW, Enyoh CE, Verla EN (2019) The importance of microplastics pollution studies in water and soil of Nigeria ecosystems. Anal Method Environ Chem J 2(3): 89-96.
- Gregory MR (2009) Environmental implication of plastic debris in marine setting-Entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philo Trans R Soc B Biol Sci 364(1526): 2013-2025
- Lozano RL, Mouat J (2009) Marine Litter in the North-East Atlantic Region: Assessment and Priorities for Response. KIMO International.
- Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, et al. (2013) Microplastic Ingestion by Zooplankton. Environ Scien & Technol 4(12): 6646-6655.
- Boucher J, Friot D (2017) Primary microplastics in the oceans: A global evaluation of sources.
- Kovochich M, Liong M, Parker JA, Oh SC, Lee JP, et al. (2021) Chemical mapping of tire and road wear particles for single particle analysis. Science of the Total Environment 757: 144085.
- Conkle JL, Báez DV, Christian D, Turner JW (2018) Are We Underestimating Microplastic Contamination in Aquatic Environments? Environ Manag 61(1): 1-8.
- Eriksen M, Lebreton LC, Carson HS, Thiel M, Moore CJ, et al. (2014) Plastic pollution in the world's oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoSone 9(12): e111913.
- Haward M (2018) Plastic pollution of the world’s seas and oceans as a contemporary challenge in ocean governance. Nat Commun 9(1): 9994.
- Watson RA, Nowara GB, Hartmann K, Green BS, Sean RT, et al. (2015) Marine foods sourced from farther as their use of global ocean primary production increases. Nat Commun 6(7365).
- Galloway TS, Cole M, Lewis C (2017) Interactions of microplastic debris throughout the marine ecosystem. Nat Ecol Evol 1(5): 116.
- Walkinshaw C, Lindeque PK, Thompson R, Tolhurst T, Cole M (2020) Microplastics and seafood: Lower trophic organisms at highest risk of contamination. Ecotoxicol Environ Saf 190: 110066.
- Lusher A (2015) Microplastics in the marine environment, distribution, interactions and effects. In Marine Anthropogenic Litter, pp. 245-307.
- Collard F, Gilbert B, Eppe G, Roos L, Compere P, et al. (2017) Morphology of the filtration apparatus of three planktivorous fishes and relation with ingested anthropogenic particles. Mar Pollut Bull 116(1-2): 182-191.
- Lihui An, Qing Liu, Yixiang Deng, Wennan wu, Yiyao Gao, et al. (2020) The handbook of environmental chemistry. Book series Vol 95. Microplastics in Terrestial Environment, pp. 143-159.
- Enyoh CE, Verla AW (2019) We are breathing Plastic; Don’t Just Look down, Look up. Presented at the 3rd IMSU World Environment Day International Conference.
- Verla AW, Enyoh CE, Verla EN (2019) The importance of microplastics pollution studies in water and soil of Nigeria ecosystems. Anal Method Environ Chem J 2(3): 89-96.
- Verla AW, Verla EN, Ajero CM, Lele KC, Stellamarris NO, et al. (2019b) Biomonitoring of Heavy Metals in Blood and Urine of African Children from Owerri Metropolis, Eastern Nigeria. J Chem Health Risk 9(1): 11-26.
- Desforges JP, Galbraith M, Dangerfield N, Ross PS (2014) Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar Pollut Bull 79(1-2): 94-99.
- Elena E (2017) Plastic pollution on the Baltic beaches of Kaliningrad region, Russia. Mar Pollut Bull 114(2): 1072-1080.
- Tiwaria M, Rathoda TD, Ajmala PY, Bhangarea RC, Sahua SK (2019) Distribution and characterization of microplastics in beach sand from three different Indian coastal environments. Mar Pollut Bull 140: 252-273.
- Dekiff JH, Remy D, Klasmeier J, Fries E (2014) Occurrence and spatial distri-bution of microplastics in sediments from Norderney. Environ Pollut 186: 248-256.
- Hirai H, Tkada H, Ogata Y, Yamashita R, Mizukawa K, et al. (2011) Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar Pollut Bull 62(8): 1683-1692.
- Enyoh CA., Verla AW, Verla EN, Ihenetu SC (2019) Macrodebris and microplastics pollution in Nigeria: First report on abundance, distribution and composition. Environmental Analysis Health and Toxicology 34(4): e2019012- e2019020.
- Briggs E, de Moura EAB, Furusawa HA, Cotrim MEB, Oguzie EE, et al. (2019) Microplastics: A Novel Method for Surface Water Sampling and Sample Extraction in Elechi Creek, Rivers State, Nigeria. Characterization of minerals, metals and materials. The minerals, metals and materials society, pp. 269-281.
- Akindele EO, Sonja ME, Jochen HE (2019) First empirical study of freshwa-ter microplastics in West Africa using gastropods from Nigeria as bio-indicators. Limnologica 78: 125708.
- Babayemi OJ, Ogundiran MB, Weber R, Osibanjo O (2018) Initial inventory of plastic import in Nigeria, bias for more sustainable management policies. J Health Pollut 8(18): 180601.
- Dumbili E, Henderson L, Letcher TM (2020) The challenge of plastic pollution in Nigeria, in: Plastic Waste and Recycling. Academic Press, pp. 569-583.
- Bashir NH (2013) Plastic problem in Africa. J Vet Res 61: S1-S11.
- Adeniran AE, Nubi AT, Adelopo AO (2017) Solid waste generation and characterization in the University of Lagos for a sustainable waste management. Waste Manag 67: 3-10.
- Okeniyi JO, Udonwan AE (2012) Solid wastes generation in Covenant University, Ota, Nigeria: characterisation and implication for sustainable waste management. J Mater Environ Sci 3(2): 419-424.
- Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M (2012) Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ Sci Technol 46(6): 3060-3075.
- Claessens M, Van Cauwenberghe L, Vandegehuchte MB, Janssen CR (2013) New techniques for the detection of microplastics in sediments and field collected organisms. Marine Pollution Bulletin 70(1-2): 227-233.
- Imhof HK, Schmid J, Niessner R, Ivleva NP, Laforsch C (2012) A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnology and Oceanography-Methods 10(7): 524-537.
- Nuelle MT, Dekiff JH, Remy D, Fries E (2014) A new analytical approach for monitoring microplastics in marine sediments. Environmental Pollution 184: 161-169.
- Hanke G, Galgani F, Werner S, Oosterbaan L, Nilsson P, et al. (2013) MSFD GES technical subgroup on marine litter. Guidance on monitoring of marine litter in European Seas. Luxembourg: Joint Research Centre–Institute for Environment and Sustainability, Publications Office of the European Union.
- Norén F, Naustvoll LJ (2010) Survey of microscopic anthropogenic particles in Skagerrak. Klimaog forurensningsdirektoratet TA, 2779-2011, pp. 1-20.
- Eriksen, M. Maximenko N. Thiel M., Cummins A., Lattin G., et al. (2013) Plastic pollution in the South Pacific subtropical gyre. Mar Pollut Bull 68(1-2): 71-76.
- Doyle MJ, Watson W, Bowlin NM, Sheavly SB (2011) Plastic particles in coastal pelagic ecosystems of the Northeast Pacific Ocean. Marine Environmental Research 71(1): 41-52.
- Law KL, Moret-Ferguson S, Maximenko NA, Proskurowski G, Peacock EE, et al. (2010) Plastic accumulation in the North Atlantic subtropical gyre. Science 329(5996): 1185-1188.
- Carpenter EJ, Smith KL (1972) Plastics on the Sargasso Sea surface. Science 175(4027): 1240-1241.
- Colton JB, Knapp FD, Burns BR (1974) Plastic particles in surface waters of the northwestern Atlantic. Science 185(4150): 491-497.
- Ng KL, Obbard JP (2006) Prevalence of microplastics in Singapore’s coastal marine environment. Marine Pollution Bulletin 52(7): 761-767.
- Dubaish F, Liebezeit G (2013) Suspended microplastics and black carbon particles in the Jade system, Southern North Sea. Water, Air, and Soil pollution 224(2): 1-8.
- Thompson RC, Olsen Y, Russell AE, Davis A, Rowland SJ, et al. (2004) Lost at sea: Where is all the plastic? Science 304(5672): 838-838.
- Moore CJ, Moore SL, Weisberg SB, Lattin GL, Zellers AF (2002) A comparison of neustonic plastic and zooplankton abundance in Southern California’s coastal waters. Marine Pollution Bulletin 44(10): 1035-1038.
- McDermid KJ, McMullen TL (2004) Quantitative analysis of small-plastic debris on beaches in the Hawaiian archipelago. Marine Pollution Bulletin 48(7-8): 790-794.
- Löder MGJ, Kuczera M, Mintenig S, Lorenz C, Gerdts G (2015) FPA-based micro- FTIR imaging for the analysis of microplastics in environmental samples. Environmental Chemistry 12(5): 563-581.
- Cooper DA, Corcoran PL (2010) Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Marine Pollution Bulletin 60(5): 650-654.
- Liebezeit G, Dubaish F (2012) Microplastics in beaches of the East Frisian Islands Spiekeroog and Kachelotplate. Bulletin of Environmental Contamination and Toxicology 89(1): 213-217.
- Andrady AL (2011) Microplastics in the marine environment. Marine Pollution Bulletin 62(8): 1596-1605.
- Cole M, Webb H, Lindeque PK, Fileman ES, Halsband C, et al. (2014) Isolation of microplastics in biota-rich seawater samples and marine organisms. Scientific Reports 4(4528).
- Norén F (2007) Small plastic particles in coastal Swedish waters. Lysekil, Sweden: KIMO Report Sweden, N-Research.
- Fries E, Dekiff JH, Willmeyer J, Nuelle MT, Ebert M, et al. (2013) Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environmental Science-Processes & Impacts 15(10): 1949-1956.
- Van Cauwenberghe L, Vanreusel A, Mees J, Janssen CR (2013) Microplastic pollution in deep-sea sediments. Environmental Pollution 182: 495-499.
- Murray F, Cowie PR (2011) Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Marine Pollution Bulletin 62(6): 1207-1217.
- Imhof HK, Ivleva NP, Schmid J, Niessner R, Laforsch C (2013) Contamination of beach sediments of a subalpine lake with microplastic particles. Current Biology 23(19): R867-R868.
- Vianello A, Boldrin A, Guerriero P, Moschino V, Rella R, et al. (2013) Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuarine, Coastal and Shelf Science 130: 54-61.
- Harrison JP, Ojeda JJ, Romero-Gonzalez ME (2012) The applicability of reflectance micro-Fourier-transform infrared spectroscopy for the detection of synthetic microplastics in marine sediments. Science of the Total Environment 416: 455-463.
- Frias J, Sobral P, Ferreira AM (2010) Organic pollutants in microplastics from two beaches of the Portuguese coast. Marine Pollution Bulletin 60(11): 1988-1992.
- Reddy MS, Basha S, Adimurthy S, Ramachandraiah G (2006) Description of the small plastics fragments in marine sediments along the Alang-Sosiya ship-breaking yard, India. Estuarine Coastal and Shelf Science 68(3-4): 656-660.
- Corcoran PL, Biesinger MC, Grifi M (2009) Plastics and beaches: A degrading relationship. Marine Pollution Bulletin 58(1): 80-84.
- FAO (2016) The state of the worlds fisheries and aquaculture, pp. 4-10.
- Witte BD, Devriese L, Bekaert K, Hoffman S, Vandermeersch G, et al. (2014) Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar Pollut Bull 85(1): 146-155.
- Wright SL, Thompson RC, Galloway TS (2013) The Physical Impacts of MPs on marine Organisms: a Review. Environ Pollut 178: 483-492.
- Moore CJ (2008) Synthetic Polymers in the Marine Environment: A Rapidly Increasing long term threat. Environmental Research 108(2): 131-139.
- Adeogun AO, Ibor OR, Khan EA, Chukwuka AV, Omogbemi ED, et al. (2020) Detection and occurrence of microplastics in the stomach of commercial fish species from a municipal water supply lake in southwestern Nigeria. Environmental Science and Pollution Research 27: 31035-31045.
- Watts AJ, Lewis C, Goodhead RM, Beckett SJ, Moger J, et al. (2014) Uptake and retention of microplastics by the shore crab Carcinus maenus. Environ Sci Technol 48(15): 8823-8830.
- GESAMP (2010) Proceedings of the GESAMP international workshop on plastic particles as a vector in transporting persistent, bio-accumulating and toxic substances in the oceans, 28–30th June 2010, UNESCO-IOC Paris. In: T Bowmer, PJ Kershaw (Eds.), GESAMP Reports and Studies, p. 68.
- Bhattacharya P, Lin S, Turner JP, Ke CP (2010) Physical Adsorption of charged plastic nanoparticles affects algal photosynthesis. J Phy Chem C 114(39): 16556-16561.
- Lee J, Hong S, Song Y, Hong S, Janga Y, et al. (2013) Relationships among the abundances of plastic debris in different size classes on beaches in South Korea. Marine Pollution Bulletin 77(1-2): 349-354.
- Pott A (2014) A new method for the detection of microplastics in the North Sea brown shrimp (Crangon crangon) by Fourier Transform Infrared Spectroscopy (FTIR). M.Sc. thesis, RWTH Aachen University/Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research, p. 61.
- Van Cauwenberghe L, Janssen CR (2014) Microplastics in bivalves cultured for human consumption. Environmental Pollution 193: 65-70.
- Eriksson C, Burton H (2003) Origins and biological accumulation of small plastic particles in fur seals from Macquarie Island. AMBIO: A Journal of the Human Environment 32(6): 380-384.
- Farrell P, Nelson K (2013) Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environmental Pollution 177: 1-3.
- Galloway TS (2015) Micro- and nano-plastics and human health. In: M Bergmann, L Gutow, M Klages (Eds.), Marine anthropogenic litter. Springer, Berlin, pp. 347-370.
- Akhbarizadeh R, Moore F, Keshavarzi B (2019) Investigating microplastics bioaccumulation and biomagnification in seafood from the Persian Gulf: a threat to human health? Food Additives & Contaminants: Part A 36(11): 1696-1708.
- Boerger CM, Lattin GL, Moore SL, Moore CJ (2010) Plastic Ingestion by Planktivorous Fishes in the North Pacific Central Gyre. Mar Pollut Bull 60(12): 2275-2278.
- Romeo T, Pietro B, Pedà C, Consoli P, Andaloro F, et al. (2015) First Evidence of Presence of Plastic Debris in Stomach of Large Pelagic Fish in the Mediterranean Sea. Mar Pollut Bull 95(1): 358-361.
- Nelms SE, Barnett J, Brownlow A, Davison NJ, Deaville R, et al. (2019) MPs in marine Mammals Stranded Around the British Coast: Ubiquitous but Transitory? Scientific Rep 9(1): 1075.
- Capone A, Petrillo M, Misic C (2020) Ingestion and Elimination of Anthropogenic Fibres and Microplastic Fragments by the European Anchovy (Engraulis Encrasicolus) of the NW Mediterranean Sea. Mar Biol 167(11): 1-15.
- Zhang C, Wang J, Pan Z, Wang S, Zhang L, et al. (2021c) A Dosage-Effect Assessment of Acute Toxicology Tests of Microplastic Exposure in Filter-Feeding Fish. Fish Shellfish Immunol 113: 154-161.
- Prata JC, da Costa JP, Duarte AC, Rocha-Santos T (2022) Suspected Microplastics in Atlantic Horse Mackerel Fish (Trachurus trachurus) Captured in Portugal. Mar Pollut Bull 174: 113249.
- Sarker S, Huda ANM, Nazmul HN, Chowdhury GW (2022) Trophic transfer of microplastics in the aquatic ecosystem of sundarbans mangrove forest, Bangladesh. Science of the Total Environment 838(2): 155896.
- Williams A, Simmons S (1996) The degradation of plastic litter in rivers: implications for beaches. J Coast Conserv 2(1): 63-72.
- Batel A, Linti F, Scherer M, Erdinger L, Braunbeck T (2016) Transfer of benzo[a] pyrene frommicroplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants: trophic transfer of microplastics and associated POPs. Environ Toxicol Chem 35(7): 1656-1666.
- Endo S, Takizawa R, Okuda K, Takada H, Chiba K, et al. (2005) Concentration of polychlorinated biphenyls(PCBs) in beached resin pellets: variability among individual particles and regional differences. Mar Pollut Bull 50(10): 1103-1114.
- Carson HS, Nerheim MS, Carroll KA, Eriksen M (2013) The plastic associated microorganisms of the North Pacific gyre. Mar Pollut Bull 75(1-2): 126-132.
- Koelmans AA, Bakir A, Burton GA, Janssen CR (2016) Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies. Environ Sci Technol 50(7): 3315-3326.
- Viršek MK, Lovšin MN, Koren Š, Kržan A, Peterlin M (2017) Microplastics as a vector for the transport of the bacterial fish pathogenspecies Aeromonas salmonicida. Mar Pollut Bull 125(1-2): 301-309.
- Pittura L, Avio CG, Giuliani ME, d'Errico G, Keiter SH, et al. (2018) Microplastics as vehicles of environmental PAHs to marine organisms: combined chemical and physical hazards to the Mediterranean mussels, Mytilus galloprovincialis. Front Mar Sci 5: 103.
- Ziccardi LM, Edgington A, Hentz K, Kulacki KJ, Kane DS (2016) Microplastics as vectors for bioaccumulation of hydrophobic organic chemicals in the marine environment: a state-of-the-science review. Environ Toxicol Chem 35(7): 1667-1676.
- Endo S, Koelmans AA (2016) Sorption of hydrophobic organic compounds to plastics in marine environments: equilibrium. In: Takada H, Karapanagioti HK (Eds.), Hazardous chemicals associated with plastics in the marine environment. Hdb Env Chem, Springer International Publishing, Switzerland, pp. 1-20.
- Possatto FE, Barletta M, Costa MF, do Sul JA, Dantas DV (2011) Plastic debris ingestion by marine catfish: an unexpected fisheries impact. Mar Pollut Bull 62(5): 1098-1102.
- Karbalaei S, Hanachi P, Walker TR, Cole M (2018) Occurrence, Sources, Human Health Impacts and Mitigation of Microplastic Pollution. Environ Sci Pollut Res 25(36): 36046-36063.
- Manzoor J, Sharma M, Sofi IR, Dar AA (2020) Plastic Waste Environmental and Human Health Impacts in Handbook of Research on Environmental and Human Health Impacts of Plastic Pollution (Hershey, USA: IGI Global), pp. 29-37.
- Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T (2020) Environmental Exposure to MPs: An Overview on Possible Human Health Effects. Sci Total Environ 702: 134455.
- Smith M, Love DC, Rochman CM, Neff RA (2018) MPs in Seafood and the Implications for Human Health. Curr Environ Health Rep 5(3): 375-386.
- Yan Z, Liu Y, Zhang T, Zhang F, Ren H, et al. (2021) Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ Sci Technol 56(1): 414-421.






























