Age and Growth of Tub Gurnard Chelidonichthys lucerna (Linnaeus, 1758) during Estuarine Occupation of a Temperate Atlantic Nursery
J Campos1*, S Costa-Dias1,2, A Bio1, P T Santos3 and I Jorge4
1 CIIMAR – UP, Interdisciplinary Centre of Marine and Environmental Research of the University of Porto, Portugal
2 ICBAS – UP, Institute of Biomedical Sciences - University of Porto, Portugal
3 FCUP – UP, Faculdade de Ciências da Universidade do Porto, R. Campo Alegre, Portugal
4 IPMA, Instituto Português do Mar e da Atmosfera (former IPIMAR – CripCentro), Canal das Pirâmides, Portugal
Submission: October 08, 2021; Published: September 12, 2022
*Corresponding author: J Campos, CIIMAR – UP, Interdisciplinary Centre of Marine and Environmental Research of the University of Porto, Terminal de Cruzeiros do Porto de Leixões, Av. General Norton de Matos, 4450-208 Matosinhos, Portugal
How to cite this article: J Campos, S Costa-Dias, A Bio, P T Santos, I Jorge. Age and Growth of Tub Gurnard Chelidonichthys lucerna (Linnaeus, 1758) 02 during Estuarine Occupation of a Temperate Atlantic Nursery. Int J Environ Sci Nat Res. 2022; 31(1): 556304. DOI 10.19080/IJESNR.2022.31.556304
Abstract
The tub gurnard Chelidonichthys lucerna is a by-catch species with an emerging economic importance, requiring information for a sustainable management. Modal progression analysis, otolith reading, length-at-age back-calculation and the von Bertalanffy model were used to trace growth patterns of C. lucerna in the Mondego estuary (Portugal) during an annual cycle. All sampled gurnards (480 individuals, 4.7-31.8cm total length, 0-5 years old) were immature and had a stable Fulton’s condition index (1.01±0.11), consistent with a nursery use of the area. Up to 5 false rings before the first winter ring appeared on 63% of the otoliths and were hypothesized to refer to ontogenic events and fluctuation in prey resources. Most juveniles entered the estuary in spring, when temperature and salinity increased, remained there for 1-2 years and migrated seawards in autumn/early winter. Older gurnards (2-5 years old) entered sporadically into Mondego, mainly in autumn and winter. During the estuarine period, length-weight relationship of the gurnards followed the equation W=0.0105*TL2.98 (r2=0.9935) and growth can be described according to the following von Bertalanffy equation:
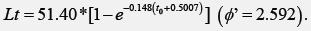
This is the first comprehensive study developed in the Atlantic region focusing on population growth patterns of young tub gurnard during the period of estuarine occupation.
Keywords: Otoliths; Tub gurnard; Nursery; Atlantic region; Von Bertalanffy growth model
Introduction
The tub gurnard Chelidonichthys lucerna is a marine fish species with a high nutritional value [1]. Besides a by-catch species in demersal fisheries [2,3], it has an emerging commercial importance in Europe, mainly due to the decline in the stocks of the traditionally targeted species [4-6]. Tub gurnards co-occur with other Triglidae with commercial interest (e.g., grey gurnard Eutrigla gurnardus), both in the Atlantic [7] and in the Mediterranean [8], which are often landed together [2]. Only 4 countries of the ICES area, however, sort gurnard landings by species at fish markets, which hinders any stock management [2,9]. Portuguese fisheries data confirm that the tub gurnard is the most valuable and frequent of the six Triglidae species landed in Portugal [9], and is the largest of them [10], though gurnards sorting at Portuguese Fish Auctions is mostly based on size, rather than on accurate species identification.
Recently, studies on otolith shape and elemental composition have revealed that tub gurnards from centre and north of Portugal belong to a single population unit that should not be managed separately [11]. Otoliths are sound transducers and play an important role in fish hearing, which is particularly relevant for gurnards, as they produce different sounds, especially under competitive feeding conditions [12,13]. The sagittal otoliths of gurnards are relatively small [14], approximately oval or triangular to oval [15]. Since its shape is species-specific [16,17], variation in otolith morphology has been used to discriminate phenotypic markers between Triglidae species, including C. lucerna [18-21]. The ultrastructure and morphology of otoliths can even reveal differences between C. lucerna juveniles and adults [21]: as the fish grow the otoliths show a relative elongation of the sulcus acusticus, an increase of excisura ostii and in structure complexity, while the fraction of organic matter decreases [18,21].
The most common utility of otoliths, however, is in fish age determination, a very useful tool for fisheries management [16,22]. The otolith’s biomineral formation is physiologically controlled and affected by environmental conditions such as temperature and prey availability [23,24]. A seasonal pattern is evident and is closely related to the changes in prey abundance and quality: an opaque ring is laid when food is abundant (spring and summer), and a translucent ring is formed when food scarce (autumn and winter) [23]. This results in optically distinct zones that can be related with the annual cycle of material deposition and otolith growth. The analysis of the alternation of rings in the macrostructure of otoliths is a reliable and routine method of aging fish, whereby a consecutive pair of hyaline and opaque rings is assumed to correspond to an annual cycle, i.e., one year of age [23].
Most information on C. lucerna has been collected in the Mediterranean Sea, where it also sustains an important fishery. In relation to Atlantic populations, a few studies have described the length-weight relationship for several Triglidae species, including C. lucerna in Portuguese waters [25-27]. From the very few studies on its growth, only one estimated the age from the fish scales [28], while most others have used the otoliths for ageing purposes. Apart from the McCarthy & Marriott [6] research in UK coastal waters, no detailed investigation has been conducted on the population biology of tub gurnard in the European Atlantic coast since the work by Baron [29,30] in the North of France. It is then evident, there is a lack of knowledge with respect to the Atlantic region, despite its need for a successful fisheries management. Namely, no information is available for the particularly sensitive period of estuarine occupation during the first years of life. This paper provides the first detailed study of the patterns of growth of young tub gurnard through otolith analysis, for a population from temperate estuarine waters of the Northeastern Atlantic coast. Four methods modal progression analysis, marginal increment analysis, the back-calculation method (for a review, see [31,32]), along with the von Bertalanffy growth model, were used to examine the growth over an annual cycle, and to compare with the existing data for southerly Mediterranean and northerly UK and French populations.
Material and Methods
Sample collection
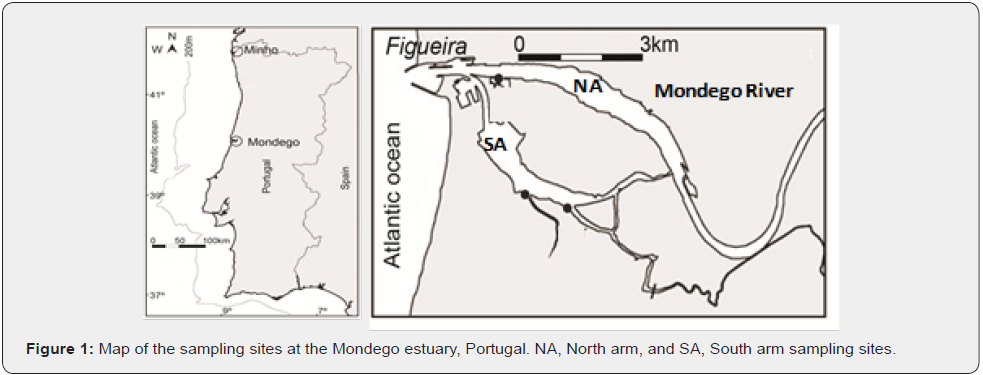
Fish were collected in two sandy sites of Mondego estuary: near the commercial harbor in the North arm (NA), and in Gala in the South arm (SA) of the river (Figure 1). The NA is deeper because the area is frequently dredged for navigation and has a larger influence of both saline intrusion from downstream, and fresh water from upstream. In contrast, the SA is silted up, and difficult to access by boat, has a larger marine influence and a greater thermal and saline homogeneity. Nevertheless, their environmental features are quite similar to the point of being considered belonging to the same spatial unit [33]. A total of nine sampling campaigns were done between July 1999 and July 2000, in each of the two first months of each season, covering the four seasons. Sampling took place during low water of spring tides. Each campaign consisted of three hauls with a 57m (3m high, 0.8cm mesh size) beach seine at the two sites; at the NA site, an extra haul was conducted with a 5.1m bottom trawl (1.8cm mesh size) during 2h only. Additionally, temperature and salinity were determined with appropriate probes, and water transparency was determined with a Secchi disc.
All animals were transported in cooling boxes and frozen in the lab. After defrosting, the total length (TL) of all tub gurnards was measured to the nearest mm. A length-stratified subsample consisting of the first four fish in each 1cm size class were retained for dissection. For this subsample, the wet weight (WW, g) and the weight of the eviscerated fish (We, g) were also registered. Whenever possible (presence of gonads), sex and maturity status were confirmed macroscopically by visual inspection of the gonads following [34]. For each individual, the sagittal otoliths were removed, cleaned and stored dried in paper envelopes for later aging. Age determination was performed by counting the number of rings (i.e., outer edges of the opaque zones) and analysis of its margin (opaque versus hyaline) following [35] and [36]. The whole left otolith was observed under binocular stereoscope with the convex side up and read under reflected light against a black background, always at the same magnitude, and immersed in 70% alcohol. Photographs were taken with a JVC TK-C1380 digital camera coupled to a Leica binocular stereoscope and digital image analysis was performed with the software Leica Qwin 500. Each consecutive pair of opaque (dark) and hyaline (light) rings together were considered as an annual growth increment [29,36,37]. At least two readings were performed to each otolith to confirm the age. The otoliths were rejected whenever the third or fourth reading were not coincident. Age determination was validated by the analysis of the otolith margin, confirming the ring formation by the deposition of an opaque and the formation of a hyaline area per year. The radius of the otolith was taken from the distance between the nucleus and the rostrum extremity because this presented a higher contrast between otolith rings, as also observed by [38]. The distance between otolith rings was also measured in this same line.
Data analysis
Observations on TL and WW (and We) were used to establish the length-weight relationships through regression, both for the overall population and separately for each sex, using the allometric growth equation W=aTLb [39,40], where W is the WW or the We, and a and b are constants. The same data were used to calculate the Fulton’s condition factor (K): K =100*WW / TL3.
The growth of the gurnards was studied through three models: 1) the modal class progression based on the analysis of the temporal series of length distributions [41]; 2) back-calculation through regression between the fish length and the otolith radius; and 3) through the von Bertalanffy growth model [40,42]. This last is considered statistically more robust and facilitates comparing with other growth studies [34].
For the modal class progression, the size class histograms were made globally and per season to determine the period of entrance of juveniles into the estuary. ‘Recruitment’ was considered to be the appearance of a new generation in the samples. The Bhattacharya method [41,43] was applied to the histograms to discriminate among normal distributions. Each mode was assumed to represent a cohort from the overall and from the seasonal size–frequency distributions. The separation index among different cohorts was estimated. Values less than 2 indicate a large overlap between cohorts and are considered statistically similar [41]. The same method was used to follow the growth of each cohort throughout the study period, using the software FISAT II [44]. The histograms also allowed validating the otolith readings and the back-calculated lengths-at-age, at least for the first age classes.
After age determination, the animals were grouped into age classes to build the length-at-age key. Back-calculation was used to reconstruct data for growth analysis, as it allows for an inference of the length of a fish at younger ages that may not be represented in the sampling, thereby increasing the number of length-at-age data to be used in fitting a growth model to the data. A prerequisite for applying this technique is the existence of a relationship between body length and otolith radius (OR) [31], which was first determined through linear regression.
Growth was described using the von Bertalanffy model equation modified by [45] and [46],
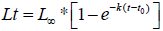
Lt is the length at age t, L∞ is the asymptotic length, k is the growth constant per year, and t0 is the age that corresponds to length zero. The von Bertalanffy growth model was fitted to four sets of data using the software FISPARM [47]: 1) the pairs age-TL i.e., length-at-age observations; 2) the back-calculated lengthsat- age with the different regression equations and respective mean values; 3) the pairs age-mean length; and 4) modal total lengths obtained through the Bhattacharya method. The growth performance index (ϕ’, phiprime) was used to compare fish growth, where φ’ = log10 K + 2*log10 L∞ [48]. It was assumed the first of January as the date of age transition for the following age class (i.e., ‘birthday’) [36].
Results
Environmental characterization
Figure 2 presents the temporal trends in abiotic conditions from summer 1999 to summer 2000. Due to extensive dredging in the area, the NA site was significantly deeper by about the double of the depth of the SA site, all along the study period. Transparency was also significantly higher in the NA (F=4.32, p=0.0026), ranging from 0.70±0.35m in the autumn 1999 to 1.85±0.17m in the summer 2000, while transparency was quite stable in the SA (mean 0.68±0.19m; F=1.14, p>0.05). The two sampling sites did not differ in salinity but salinity in the NA varied over time (F=25.69, p=0.0046), while in the SA seasonal differences were not significant (F=2.80, p>0.05), though varying between 11.9±0.1 and 29.3±0.0. For the NA, salinity ranged between 7.6±6.7 in the rainy spring 2000 and 27.2±4.0 in the dry summer 2000. Trends in temperature were also similar in the two sites (F=0.01, p>0.05) with significant differences between the seasons (NA: F=127.74, p<0.0001; SA: F=9.69, p=0.0037), following the expected fluctuations: lower in winter (min: 10.9±0.6ºC and 10.0±1.6ºC, respectively in the NA and in the SA) and higher in the summer (max: 21.3±0.6ºC and 21.4±0.0ºC, respectively in the NA and in the SA).

A total of 480 gurnards (28.3Kg of biomass) were sampled and measured. Despite 60% of the gurnards were caught in the NA, considering only the catches with the beach seine used in both sites, catches rendered about 5.5 times higher in the SA site, both in number and in total biomass (192 gurnards, 11.7Kg in the SA versus 35 gurnards, 2.1Kg in the NA). Altogether catches were higher in the summer (177 ind, 11.6Kg) and autumn 1999 (99 ind, 10.3Kg), both in number and in biomass, and lower in the winter 2000 in number (46 ind), and in spring 2000 in biomass (1.5Kg) (Figure 3).
Size distribution and modal class progression
The size of the gurnards ranged from 4.7 to 31.8cm TL. More than half of the sampled gurnards were not sexed as they were sexually immature, and hence data was treated globally considering them as juveniles or immature. Table 1 presents the length-weight relationships. For all equations, the exponent ‘b’ was close to 3, typical of isometric growth in the dimensions considered. The Fulton’s condition factor K was stable (i.e., standard deviation <15% of the mean) during the study period with a mean of 1.01±0.11 (F=2.16, p>0.05).
In the overall sample, the most evident modal class was the 18.0cm with 45 gurnards. Four other less evident modal classes could be depicted from the size distribution histogram (Figure 3): 5.0cm, 9.0cm, 23.0cm, and 26.0cm. Yet, the direct analysis of the seasonal histograms did not allow a proper evaluation of the gurnards’ growth due to the low numbers per size class. Moreover,no clear modal class was present in most seasons making it difficult to detect the modal class progression. Nevertheless, in autumn 1999, size range of the gurnards which appeared in the estuary was larger than in summer 1999, though in much lower numbers. In the winter, numbers continued decreasing but even smaller gurnards entered the estuary. They continued entering in higher numbers in the spring 2000, when the modal class appeared to drawback to the 5.0cm and 9.0cm size classes. The modal class proceeded to 16.0cm in the next season (summer 2000). Yet, numbers were lower than the year before because only one month was sampled instead of two.
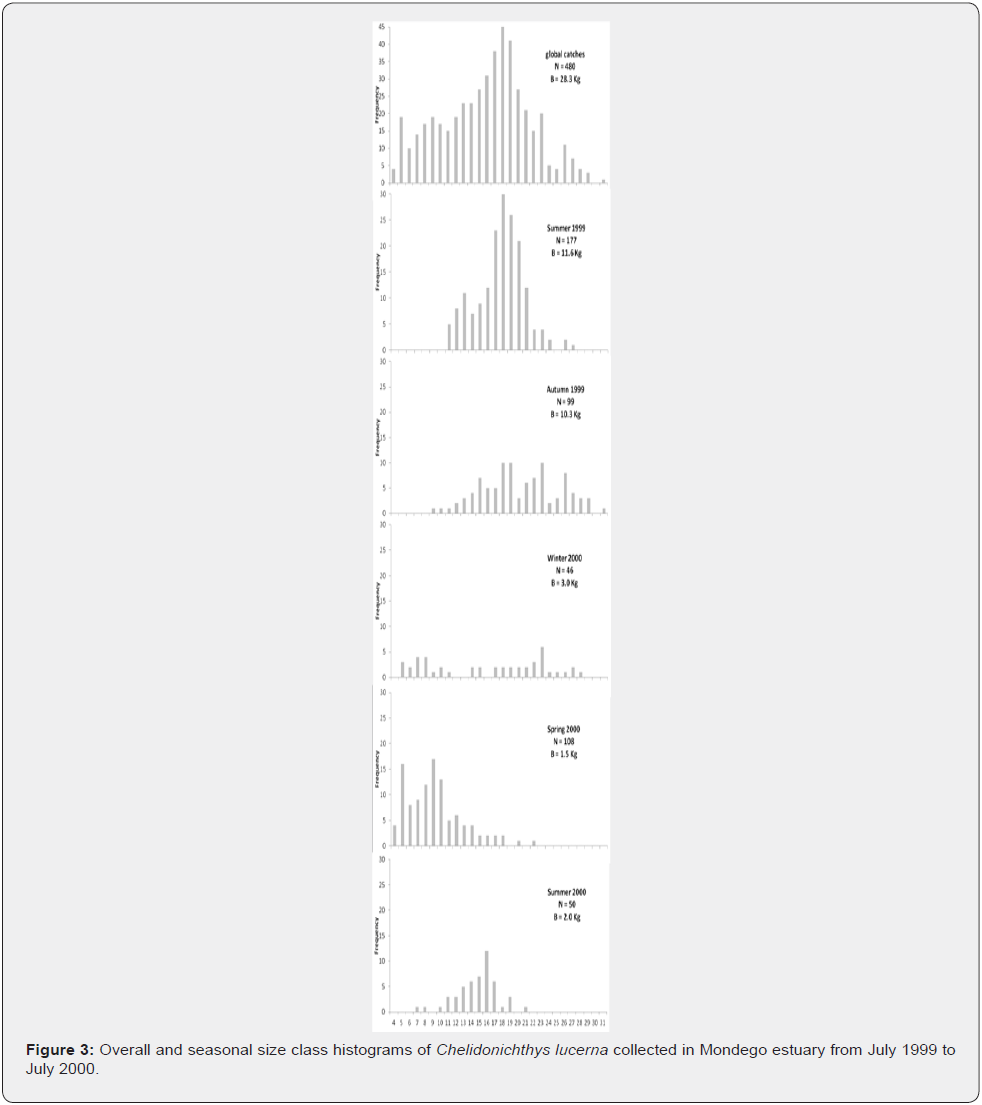
The Bhattacharya method was applied to improve the group discrimination. Modal classes, standard deviation and separation index (SI) are presented in Table 2. These modal classes per season enabled an approximate age to be attributed to the mean modal length (Table 3) which gives already a first approach to the growth of C. lucerna in Mondego estuary. All SI but one were over 2 indicating a statistically-sound separation between the cohorts [41].
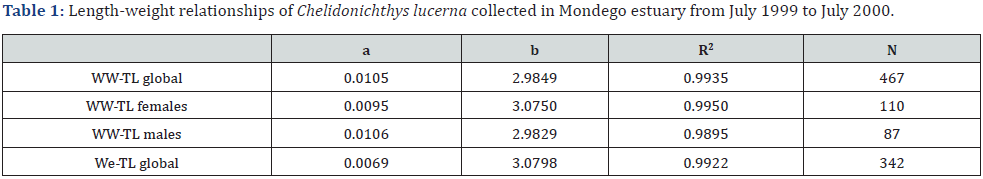
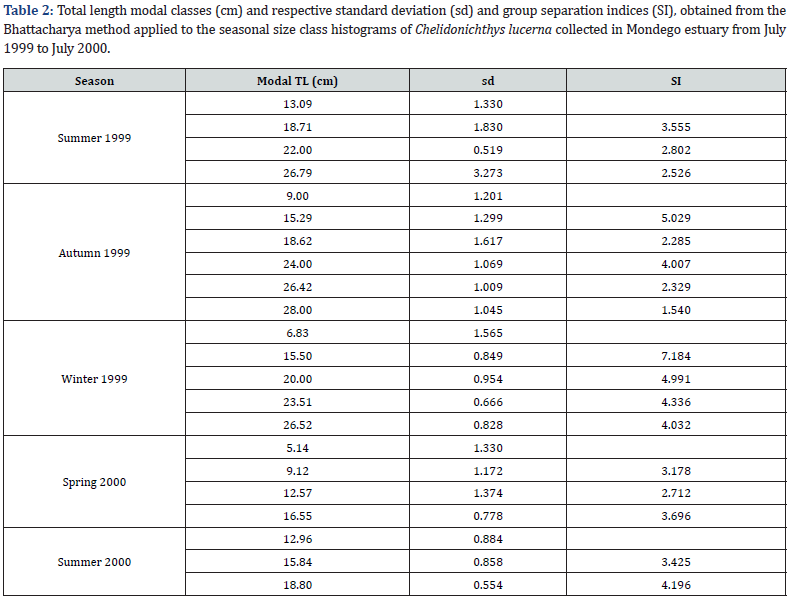
Otoliths’ age estimation
A total of 327 pairs of otoliths were collected. The legibility was 93.7% as 25 otoliths were rejected due to inconsistent readings. For most of the otoliths, three to four readings were necessary due to the presence of false rings.
Most otoliths presented a hyaline margin (always over 75%).
The percentage of otoliths with opaque margin was highest in summer 1999 (30%) but higher in the winter 1999 (24%) than in the following warmer seasons (17-20%) (X2 = 14.45, p<0.01). In the majority of the otoliths, the nucleus was easilly distinguishable, even with no manipulation of the image contrast. The nucleus was located in the otolith center, in the confluence of the sulcus which crosses the otolith. Most otoliths (251, 63% of total) presented false rings, but the distance to the nucleus was only possible to measure in 138 of them (35% of total) due to the opacity of the region. The first false ring appeared close to the nucleus between 215.32 and 621.05μm (mean 470.92±170.83μm) following the relation:
Mean Radius of 1st false ring =1.4935*TL + 447 (r2 = 0.0082, N =138).
Other false rings (up to 5) were observed in the otoliths before the first winter ring, but at lower frequencies: a second false ring occurred in 49%, and a third false ring occurred in 13% of the otoliths. Their distance to the nucleus was not possible to measure because they had no clear edges.
In contrast to the false rings, the ring corresponding to the first winter was always easily distinguished as a hyaline band all around the otolith, larger than any of the false rings. The distance to the nucleus was 386.35 to 1272.71μm (mean = 1064.32±168.78μm). The distance to the nucleus of the subsequent winter rings increased with the increase in the number of rings and the difference was significant (F=444.14, p<0.0001, Figure 4).
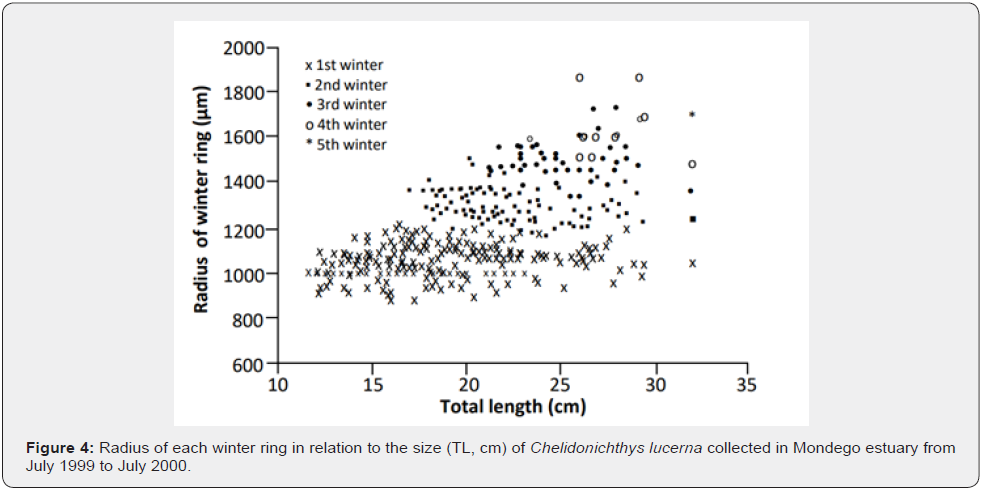
One year of age was attributed to each set of consecutive winter and summer rings. The best represented age classes were age classes 0 and 1, with the number of gurnards per age class decreasing with increasing age with only a single 5 years-old individual (Figure 4), i.e., the same number of age classes as the number of age groups determined by the Bhattacharya method (Table 3). Mean size-at-age (Table 3) was significantly different (F=338.23, p<0.0001) and slightly larger than the ones estimated through the Bhattacharya method.
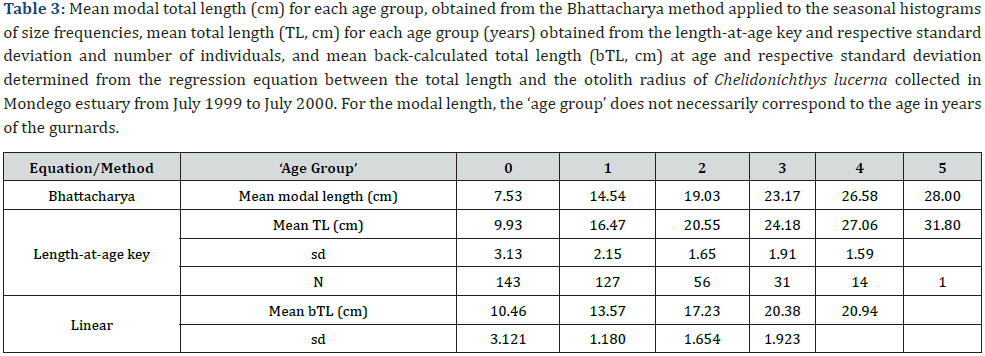
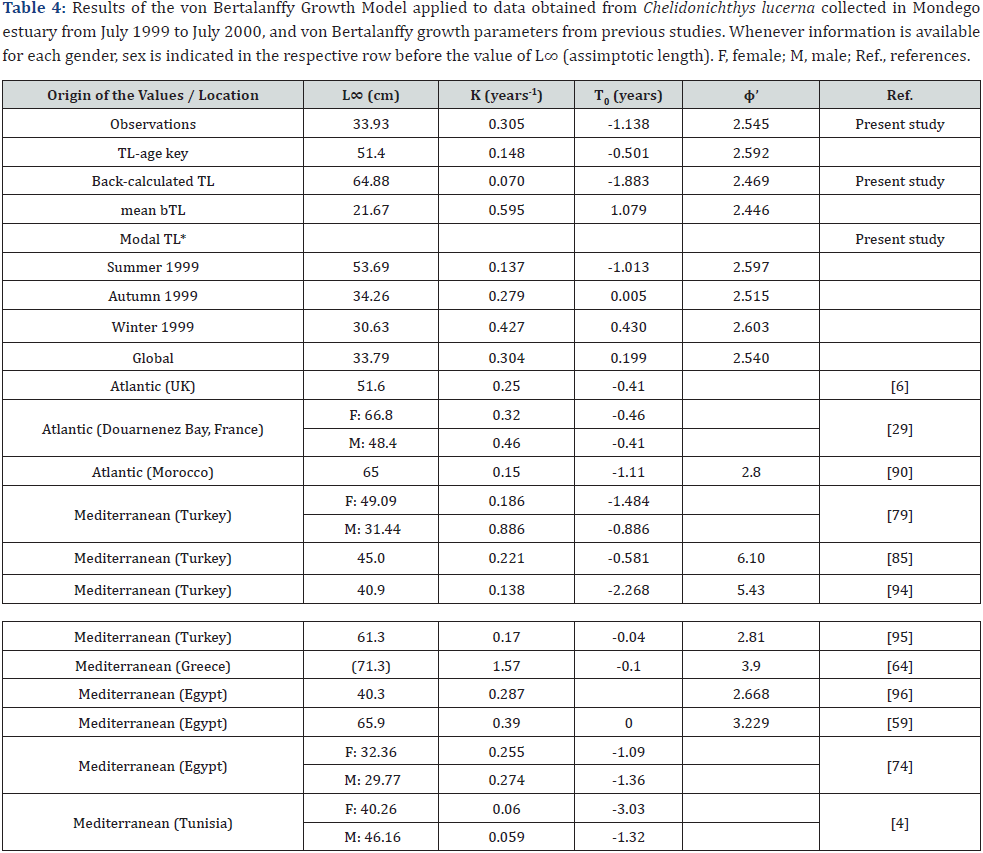
* In Spring 1999 and Summer 2000, data was not sufficient to estimate the modal TL, and hence the von Bertalanffy model was not applied.
The equation describing the relationship between the fish total length (TL, cm) and the otolith radius (OR, μm) was as follows: TL = 0.0619*OR − 6.1201, R2 = 0.9275, N = 302
From this equation, the back-calculated mean lengths and respective standard deviations were determined for each age class (Table 3).
Von Bertalanffy model
The outputs using the von Bertalanffy model for the present study are shown in Table 5, along with similar information from previous studies. Asymptotic length was maximum using backcalculated set of data. Most of the values of the growth constant were over 0.275, while t0 ranged from -1.883 to 1.079. All models had ϕ’ value larger than 2.400, but the models obtained from the observations and from the modal lengths had the higher scores of this index.
Discussion
This work successfully investigated the growth of the tub gurnard C. lucerna in the Mondego estuary. The tub gurnard was present all year round within the estuary as juveniles or immature animals, confirming the use of the area as a nursery ground. The alternation of seasons, and hence of years, marked through hyaline and opaque rings in the otoliths, enabled estimating the fish age. It revealed that estuarine occupation occurred mainly during the first 1-2 years of life. Occasionally older gurnards of up to 5 years old also entered in the estuary. Up to 5 false rings were marked in the otoliths before the first winter ring which might be related with critical periods of the gurnards’ early life. The present study further determined the von Bertalanffy growth parameters for C. lucerna from the Mondego population, which are of upmost importance to develop stock management strategies for sustainable fisheries.
Seasonality in the estuarine occupation
The distribution of tub gurnards seems more closely related with temperature than other abiotic factors such as depth [7]. Yet, a certain degree of spatial segregation exists between juveniles and adults [50]: larger and older adults are often found at depths over 100m - up to 250m in Galician [7] and 320m in Greek waters [51] -, while juveniles prevail at shallower coastal waters up to 20m, including within estuaries [4,20,52-60]. Young gurnards use these shallow areas as nursery grounds, where they mainly feed on the brown shrimp Crangon crangon [61]. In Portugal, the estuaries of the rivers Arade, Tagus and Mondego have been identified as nurseries for the tub gurnard [33,53,60,62,63].
In the present work, the gurnards caught in the Mondego estuary were all juveniles or immature, which is consistent with a nursery role. The stability in their condition over the study period, measured through the Fulton’s K, reflects the overall fitness of the population, and is also consistent with a nursery use: on the one hand, it probably derived from the fact that all gurnards were immature, and hence the condition did not reflect the fluctuations in the reproductive investment (i.e. gonads’ maturation); on the other hand, a nursery ground is expected to provide plenty of food, resulting in a healthy and stable condition.
Migration of young gurnards into the estuarine nursery was reflected in spring catches in terms of number and size range, though the total biomass did not reveal this entrance because the fish were mainly small juveniles, and hence of low weight. In turn, the decrease in the catches from the summer 1999 to the following winter, probably reflected the seawards migration of the larger sized gurnards. During winter, rain is more abundant, decreasing the estuarine salinity, temperature decreases and prey is scarce, and all favor the seawards migration. Identical seasonal migratory movements are documented in other regions: concentration in shallower waters in spring and summer, and movements towards deeper areas in winter, as gurnards grow [36,64,65].
Age determination using otoliths
Similar to other gurnards (Aspitrigla cuculus [29], Aspitrigla cuculus[37], Eutrigla gurnardus [66], Trigla lyra [67]), C. lucerna produces one opaque and one hyaline ring in the otoliths each year [4,36]. The hyaline ring usually corresponds to the period of slow growth during autumn and winter, when food supply is scarce, while the opaque ring is formed during fast growth [36] from June to September [4]. Yet, the marginal increment analysis of Mondego gurnards’ otoliths was not entirely clear. The relatively high percentage of otoliths with an opaque edge found in winter might be related with the nursery use, as within the estuary they benefit from better feeding conditions, resulting in material deposition in the otolith. In contrast, a higher number of otoliths with an opaque edge was expected in the warmer seasons. The relatively low numbers in summer 2000, though, might reflect the recent entrance of the animals in the estuary which had not time yet to start depositing a clear opaque ring.
Otolith readings can be complicated due to the presence of the so-called ‘false rings’ [68], i.e., rings which do not correspond to seasonal growth but result from material deposited during critical events of the fish life. Metamorphosis, sexual maturity, habitat changes, migrations are all critical events that occur in the life of a fish that are known to be recorded as marks in the otoliths [23], and even the diel patterns of rest and activity can produce marks [69]. Up to 5 false rings before the first winter ring were observed in 63% of the Mondego gurnards, complicating age readings. False rings are common in C. lucerna [36] and in other gurnards as well (A. cuculus [37]) and have been related to ontogenic processes such as the passage from pelagic to benthic life modes, with recruitment and reproduction, with changes in habitat or migrations, and with environmental stress [36]. In red gurnards from the Mediterranean, a rapid change in the feeding strategy coincides with the size of first maturity and is mirrored in the otolith macrostructure as large false rings [37]. Besides the presence of false rings, another difficulty in the otolith reading was related to the location of its core or nucleus, due to the high opacity. This is probably linked to protein deposition in early development, as reported for gurnards including C. lucerna [20,70].
To improve the consistency of age determinations, it has been recommended that a combination of methods is used [71]. Some studies on aging gurnards have used otoliths burned at 200 to 450ºC to increase the visibility of the annual growth increments [38,56,66,67]. In the present work, however, no preparation method was applied to the otolith to prevent them breaking as suggested by [36]. Despite some difficulties, namely due to false rings and to opacity of the otolith core, a high degree of consistency was obtained in the systematic readings. Difficulties apart, it was possible to attribute an age to almost all gurnards (93.7%).
Young gurnards up to one year old, dominated the estuarine population, with decreasing numbers of fish of older age up to a single 5 years old gurnard. Juveniles entering the estuary in spring 2000 were mostly of age class 0, and hence probably born in the previous winter, as suggested in earlier studies [33,62].
However, since small age class 0 gurnards occupied the estuary also in the winter time, and even a few in the autumn, it does not exclude the possibility of an extended reproduction period, as in the North coast of France [30] and in Greek waters [55,64], starting in late summer. Maturation of gonads indicate that the reproduction period ranges from spring to summer up to autumn in the North of France [29], and from autumn to early spring with a peak in January in the Mediterranean [72,73,74], while in Atlantic waters, reproduction extends from December to July [75].
Larger juveniles continue migrating into the estuary, but the majority were born the year before (i.e., with 1 year old). In summer, most of the gurnards in the estuary were older than one year. The abrupt decrease in number of Mondego gurnards with increasing age from 1 to 2 years old, suggests that these older gurnards have migrated seawards. Therefore, the residency period within the estuary seems to be close to 2 years, though a period of 3 years has been reported before [33]. Older gurnards can sporadically enter in the estuary as well, mainly during autumn and winter, where they benefit from the more stable environmental conditions.
Growth
In contrast to more detailed studies of the population biology, information on length-weight relationships of C. lucerna and other Triglidae is quite abundant, specially for Mediterranean populations [74,76-85], but also for populations from the Atlantic region [25,26,27,63,86,87]. These relations are generally used to estimate the weight of a fish corresponding to a given length and as indicators of the overall growth and fish condition [39,88]. The parameter ‘b’ is related with the fish shape: when close to 3, it signifies that the fish grew without changing its shape, i.e., isometric growth [88], as was observed in the Mondego population, in juveniles from other C. lucerna populations [82,63] and in other Triglidae, while allometric growth is common in adults, at least in the Mediterranean [19,82].
The size range in this study was similar to that of [33] and larger than the observed in another nearby nursery, the Tagus estuary, where gurnards attained only 22.0cm standard length and minimum size was 10.6cm [62]. Further South, in the Arade estuary, tub gurnards ranged from 6.0 to 32.4cm [63], which is in line with present observations.
Again, similar to other Triglidae [34,37,56,89], C. lucerna is a moderately long-living species and relatively fast-growing, especially in males during the first years [4,64,85]: in the first year, the male tub gurnard grows about 55-62% of their maximum size, while the female grows about 30-52% [4,64]. Yet, in the present work growth patterns were not differentiated between sexes because gurnards were all immature.
In the otoliths of Mondego gurnards, the first growth ring was formed when the fish were about 13.5 to 16.5cm total length, which is in line with previous references: 12.6cm in the Catalan Sea [28], and 17.0cm in Greek and Moroccan waters [64,90], but under the 21cm observed in the North of France [29], while in the Tagus estuary 23cm gurnards belong to the 0+ age class still [62]. The largest reference for the size of age class 0+ is about 27cm in the Adriatic Sea [52].
Growth slowed down greatly relative to observations in Morocco, as here they attain 25cm in the second year of life [90], while in Mondego this size was only achieve after 3 years. Yet, this might be related with the migration seawards of older,larger and faster growing gurnards. If so, mean length-at-age was underestimated for ages older than one year. In general, from age class 1+ onwards, the growth rate decreases considerably, still with gender differences, reflecting a shift from somatic to reproductive growth as in other Triglidae [34,37,56,64,66,89]: males grow 15% of their maximum size and females only 6-9% [4,64,74]. Curiously, although males attain their maximum size faster, and hence earlier than females, since their maximum size is shorter than that of females –32.7cm and 67 cm respectively for males from Greece and the Morocco versus 76.7cm and 61.0 cm for females, respectively for the same locations [28,64] -, the annual size increment is larger in females [64].
The same way, females’ longevity is referred to be larger than that of males, though both can live quite long: 13 and 14 years, respectively for males and females [64]. In fact, the tub gurnard is described as the largest of the European Triglidae [7], as it reaches a maximum size of 75cm and 15 years old (Baron, 1985a). Yet, males older than 3 [6] to 4 years old are rare [64]. In Mondego, a small share of the gurnards (about 20%) was older than one year of age and just 9.5% were older than 3 years. Similar to other Triglidae, male sexual maturity is also attained at smaller size and younger age than in females [37,50] and even earlier in each reproductive season [30,64]. Size at first maturity varies across the geographic range: 14 and 15cm total length, respectively in males and females, in France and Libyan Mediterranean Sea [30,73], and 21 and 23cm in UK waters [6], while the age at first maturity is 2–3 years in Italian waters [91]. This means that some of the gurnards from Mondego could be part of the breeders’ pool, though they were all sexually immature still.
Finally, the asymptotic length determined applying the von Bertalanffy model (L∞ = 22 to 54cm) was far from the species maximum size of over 75cm - a maximum record of 81.0cm and 82.8cm was reported in the North of Portugal [5] and in the Black Sea [92], respectively. However, this is in line with a juvenile population which has still much to grow. In contrast, the growth constant K, depending on the dataset used for its determination (0.148 to 0.595 years-1), was mostly over the value for other Atlantic and Mediterranean locations, confirming a fast growth, as expected for such a young population.
Conclusion
In conclusion, this paper presents the growth patterns for an Atlantic population of tub gurnard during estuarine occupation. ICES has identified tub gurnard as a new MoU (Memorandum of Understanding) species and has recommended regular monitoring of biological parameters to define stock characteristics and assist in the sustainable fisheries’ management [93]. The results of this paper contribute to provide such information in relation to growth during early life within a nursery area. The present work further showed the importance of developing a standard aging protocol adapted for this species to overcome certain particularities in the otolith readings (presence of false rings, core opacity). Feeding habits and reproductive biology should be investigated to clarify the origin of the marks in C. lucerna otoliths
Acknowledgment
The research was supported by the project RecBio – Operation MAR-01.04.02-FEAMP-0025, co-founded by the European Maritime and Fisheries Fund (EMFF), through the Operational Program MAR2020. J. Campos acknowledges the collaboration of Preciosa Sobral and Laurinda Paiva in the laboratory work, the fisherman Raul in the sampling campaigns, and the financial support with national funds by FCT - Foundation for Science and Technology through the projects UIDB/04423/2020 and UIDP/04423/2020.
Ethics Declaration
Sample collection
Conflict of interest: The authors declare that they have no conflict of interest.
Ethical statement: All applicable international, national and institutional guidelines for the care and use of animals were followed. The care and use of experimental animals complied with animal welfare laws, guidelines and policies as approved by the European Union (2010-63-EU Directive).
Informed consent: This article does not contain any studies with human participants.
References
- Roncarati A, Felici A, Mariotti F, Melotti P (2014) Flesh qualitative traits of tub gurnard (Chelidonichthys lucerna), a promising species candidate for aquaculture, captured in the middle Adriatic Sea in different seasons. Ital J Anim Sci 13(2): 352-356.
- ICES (2007) Report of the Working Group on Assessment of New MOU Species (WGNEW). ICES CM 2007/ACFM 01.
- Deval MC, Bök T, Ates C, Özbilgin H (2007) Size selectivity of three diamond mesh codends for the European hake (Merluccius merluccius) and the tub gurnard (Trigla lucerna) in the Sea of Marmara, Turkey. J Appl Ichthyol 23(2): 167-172.
- Boudaya L, Neifar L, Rizzo P, Badalucco C, Bouain A, et al. (2008) Growth and reproduction of Chelidonichthys lucerna (Linnaeus) (Pisces: Triglidae) in the Gulf of Gabès, Tunisia. J Appl Ichthyol 24(5): 581-588.
- Feijó D, Rocha A, Santos P, Saborido-Rey F (2008) Statistical Species characterization of Gurnard Landings in North of Portugal. Conference handbook (ICES CM 2008/K:15) ICES Annual Science Conference, Canada.
- McCarthy I, Marriott A (2018) Age, growth and maturity of tub gurnard (Chelidonichthys lucerna Linnaeus 1758: Triglidae) in the inshore coastal waters of Northwest Wales, UK. J Appl Ichthyol 34(3): 581-589.
- Lopez-Lopez L, Preciado I, Velasco F, Olaso I, Gutiérrez-Zabala JL (2011) Resource partitioning among five coexisting species of gurnards (Scorpaeniforme: Triglidae): role of trophic and habitat segregation. J Sea Res 66(2): 58-68.
- Colloca F, Milisenda G, Capezzuto F, Cau A, Garofalo G, et al. (2019) Spatial and temporal trend in the abundance and distribution of gurnards (Pisces: Triglidae) in the northern Mediterranean Sea. Sci Mar 83S1: 101-116.
- Feijó D, Rocha A, Silva C (2014) Grey Gurnard: Portuguese data for Division IXa. Working Document, Lisbon, 7-13 May, ICES Working Group on the Assessment of Biscay and Iberian Ecoregion (WGBIE).
- Rocha A, Feijó D, Santos P (2008) An insight on gurnard Fisheries in North of Portugal. Foro dos Recursos Mariños e da Acuicultura das Rías Galegas 10: 609-615.
- Ferreira I, Santos D, Moreira C, Feijó D, Rocha A, et al. (2019) Population structure of Chelidonichthys lucerna in Portugal mainland using otolith shape and elemental signatures. Mar Biol Res 15(8-9): 500-512.
- Amorim MCP, Stratoudakis Y, Hawkins AD (2004) Sound production during competitive feeding in the grey gurnard. J Fish Biol 65(1): 182-194.
- Amorim MCPM, Hawkins AD (2005) Ontogeny of acoustic and feeding behavior in the grey gurnard, Eutrigla gurnardus. Ethology 111(3): 255-269.
- Lombarte A, Cruz A (2007) Otolith size trends in marine fish communities from different depth strata. J Fish Biol 71(1): 53-76.
- Tuset VM, Lombarte A, Assis CA (2008) Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Sci Mar 72S1: 1-203.
- Campana SE, Casselman JM (1993) Stock discrimination using otolith shape analysis. Can J Fish Aquat Sci 50(5): 106-108.
- Ferri J, Bartulin K, Škeljo F (2018) Variability of otolith morphology and morphometry in eight juvenile fish species in the coastal eastern Adriatic. Croatian Journal of Fisheries 76(3): 91-98.
- Montanini S, Stagioni M, Benni E, Randi M, Vallisneri M (2015a) Using otolith shape for intraspecific discrimination: the case of gurnards (Scorpaeniformes, Triglidae). Frontiers in Marine Science, XV European Congress of Ichthyology, Porto, Portugal.
- Montanini S, Stagioni M, Valdrè G, Tommasini G, Vallisneri M (2015b) Intra-specific and inter-specific variability of the sulcus acusticus of sagittal otoliths in two gurnard species (Scorpaeniformes, Triglidae). Fish Res 161: 93-101.
- Montanini S (2015c) Demersal communities in the Mediterranean Sea: a case study of Triglidae (Osteichthyes, Scorpaeniformes) on the conservation and sustainable use of marine resources. PhD Thesis, Bologna University, Italy.
- Montanini S, Stagioni M, Benni E, Vallisneri M (2017) Ontogenetic changes in otolith morphology and shape analyses in Chelidonichthys cuculus (Linnaeus, 1758) and Chelidonichthys lucerna (L., 1758). J Appl Ichthyol 33(2): 217-220.
- Campana SE (2005) Otolith science entering the 21st century. Mar Freshw Res 56(5): 485-495.
- Pannella G (1971) Fish otoliths: Daily growth layers and periodical patterns. Sciences 173(4002): 1124-1127.
- Parmentier E, Cloots R, Warin R, Henrist C (2007) Otolith crystals (in Carapidae): Growth and habit. J Struct Biol 159(3): 462-473.
- Santos MN, Gaspar MB, Vasconcelos P, Monteiro CC (2002) Weight-length relationships for 50 selected fish species of the Algarve coast (Southern Portugal). Fish Res 59(1-2): 289-295.
- Mendes B, Fonseca P, Campos A (2004) Weight-Length relationships for 46 species of the Portuguese West Coast. J Appl Ichthyol 20(5): 355-361.
- Olim S, Borges TC (2006) Weight-length relationships for eight species of the family Triglidae discarded on the South coast of Portugal. J Appl Ichthyol 22(4): 257-259.
- Mouneimne N (1971) Les triglidae de la mer Catalane. Thesis Doctorale, Université de Paris, France.
- Baron J (1985a) Les Triglidés (Teleostéens, Scorpaeniformes) de la Baie de Douarnenez. I. La croissance de Eutrigla gurnardus, Trigla lucerna, Trigloporus lastiviza et Aspitrigla cuculus. Cybium 9: 127-144.
- Baron J (1985b) Les Triglidés (Teleostéens, Scorpaeniformes) de la Baie de Douarnenez. II. La reproduction de Eutrigla gurnardus, Trigla lucerna, Trigloparus lastoviza et Aspitrigla cuculus. Cybium 9: 255-261.
- Francis RICC (1990) Back-calculation of fish length: a critical review. J Fish Biol 36(6): 883-902.
- Vigliola L, Meekan M (2009) The Back-Calculation of Fish Growth from Otoliths. In: BS Green, et al. (Eds.), Tropical Fish Otoliths: Information for Assessment, Management and Ecology, Reviews: Methods and Technologies in Fish Biology and Fisheries. 11: 175-211.
- Jorge I (1991) Contribuição para o conhecimento da ictiofauna do Estuário do Mondego. Relatório Técnico Científico INIP, 44, Lisbon (in Portuguese).
- Booth AJ (1997) On the life history of the lesser gurnard (Scorpaeniformes: Triglidae) inhabiting the Agulhas Bank, South Africa. J Fish Biol 51(6): 1155-1173.
- Marriott AL, Latchford JW, McCarthy ID (2010) Population Biology of the red gurnard (Aspitrigla cuculus; Triglidae) in the inshore waters of Eastern Anglesey and Northwest Wales. J Appl Ichthyol 26(4): 504-512.
- Carbonara P, Follesa MC (2019) Handbook on fish age determination: a Mediterranean experience. Studies and Reviews. FAO No. 98, Rome.
- Colloca F, Cardinale M, Marcello A, Ardizzone GD (2003) Tracing the life history of red gurnard (Aspitrigla cuculus) using validated otolith annual rings. J Appl Ichthyol 19(1): 1-9.
- Papaconstantinou C (1981) Age and growth of piper, Trigla lyra, in Saronikos Gulf (Greece). Cybium 5(2): 73-87.
- Froese R (2006) Cube law, condition factor and weight-length relationships: history, meta-analysis and recommendations. J Appl Ichthyol 22(4): 241-253.
- King M (2007) Fisheries biology, assessment and management. Blackwell Publishing, Oxford.
- Sparre P, Ursin A, Venema SC (1989) Introduction to Tropical Fish Stock Assessment. Part 1, Manual. FAO Fisheries Technical Paper, 306 Rome.
- Von Bertalanffy L (1938) A quantitative theory of organic growth (inquiries on growth laws II). Hum Biol 10(2): 181-213.
- Bhattacharya CG (1967) A simple method of resolution of a distribution into Gaussian components. Biometrics 23(1): 115-135.
- Gayanilo FC, Sparre P, Pauly D (2005) FAO-ICLARM stock assessment tools II—revised version. FC - FAO, Rome.
- Beverton RJH (1954) Notes on the use of theoretical models in the study of the dynamics of exploited fish populations. Miscellaneous Contribution, United States Fishery Laboratory, Beaufort, North Carolina.
- Beverton RJH, Holt SJ (1957) On the dynamics of exploited fish populations. United Kingdom Ministry of Agriculture; Fisheries.
- Prager M, Saila S, Recksiek C (1987) A microcomputer program for parameter estimation of nonlinear models in fishery science. Old Dominion University Research Foundation, Technical Report, 87/10.
- Moreau J, Bambino C, Pauly D (1986) Indices of overall growth performance of 100 Tilapia (Cichlidae) populations. In: Maclean JL, LB Dizon, LV Hosillos (Eds.), The first Asian Fisheries Forum. Asian Fish Society, Manila, Philippines, pp. 201-206.
- R Development Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0.
- Vallisneri M, Stagioni M, Montanini S, Tommasini S (2011) Body size, sexual maturity and diet in Chelidonichthys lucerna (Osteichthyes: Triglidae) from the Adriatic Sea, north eastern Mediterranean. Acta Adriat 51(1): 141-148.
- Mytilineou C, Politou CY, Papaconstantinou C, Kavadas S, D'Onghia G, et al. (2005) Deep-water fish fauna in the Eastern Ionian Sea. Belg J Zool 135(2): 229-233.
- Froglia C (1976) Osservazioni sull’alimentazione dei giovani di Trigla lucerna della classe di età nel Medio Adriatico (Pisces: Triglidae). Arch Oceanogr Limnol 18: 365-373.
- Costa MJ (1982b) Tagus Estuary as a nursery. In: Proceedings of UNESCO/IOC/CNA Workshop on Estuaries Processes: An application to the Tagus Estuary, Lisboa, Portugal.
- Costa MJ (1988) Ecologie alimentaire des poissons de l'estuaire du Tage. Cybium 12(4): 301-320.
- Papaconstantinou CA (1983a) Observations on the ecology of gurnards (Pisces, Triglidae) of the Greek seas. Cybium 7: 71-88.
- Papaconstantinou C (1983b) Aspect on the biologie of Aspitrigla cuculus (Pisces: Scorpaeniformes) from the Saronikos Gulf. Thalassographica 6: 49-75.
- Hureau JC (1986) Triglidae. In: Whitehead PJP, ML Bauchot, JC Hureau, J Nielsen, Tortonese E (Eds.), Fishes of the Northeastern Atlantic and the Mediterranean. Vol 3, Paris, Unesco.
- Tsimenides N, Machias A, Kallianiotis A (1992) Distribution patterns of triglids (Pisces: Triglidae) on the Cretan shelf (Greece), and their interspecific associations. Fish Res 15(1-5): 83-103.
- Serena F, Voliani A, Auteri R (1998) Nursery areas and some biological information of tub gurnard (Trigla lucerna, 1758) off Tuscany coast (Italy). Rapports et Proces-verbaux des Réunions – Commission Internationale pour l’Exploration Scientifique de la Mer Méditerranée 35: 482-483.
- Costa MJ, Cabral HN (1999) Changes in the Tagus nursery function for commercial fish species: some perspectives for management. Aquat Ecol 33: 287-292.
- Costa MJ, Bruxelas A (1989) The structure of fish communities in the Tagus Estuary, Portugal, and its role as a nursery for commercial fish species. Sci Mar 53: 561-566.
- Costa MJ (1982a) Contribution à l’étude de l’écologie des poissons de l’estuaire du Tage (Portugal). Thèse Doctorale, Université Paris VII.
- Veiga P, Machado D, Almeida C, Bentes L, Monteiro P, et al. (2009) Weight–length relationships for 54 species of the Arade estuary, southern Portugal. J Appl Ichthyol 25(4): 493-496.
- Papaconstantinou C (1984) Age and growth of the yellow gurnard (Trigla lucerna1758) from the Thermaikos gulf (Greece) with some comments on its biology. Fish Res 2(4): 243-255.
- Richards WJ, Saksena VP (1990) Triglidae. In: Quero JC, JC Hureau, C Karrer, A Post, L Saldanha (Eds.), Check-list of the fishes of the eastern tropical Atlantic (Clofeta). JNICT, Lisbon, SEI, Paris and Unesco 2: 680-684.
- Papaconstantinou C (1982) Age and growth of grey gurnard (Eutrigla gurnardus) in Pagassitikos Gulf (Greece). Invest Pesq 46(2): 191-213.
- Sousa I (2019) Life history parameters and valorisation of a low commercial value fish species: the case study of piper gurnard, Trigla lyra Linnaeus 1758. PhD dissertation, University of Algarve, Portugal.
- Morales-Nin B (1992) Determination of Growth in Bony Fishes from Otolith Microstructure. FAO Fish. Technical Paper.
- Radtke RI, Morales-Nin B (1989) Mediterranean juvenile bluefin tuna: life history patterns. J Fish Biol 35(4): 485-496.
- Vallisneri M, Montanini S, Randi MR, Reggi S, Tommasini M, et al. (2014) Preliminary results on organic and mineral fractions in otoliths of three fish species from Adriatic Sea. Biol Mar Mediterr 21: 310-312.
- Campana SE (2001) Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation method. J Fish Biol 59(2): 197-242.
- Abdallah M, Faltas SN (1998) Reproductive Biology of Trigla lucerna and Trigloporus lastoviza in the Egyptian Mediterranean Waters. Bull Natl Inst Oceanogr Fish (Egypt) 24: 285-304.
- Ahmed AI (2012) Reproductive biology of the tub gurnard Trigla lucerna (Linnaeus, 1978), in the Libyan eastern coast of Mediterranean Sea. Egypt. J Aquat Biol Fish 16(1): 95-104.
- El-Serafy S, El-Gammal FI, Mehanna FS, Abdel-Hamid NH, El-Sayed Farrag FE (2015) Age, growth and reproduction of the tub gurnard, Chelidonichthys lucerna (Linnaeus, 1758) from the Egyptian Mediterranean waters off Alexandria. Int J Fish Aquat Sci 4: 13-20.
- Richards WJ (1968) Eastern Atlantic Triglidae (Pisces, Scorpaeniformes). Atlantide Report 10: 77-114.
- Abdallah M (2002) Length-weight relationship of fishes caught by trawl off Alexandria, Egypt. ICLARM Quarterly 25(1): 19-20.
- Çiçek E, Avşar D, Yeldan H, Özütok M (2006) Length-weight relationships for 31 teleost fishes caught by bottom trawl net in the Babadıllimanı Bight (Northeastern Mediterranean). J Appl Ichthyol 22(4): 290- 292.
- Sangun L, Akamca E, Akar M (2007) Weight-length relationships for 39 fish species from the North-Eastern Mediterranean coast of Turkey. Turkish J Fish Aquat Sci 7: 37-40.
- Uckun DI, Togulga M (2007) Age, growth and reproduction of tub gurnard Chelidonichthys lucernus Linnaeus, 1758 (Osteichthyes: Triglidae) from Izmir Bay, Aegean Sea, Eastern Mediterranean. Acta Adriat 48: 173-184.
- Ilkyaz AT, Metin G, Soykan O, Kinacigil HT (2008) Length– weight relationship of 62 fish species from the Central Aegean Sea, Turkey. J Appl Ichthyol 24(6): 699-702.
- Mata AJ, Morales J, Márquez L (2008) Weight–length relationships for 26 demersal fish species of the Spanish South-Atlantic coastal waters. J Appl Ichthyol 24(3): 330-333.
- Vallisneri M, Montanini S, Stagioni M (2010) Length-weight relationships for the family Triglidae in the Adriatic Sea, northeastern Mediterranean. J Appl Ichthyol 26(3): 460-462.
- Bök TD, D Göktürk, AE Kahraman, TZ Alıçlı, T Acun, et al. (2011) Length-weight relationships of 34 fish species from the Sea of Marmara, Turkey. J Anim Vet Adv 10: 3037-3042.
- Demirel N, Dalkara EM (2012) Weight-length relationships of 28 fish species in the Sea of Marmara. Turk J Zool 36(6): 785-791.
- İşmen A, İşmen P, Baflusta N (2004) Age, growth and reproduction of tub gurnard (Chelidonichthys lucerna 1758) in the bay of Iskenderun in the Eastern Mediterranean. Turk J Vet Anim Sci 28(2): 289-295.
- Borges TC, Olim S, Erzini K (2003) Weight–length relationships for fish species discarded in commercial fisheries of the Algarve (Southern Portugal). J Appl Ichthyol 19(6): 394-396.
- Silva JF, Ellis JR, Ayers RA (2013) Length-weight relationships of marine fish collected from around the British Isles. Science Series Technical Report, Cefas, Lowestoft, 150.
- Folkvord A, Mosegaard H (2002) Chapter V-A: growth and growth analysis. In: Panfili J, H Pontual, H Troadec, PJ Wright (Eds.), Manual of fish sclerochronology. Ifremer-IRD coedition, Brest, France.
- Elder RD (1976) Studies on age and growth, reproduction and population dynamics of red gurnard, Chelidonichthys kumu in the Hauraki Gulf. N. Z. Fish Res Bull 12: 1-77.
- Collignon J (1968) Les trigles des eaux marocanies lére note: Généralités - Trigla hirundo. Bull Inst Pêch Maroc 16: 3-33.
- Bombace G, Lucchetti A (2011) Elementi di Biologia Della Pesca. Edagricole, Milano.
- Hasimoğlu A, Ak O, Kasapoğlu N, Atılgan E (2016) New maximum length report of Chelidonichthys lucerna (Linneaus, 1758) in the Black Sea, Turkey. J Black Sea/Mediterranean Environment 22: 149-154.
- ICES (2006) Report of the Working Group on Assessment of New MOU Species (WGNEW). ICES CM 2006/ACFM 11.
- Altun A, Göksu MZL, Türeli C, Erdem Ü (1997) Yumurtalık (Adana) Körfezinde Dil Balığı (Solea vulgaris) ve Kırlangıç Balığı (Trigla lucerna) Türlerinin Bazı Biyolojik Özellikleri. XIII. Ulusal Biyoloji Kongresi, İstanbul 5: 147-158.
- Eryilmaz L, Meriç N (2005) Some biological characteristics of the tub gurnard, Chelidonichthys lucerna (Linnaeus, 1758) in the Sea of Marmara. Turk J Vet Anim Sci 29: 367-374.
- Faltas SN, Abdallah M (1997) Growth, mortality and relative yield per recruit of two Triglid species from the Egyptian Mediterranean, off Alexandria. Bull Nat Inst Oceanogr & Fish 23: 473-484.






























