Nutritional Status of Micropropagated Plantlets of Two Varieties of Stevia rebaudiana Bert. under Different Culture Conditions
Vilariño Susana1, Florido María del Carmen2, García José Luis3 and Cantos Manuel3*
1Vitrosur Lab SLU. Algodonera del Sur, Spain
2Departmant of Cristalografía, Mineralogía y Química Agrícola, Universidad de Sevilla, Spain
3IRNAS (CSIC), Spain
Submission: November 11, 2021; Published: December 06, 2021
*Corresponding author: Manuel Cantos, Institute of Natural Resources and Agrobiology of Seville (IRNAS), State Agency Spanish National Research Council (CSIC), Av. Reina Mercedes 10, 41012 Sevilla, Spain
How to cite this article: Vilariño S, Florido MC, García JL, Cantos M. Nutritional Status of Micropropagated Plantlets of Two Varieties of Stevia rebaudiana 02 Bert. under Different Culture Conditions. Int J Environ Sci Nat Res. 2021; 29(2): 556263. DOI: 10.19080/IJESNR.2021.29.556263
Abstract
Extracts obtained from plants of Stevia rebaudiana Bert have high sweetening capacity and without risks for health. The sexual reproduction of this species is characterized by the low germination efficiency of its seeds and the high heterogeneity of the resulting populations. Thus, asexual propagation, particularly micropropagation, is the preferred tool for obtaining a large number of healthy and genetically homogeneous plants in a short time. To study the effects of the substrate composition and culture system on the nutrient contents of resulting plantlets, two stevia varieties (Criolla and Morita), three culture media (Murashige and Skoog and two modifications of this medium) and two culture systems (Temporal Immersion System-TIS- and solid medium) were used. After 30 days of culture the stem length and leaf nutritional status were tested. The nutrient contents of the in vitro well-developed plants are clearly related with the substrate compositions. Except for manganese no differences were observed between both varieties in terms of macro or micronutrients levels. In most cases the nutrient levels were higher in the microplants grown in solid medium. The macro and micronutrient levels of the resulting plantlets reported and discussed here, for the first time, may be useful for future studies in order to establish normal nutritional status of reference for researchers working in stevia micropropagation.
Keywords: Criolla; Morita; Solid medium; Temporal immersion system; PLANTFORM
Abbreviations: TIS: Temporary Immersion System; MS: Murashige Skoog; MSM: Modified MS; G: Grapevine Medium; BA: 6-benzylaminopurine; NAA: Naphthaleneacetic Acid
Introduction
The extract obtained from stevia (Stevia rebaudiana, Bert) leaves is remarkable due to its high sweetening capacity and very low-calorie content. Since no side effects from its consumption have been described [1], S. rebaudiana has become a real alternative sweetener on the market.
Stevia sexual reproduction is characterized by low germination and a high heterogeneity in the resultant populations from its seeds. For these reasons, asexual propagation methods, mainly micropropagation, have been preferred [2,3]. For this purpose Murashige and Skoog medium (MS) [4] has been routinely applied as basal medium for the micropropagation from buds [5,6]. However, the high levels of nitrogen, chlorine and molybdenum in MS at full strength suggest, according to some authors as Chee & Pool [7], the possibility of reducing the concentration of MS salts to a half or a third of its usual concentration.Consequently, this substrate could be improved for certain species as stevia.
In micropropagation, mainly for commercial purposes, solid culture media tend to be more costly due to the presence of agar. Accordingly, culture in liquid substrate performed by temporary immersion system (TIS) may be a suitable alternative [8] avoiding continuous contact with the liquid substrate. This system is considered to be highly automatable with important reductions in both costs and contamination [9]. Since the purpose of this study was to answer the question: How the cultured system and nutritional conditions assayed affect the nutritional status of the obtained microplant?
Material and Methods
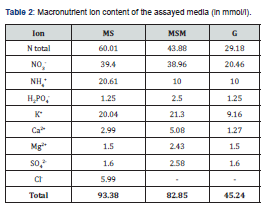
Plantlets grown previously under in vitro conditions of two Stevia rebaudiana (Bert) varieties, Criolla and Morita III, were cut into uninodal explants (1-2cm in length with two axillary buds) and subcultivated in two different culture systems: medium solidified by agar and TIS. Three substrates were assayed in each culture systems: MS; MS modified (MSM); and G, also deriving from MS and adapted for in vitro grapevine culture [10]. MSM and G substrates were modified in relation to MS at macronutrient level, consisting mainly of a gradual reduction in nitrogen content (Table 1 & 2).
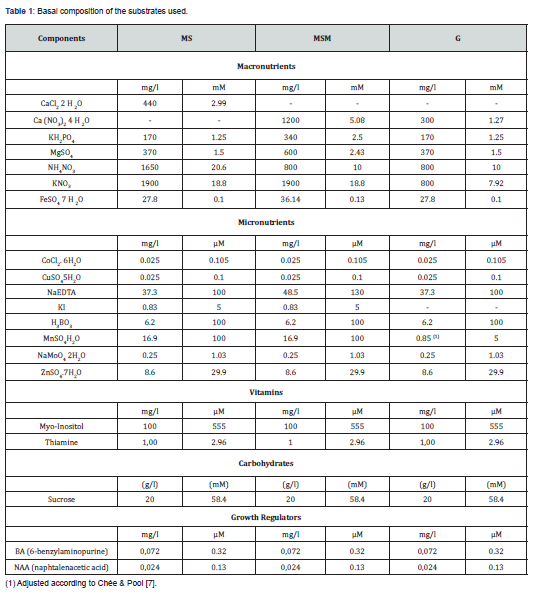
Six treatments using the three substrates mentioned were assayed: three for solid medium adding agar (7gL-1) (MSS, MSMS and GS) and three for TIS system (MSTIS, MSMTIS and GTIS). In all situations, the pH was adjusted to 5.7 prior to autoclaving. The different culture media were distributed in each flask (600mL) (100mL per flask) for solid conditions. In the case of the TIS treatments PLANTFORM bioreactors [9,11] (4000mL) (500mL substrate per bioreactor) were used. After substrate distribution the flasks and bioreactors were closed and autoclaved for 20min (120ºC, 1kg/cm2 of pressure). Ten explants per flask, in the case of the solid medium and 50 per PLANTFORM bioreactor were established in order to provide 10mL of substrate per explant in both cases. Cultures were incubated in a growth chamber at 26±1 oC, 30μEm-2s-1 and for a 16h. photoperiod. In the case of the PLANTFORM bioreactors gas exchange sterilized by filters was controlled through three inlets/outlets anchored to the side. The immersion frequency was 2 min every 8h, as described elsewhere [12,13]. Each treatment was repeated 5 times.
At 30 days of culture the aerial plant size (length in cm) was measured and the nutrient status of the obtained material was evaluated. The leaf samples were carefully washed with distilled water and then dried at 80℃ for 48h and ground. Individual 500mg samples were then digested by wet oxidation with concentrated HNO3 under pressure in a microwave oven to obtain the extract. Concentrations of P, K, Ca, Mg S, B, Cu Fe, Mn, Na, Mo and Zn in the extracts were determined by inductively coupled plasma optical spectroscopy (ICP-OES) (MS 3.5). Total N concentration was determined by Kjeldahl digestion using an elemental analyzer (Leco CHNS-932, Spain). Analyses were repeated three times.
Statistical analysis was carried out using IBM SPSS Statistics v.25. Three- and one way analyses of variance (F-test) and the Bonferroni test for average comparison were employed. Data were tested for normality using the Kolmogorov–Smirnov test and for homogeneity of variance using the Levene test. The Tukey tests were applied to significant test results for the identification of important contrasts.
Results
Growth
Root presence and absence of visual hyperhydricity symptoms were observed in all the plantlets developing from explants.
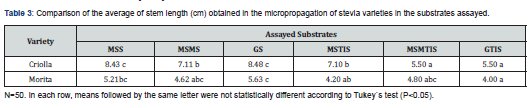
Table 3 summarizes the results of the average of stem length (cm) obtained.
As regards stem length in the Criolla plantlets, three statistically well-differentiated groups were observed (Table 3):
a) The two longest stems were grown in MSS and GS;
b) Two stems of intermediate length - MSMS and MSTIS - were obtained; and
c) The two shortest stems were grown on MSMTIS and GTIS. Less homogeneity in length was found in the Morita plantlets.
As with the Criolla plantlets, the longest average stem length was obtained in those grown in GS medium, but similar values were obtained for stem grown in MSS, MSMS and MSMTIS. In contrast, shorter stems were quantified in MSTIS and GTIS media (Table 3). Consequently, considering the combined results for Criolla and Morita, the stems were longer (P<0.05) in solid substrates (average 6.60cm) than with TIS (average 5.21cm).
When comparing the behavior of both stevia varieties and the three substrates (MS, MSM and G) independently of the culture system used (solid medium or TIS), the best development in length was found in stems grown in MS medium (average of 6.33cm), similar statistically to the length achieved in G (average of 5.9cm) and shorter (P<0.05) than the lengths of stems grown in MSM (average of 5.52cm). In general, considering the different averages for all substrates in both solid and TIS, longer stems were obtained for Criolla (7.02cm) (P<0.05) than Morita (4.77cm), respectively.
Nutritional status
Macronutrients
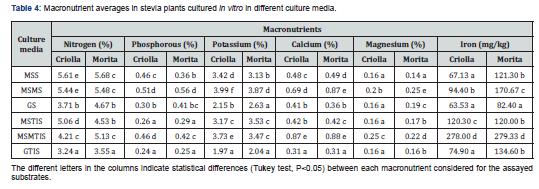
In general, no differences were observed between both varieties in terms of macro or micronutrients levels. Only manganese levels in leaves were higher (Student`s t-test P<0.05) in the Morita plantlets (411.45mg/kg) than in the Criolla variety (389.68mg/kg). However, the macronutrient analysis revealed statistical differences (ANOVA Tuckey test P<0.05) among the assayed substrates. Thus, statistically highest nitrogen percentages were found in plants developed in MSS and MSMS, followed by the same TIS substrates (Table 4). The G substrate presented the lowest (ANOVA, Tuckey test P<0.05) concentrations of this element in both solid and TIS conditions, although concentrations were statistically higher in the former than in the latter. As regards nitrogen content, significant differences (Student’s t-test (P<0.05)) between solid and TIS systems were observed: 5.12 % and 4.33%, respectively.
In terms of phosphorous and potassium levels, plantlets cultured in MSS, MSMS and MSMTIS substrates contained the highest percentages (ANOVA Tuckey test P<0.05). In contrast, the plantlets with the lowest phosphorous and potassium contents were those cultured in GTIS and MSTIS. The phosphorous level registered in leaves of plantlets grown in solid media (0.43%) was significantly higher (Student's t-test (P<0.05) than those found in plantlets developed under TIS conditions, (0.32%). Foliar analysis revealed the highest calcium and magnesium content in plantlets grown in MSMTIS, although in the Morita plantlets the highest percentage (ANOVA Tuckey test P<0.05) was found in MSMS medium (Table 4). For leaves from the both varieties, the highest (ANOVA Tuckey test P<0.05) iron content was obtained in the plantlets grown in MSMTIS medium. In TIS conditions more iron was accumulated in leaves, (171.25mg/kg) than in solid media (108.21mg/kg) (Student's t-test (P<0.05).
Micronutrients
In relation to micronutrients (Table 5), with exception of molybdenum with substrate averages of 12.98 and 149.45mg/kg in Criolla and Morita leaves, respectively (Student's t-test P<0.05), the other microelements analyzed did not present any differences in contents for either variety.
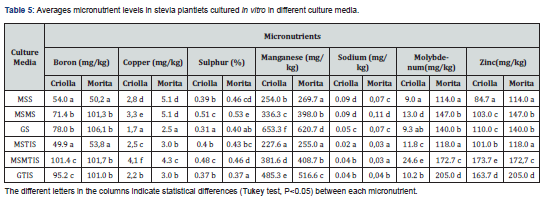
Boron content was higher in the leaves of Criolla plantlets grown in MSMTIS and GTIS media (ANOVA Tuckey test P<0.05). The pattern was similar for the Morita variety, but in this case analogous levels were obtained for both media and also in solid conditions (Table 5).
The highest copper content quantified in Criolla leaves was obtained in the plantlets cultured in MSMTIS medium (ANOVA Tuckey test P<0.05). However, in the Morita variety these contents were significantly higher (P<0.05) in plantlets cultured in the MSS and MSMS media. The highest concentrations of sulphur (ANOVA Tuckey test P<0.05) were found in the plantlets growing in the MSMS and MSMTIS substrates, while the lowest contents were quantified in plantlets grown in the GS and GTIS substrates (Table 5). The highest levels of manganese were found in plantlets from the two varieties grown in GS and the lowest in MSMTIS (ANOVA Tuckey test P<0.05). Significant differences (ANOVA Tuckey test P<0.05) in sodium content were also observed, with maximum values in plantlets multiplied in MSS and MSMS media (ANOVA Tuckey test P<0.05). In this case, the Na concentration identified in leaves of plantlets developed in solid conditions (0.082mg/kg) was higher than in those quantified in TIS (0.033mg/kg) (Student's t-test (P<0.05)). For molybdenum and zinc, the highest values detected in Criolla plantlets corresponded to those grown in MSMTIS medium. However, for Morita plantlets the highest levels of both elements were found in GTIS medium. Leaves developing in TIS presented higher levels of zinc (158.82mg/kg) than those of plantlets grown in solid substrates (122.34mg/kg) (Student's t-test (P<0.05)).
Discussion
In general, the length ranges obtained are in accordance with the normal length found by other authors micropropagating stevia explants [3,6]. However, it may be suggested for stem length that the best combination consisted of Criolla plantlets growing in solid MS or G media. Many publications on steviosides and rebaudiosides, as main responsible for sweet taste of stevia plantlets cultured in vitro, can be found in current literature. On the contrary, no references about macro and micronutrient status of leaves from micropropagated plants have been found. For this reason, the nutrient levels reported here may be useful as references for future studies. In general, no differences were observed between both varieties in terms of macro or micronutrients levels. Only manganese levels in leaves were higher (Student`s t-test P<0.05) in the Morita plantlets (411.45mg/kg) than in the Criolla variety (389.68mg/kg). However, the macronutrient analysis revealed statistical differences (ANOVA Tuckey test P<0.05) among the assayed substrates. Thus, statistically highest nitrogen percentages were found in plants developed in MSS and MSMS, followed by the same TIS substrates (Table 4). The G substrate presented the lowest (ANOVA, Tuckey test P<0.05) concentrations of this element in both solid and TIS conditions, although concentrations were statistically higher in the former than in the latter. These N contents are clearly related with the substrate compositions (Table 1 & 2), r=0.7544 (P<0.01). As regards nitrogen content, significant differences (Student’s t-test (P<0.05)) between solid and TIS systems were observed: 5.12% and 4.33%, respectively. A similar result was found by Deng et al. [14] in explants of Fraxinus mandshurica that the absorption of nitrogen was favored in agar gelled culture media when compared with plants grown in TIS.
In terms of phosphorous and potassium levels, plantlets cultured in MSS, MSMS and MSMTIS substrates contained the highest percentages (ANOVA Tuckey test P<0.05). In contrast, the plantlets with the lowest phosphorous and potassium contents were those cultured in GTIS and MSTIS. Levels of both macronutrients were directly correlated with its contents in the substrate (r=0.7521 and r=0.9649, P<0.01) respectively. This strongest correlation in the case of potassium indicate that a dose almost three fold added to the MSM substrate may be suitable for good in vitro development of stevia plantlets. The phosphorous level registered in leaves of plantlets grown in solid media (0.43%) was significantly higher (Student's t-test (P<0.05) than those found in plantlets developed under TIS conditions, (0.32%). In a similar way, K content in leaves of both stevia varieties was slightly higher (3.18%) when cultured in solid substrates than in TIS (2.96%). Foliar analysis revealed the highest calcium and magnesium content in plantlets grown in MSMTIS, although in the Morita plantlets the highest percentage (ANOVA Tuckey test P<0.05) was found in MSMS medium (Table 4). Calcium levels showed a very strong correlation with the calcium added to the substrates (r=0.9134, P<0.01). George et al. [15] indicated that calcium concentrations within in vitro plant tissue culture tend to be proportional to those in soil. Magnesium levels were also clearly related with the supply of Mg in the substrates (r=0.8911, P<0.01). For leaves from both varieties, the highest (ANOVA Tuckey test P<0.05) iron content was obtained in the plantlets grown in MSMTIS medium. However, the direct correlation with the Fe in the substrates was maintained (r=0.7118, P<0.05). In TIS conditions more iron was accumulated in leaves, (171.25mg/kg) than in solid media (108.21mg/kg) (Student's t-test (P<0.05).
In relation to micronutrients (Table 5), with exception of molybdenum with substrate averages of 12.98 and 149.45mg/kg in Criolla and Morita leaves, respectively (Student's t-test P<0.05), the other microelements analyzed did not present any differences in contents for either variety.
Boron content was higher in the leaves of Criolla plantlets grown in MSMTIS and GTIS media (ANOVA Tuckey test P<0.05). The pattern was similar for the Morita variety, but in this case analogous levels were obtained for both media and also in solid conditions (Table 5).
The highest copper content quantified in Criolla leaves was obtained in the plantlets cultured in MSMTIS medium (ANOVA Tuckey test P<0.05). However, in the Morita variety these contents were significantly higher (P<0.05) in plantlets cultured in the MSS and MSMS media. The highest concentrations of sulphur (ANOVA Tuckey test P<0.05) were found in the plantlets growing in the MSMS and MSMTIS substrates, while the lowest contents were quantified in plantlets grown in the GS and GTIS substrates. No differences were observed between the solid and TIS systems (0.43 and 0.42%, respectively). The highest levels of manganese were found in plantlets from the two varieties grown in GS and the lowest in MSMTIS (ANOVA Tuckey test P<0.05). This was the only element significantly inversely correlated with its concentration in the substrates (r= -0.8653, P<0.01). This result can be explained by the antagonism between K content in the substrate and Mn in leaves. Tavares et al. [16] reported a significant Mn decrease in leaves of Aechmea blanchetiana micropropagated in medium with high K levels. According to this antagonism, our results indicate a similar inverse correlation between K added to the medium and Mn concentration in leaves (r= - 0.8252, P<0.01). Significant differences in sodium content were also observed, with maximum values in plantlets multiplied in MSS and MSMS media (ANOVA Tuckey test P<0.05). In this case, the Na concentration identified in leaves of plantlets developed in solid conditions (0.082mg/kg) was higher than in those quantified in TIS (0.033mg/kg) (Student's t-test (P<0.05)). For molybdenum and zinc, the highest values detected in Criolla plantlets corresponded to those grown in MSMTIS medium. However, for Morita plantlets the highest levels of both elements were found in GTIS medium. Leaves developing in TIS presented higher levels of zinc (158.82mg/kg) than those of plantlets grown in solid substrates (122.34mg/kg) (Student's t-test (P<0.05)) [17-20].
Acknowledgment
We are grateful to María del Mar Parra Alejandre for skillful technical assistance.
Compliance with Ethical Standards
Funding
Project No 2017 6043. Vitrosur Lab SLU. (Algodonera del Sur).
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
All authors designed the performed experiments according to the consensual conception of the study. Material preparation and data collection were performed by S.V. Statistical analysis was performed by M.C.F. The first draft of the manuscript was written by M.C. and J.L.G. All authors contributed to the revision of scientific contents and approved the final version of the drafted manuscript.
References
- Thiyagarajan M, Venkatachalam P (2012) Large scale in vitro propagation of Stevia rebaudiana (bert) for commercial application: Pharmaceutically important and antidiabetic medicinal herb. Ind Crops Prod 37(1): 111-117.
- Casaccia J, Álvarez E (2006) Recomendaciones técnicas para una producción sustentable del Ka’a He’e (Stevia rebaudiana (Bertoni) Bertoni) en el Paraguay. Manual Técnico No 8. Caacupé (Paraguay)
- Ibraahim IA, Nasr MI, Mohammedm BR, El-Zefzafi MM (2008b) Nutrient factors affecting in vitro cultivation of Stevia rebaudiana. Sugar Tech 10: 248-253.
- Murashige T, Skoog F (1962) A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol Plant 15: 473-497.
- Ibrahim IA, Nasr MI, Mohammed BR, El-Zefzafi MM (2008a) Plant growth regulators affecting in vitro cultivation of Stevia rebaudiana. Sugar Tech 10: 254-259.
- Khalil SA, Zamir R, Ahmad N (2014) Selection of suitable propagation method for consistent plantlets production in Stevia rebaudiana (Bertoni). Saudi J Biol Sci 21(6): 566-573.
- Chée R, Pool RM (1987) Improved inorganic media constituents for in vitro shoot multiplication of Vitis. Sci Hortic (Amsterdam) 32: 85-95.
- Etienne H, Berthouly M (2002) Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult 69: 215-231.
- Benelli C, De Carlo A (2018) In vitro multiplication and growth improvement of Olea europaea L. cv Canino with temporary immersion system (PlantformTM). 3 Biotech 8: 317.
- Cantos M, Arroyo-García R, García JL, Lara M, Morales R, et al. (2017) Current distribution and characterization of the wild grapevine populations in Andalusia (Spain). C R Biol 340(3): 164-177.
- Welander M (2011) Teknisk utveckling av bioreaktorer för storskalig mikroförökning. slutredovisning av partnerskapsprojekt 2008-2010.
- Mosqueda MAR, Andreu LGI, Madero GR, Rincón EUH (2016) Micropropagation of Stevia rebaudiana Bert. in temporary immersion systems and evaluation of genetic fidelity. South African J Bot 106: 238-243.
- Vives K, Andújar I, Lorenzo JC, Concepion O, Hernandze M, et al (2017) Comparison of different in vitro micropropagation methods of Stevia rebaudiana B. including temporary immersion bioreactor (BIT®). Plant Cell Tissue Organ Cult 131: 195-199.
- Deng Z, Chu J, Wang Q, Wang L (2012) Effect of different carbon sources on the accumulation of carbohydrate, nutrient absorption and the survival rate of Chinese Ash (Fraxinus mandshurica) explants in vitro. African J Agric Res 7: 3111-3119.
- George EF, Hall MA, De Klerk GJ (2008) The Components of Plant Tissue Culture Media I: Macro- and Micro-Nutrients. In: George EF, Hall MA, Klerk GJ De (Eds.), Plant Propagation by Tissue Culture. Springer Netherlands, Dordrecht, pp. 65-113.
- Tavares AR, Kanashiro S, Jocys T, Ribeiro RCS, Goncalves AN, et al. (2014) Effect of potassium on the contents of micronutrients on in vitro culture of Aechmea blanchetiana (Bromeliaceae). Acta Hortic 201-206.
- Bondarev N, Reshetnyak O, Nosov A (2002) Features of Development of Stevia rebaudiana Shoots Cultivated in the Roller Bioreactor and their Production of Steviol Glycosides. Planta Med 68(8): 759-762.
- Hossain M, Shamim Kabir A, Jahan T, Hasan M (2008) Micropropagation of Stevia. Int J Agric Sustain Crop Prod 3: 1-9.
- Lyakhovkin AG, Long TD, Titov DA, Anh MP (1993) Cultivation and utilization of Stevia (Stevia rebaudiana Bertoni). Agricultural Publishing House, Hanoi.
- Sivaram L, Mukundan U (2003) In vitro culture studies on Stevia rebaudiana. In Vitro Cell Dev Biol - Plant 39: 520-523.






























