Seagrass Loss and Sub-Surface Carbon Fate: Insights from a Long-Term Experimental Removal in Gazi Bay, Kenya
Charles Cadier1* and Michael N Githaiga2
1Australian Rivers Institute, Griffith University, Nathan, Australia
2Department of Biological Sciences, University of Embu, Kenya
Submission: July 06, 2021; Published: August 04, 2021
*Corresponding author: Charles Cadier, Australian Rivers Institute, Griffith University, 170 Kessels Rd, Nathan, QLD 4111, Australia
How to cite this article: Charles C, Michael N G. Seagrass Loss and Sub-Surface Carbon Fate: Insights from a Long-Term Experimental Removal in Gazi Bay, Kenya. Int J Environ Sci Nat Res. 2021; 28(4): 556243. DOI: 10.19080/IJESNR.2021.28.556243
Abstract
Seagrass meadows are considered as global hotspots of blue carbon stocks. However, they suffer global cover loss mainly due to anthropogenic activities. Few is known on the impact of seagrass loss on their blue carbon stocks. This study investigates the impact of seagrass removal on soil organic carbon stocks two years after initial perturbation, and the potential bioturbation activity of co-existing burrowing shrimps in Gazi Bay, Kenya. Seagrass aboveground biomass was removed for a period of 18 months and organic carbon samples were taken 24 months after the first harvested at three depth layers (0-5cm, 5-10cm, 10-15cm). Results indicated that organic carbon was significantly lower in the 15cm depth profile sampled in harvested seagrass meadows. The sediment turnover rate of Callianassidae present in the bay was estimated at 948 ± 342 (SE) g.DW.d-1. This bioturbation activity is assumed to play an important role in the potential release of sediment organic carbon stock from harvested plots. This study demonstrates the significant sub-surface organic carbon loss after seagrass removal, and the potential for burrowing shrimp to enhance organic carbon remineralisation. Further studies on tropical seagrass meadows organic carbon fate after seagrass loss to account for blue carbon budget.
Keywords: Seagrass meadows; Blue carbon; Bioturbation; Western indian ocean
Introduction
Seagrass meadows are one of the most productive coastal ecosystems worldwide. They provide a wide range of ecosystem services for coastal populations providing a habitat for many coastal fauna including commercially important fish species, preventing erosion and sea surface elevation, and reducing eutrophication through their denitrification potential [1]. Recently, there has been increased scientific attention to their capacity to sequester carbon via the development of the notion of “Blue carbon ecosystems” [2]. These ecosystems are sinks of organic carbon (Corg), therefore regulating climate change by limiting the release of carbon into the atmosphere [3]. Global estimates of Corg storage in the top metre of seagrass sediments range from 4.2 to 8.4Pg C [4]. While seagrass meadows only occupy 0.1% of ocean surface, they store 10-18% of the total Corg trapped in the ocean and are therefore considered Blue carbon hotspots [5]. In total, seagrass Corg stocks are comparable to the quantity stored in both saltmarshes and mangroves (~10Pg C) and their annual carbon accumulation rate of ∼83gC.m−2 yr−1 is larger than that of most terrestrial ecosystems [4,6].
Despite the importance of seagrass ecosystems, their global cover has been decreasing at a rate estimated to be around 7% yr-1 since 1990 [7]. This decline is due to global and local threats, such as marine heatwaves or nutrients runoff [8,9]. The shift from vegetated habitat to unvegetated is leading to the erosion of sediment and to the remineralisation of the Corg stored in the soils, with an estimation of between 0.05 and 0.33Pg CO2 yr-1 released by land-use change in coastal ecosystems globally [3,10].
Seagrass loss can also lead to alterations in faunal communities with a shift toward a domination of bioturbator organisms, therefore accelerating the remineralisation of Corg [11,12]. In the sub-tropical and tropical regions of the world, the main macrofaunal bioturbators are Callianassidae shrimps (Decapoda : Axiidea) [13,14]. They co-exist within seagrass meadows and often control their lower and upper boundaries [15]. They are considered as upward conveyors regarding their bioturbation activity which means they drag deep sediments to the surface [16]. Their bioturbation activity has been estimated in a laboratory experiment to lead to a 2 to 5-fold increase in total carbon dioxide (CO2) release from seagrass sediment [12]. It is therefore necessary to investigate their bioturbation activity in the field in order to account for their impact on the blue carbon storage potential of seagrass meadows.
Loss of seagrass has been claimed to be the main cause of subsequent loss of Corg [9,17,18]. However, two experimental studies [19,20] found no Corg loss following disturbance of seagrass. These experiments were performed at a small scale and over short period of time, which could have led to the lack of effect of seagrass loss on their sediment Corg stock. There is therefore the need to produce large scale and long-term experimental studies to understand the effect of seagrass loss on their sediment Corg stocks.
A recent study developed by Githaiga et al. [21] in a tropical seagrass meadow in the Western Indian Ocean, in Gazi Bay, Kenya, has developed a large-scale experimental study on the impact of seagrass loss on environmental conditions, including blue carbon fate. Gazi bay seagrass meadows Corg storage have been estimated to range from 160.7 to 233.8Mg.C.ha-1, storing 4 to 6 times more carbon than surrounding unvegetated areas [22]. In Kenya, seagrass meadows cover is declining with increasing rates since 2000, reaching 1.59% yr-1 in 2016 [23]. In Gazi bay where seagrass cover loss is faster with a rate of 1.69%, small scale fisheries are assumed to be the main drivers of this decline [23]. It is therefore urgent to account for the impact of these tropical seagrass meadows loss on the Corg stored in their sediment.
The study presented in this paper is a prolongation of this experiment, as the aim was to enhance our knowledge on the long-term effect of seagrass loss on sub-surface carbon stocks. It has been conducted in parallel with the seagrass macrofauna experiment presented in Cadier & Frouws [11]. While plots have been continuously harvested for 18 months preceding Githaiga sampling, they were left undisturbed until this sampling which happen 6 months after. We were also interested in the bioturbation potential of the burrowing shrimps present in the bay, as it may impact the fate of the Corg present in the sediment.
Material and Methods
Study area
This study took place in tropical seagrass meadows located in Gazi Bay, Kenya (S 4°25’27.841’’ E 39°30’23.365’’) (Figure 1). The Bay is shallow and semi-enclosed, covering a total area of approximately 13.5km2 with a maximum depth of 8m [24]. The surrounding vegetation is predominantly large mangrove [25]. The Kidogoweni and the Mkurumuji rivers are the main input of freshwater discharge in the bay. Seagrasses form dense beds covering around 70% of the bay area. Gazi Bay hosts twelve seagrass species, with a dominance of Enhalus acoroides and Thalassia hemprichii in the intertidal zone [21]. Seagrass belowground soil have been estimated to store significantly more Corg than unvegetated areas present in the meadow [22]. Vast areas of the bay are exposed during low spring tides, displaying seagrass meadows in tidal pools. Those tidal pools are formed by the presence of regular mounds (10-20cm in height) which are created by the activity of burrowing shrimps.
Study design and sampling
In February 2015, eight experimental plots of 3m × 2m were randomly selected in the intertidal seagrass meadows of Gazi Bay, as described in the Figure 1 & 2 from Githaiga et al. [21]. Plots were dominated by either Enhalus acoroides or Thalassia hemprichii. Harvested treatment concerned four plots, where aboveground seagrass was clipped and removed on a monthly basis, without trampling in the plots. The remaining four plots were left undisturbed as control sites. Seagrass clipping then stopped, leaving the plots undisturbed, allowing for natural regeneration of seagrass. However, seagrass had not recolonised the plots after this period.
Carbon cores were sampled in March 2017 in the plots created for the study performed by Githaiga et al. [21]. Three surface (0-5cm) cores were taken in each plot, alongside two deep (0-15cm) cores (2.7cm diameter). The deep cores were subdivided in three parts (0-5cm; 5-10cm; 10-15cm) to display a depth profile (n=8 for each layer). Surface sediment results were obtained by merging the data from the surface cores and deep cores of equivalent depth (n=20). All cores were dried in the oven at 60°C for 48h, then sieved to eliminate seagrass belowground biomass and dead shells. Dry bulk density was calculated from the formula: DBD (g/cm3) = Dry weight/Original volume of the sediment. Samples were then transported to the Kenyan Marine Fisheries and Research Institute in Mombasa where they were combusted in a muffle furnace at 450°C over 6 hours to obtain Loss on Ignition (LOI) values. The weight changes following combustion gave us the percentage of organic matter in the samples using the formula from Bowden et al. [26]: % LOI = [(Initial dry weight–Weight remaining after ignition)/Initial dry weight] × 100). The %LOI resulting from the samples being higher than 0.2, we used the following formula from Fourqurean et al. [4] to obtain the percentage of Corg of the samples: % Corg = 0.43 * % LOI – 0.33.

To estimate the burrowing shrimp bioturbation activity, a direct entrapment method was set up on the top of burrowing shrimp mounds to estimate their sediment turnover rate [15,27,28]. We did not obtain the authorization to conduct this experiment in the plots as it would have highly disturbed them. Therefore, it was conducted in the nearby intertidal seagrass area to obtain an estimation of local burrowing shrimps bioturbation activity. Ten buckets of 10 litres (27cm diameter) with a 4cm hole drilled in their bottom were used. The top of each bucket top was fitted with a gauze mesh with a diameter of between 63µm and 500µm to avoid sediment deposition from the water column and sediment resuspension from the bucket (Appendix 1). Five buckets were set up on mounds directly surrounded by seagrass meadows, and five were set up on mounds surrounded by barren areas of at least 1m2 to account for the heterogeneity of the landscape. To ensure sediment input was coming from the burrowing shrimp bioturbation activity, a plastic tube was inserted in the mouth of the mound and connected to the bucket hole. As this could have affected shrimp behaviour, we only collected and analysed buckets which were still active after 24h, which we assumed were not stressed by the set up. Furthermore, the tide affected this experiment and some buckets were lifted from their position. These two issues led to the collection of only four buckets which were removed from their position after 24h, and their content was transported to the laboratory. The weight of each bucket’s sediment sample was measured after being dried at 60°C for 48h. To identify the family of burrowing shrimp responsible for the mounds, burrowing shrimps were collected using a plastic tarp following the method described by Priosambodo et al. [29].
Data analysis
Assumption of normality was inspected by displaying a normal probability plot of residuals to compare the dataset with normal distribution, using the standardized residual of the linear regression model. Corg was compared between treatments for sediment surface (0-5cm) using a one tailed test (Wilcoxon signed rank), while a repeated measures ANOVA was used to compare treatments along the depth profile (0-5cm, 5-10cm, 10-15cm). Statistical analyses were performed in R [30].
Results
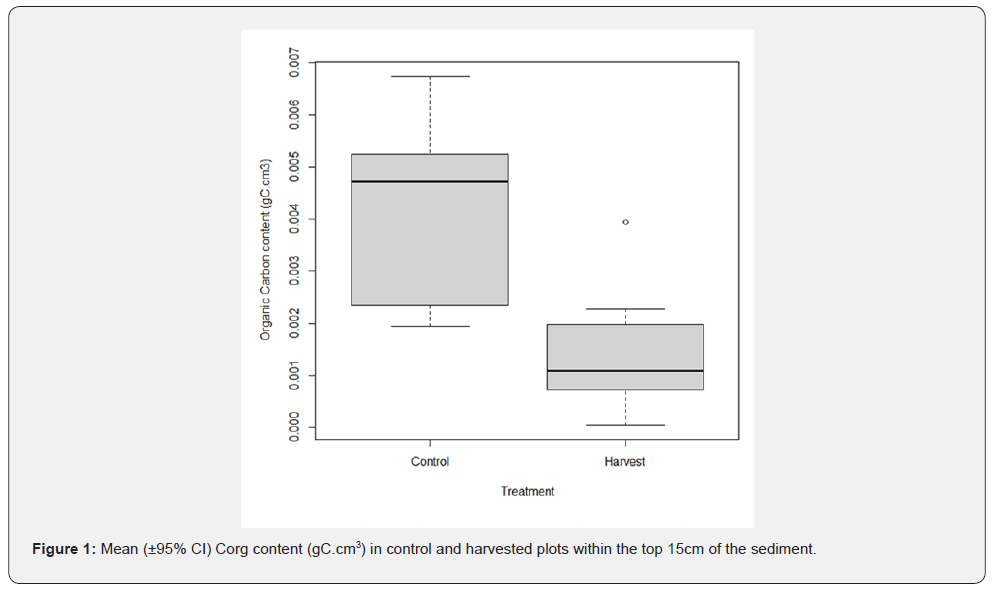
Mean Corg (±SD) content in the top 15cm of control plots was0.0043 (± 0.0021) gC.cm3 and 0.0014 (± 0.0016) gC.cm3 for harvested plots (Figure 1). This corresponded to an average Corg content over the top 15cm of the sediment of 32Mg C.ha-1 in control plots and 11Mg C.ha-1 in harvested plots. Harvested plots contained 66.88 % less Corg content than control plots.
The sediment Corg content depth profile displays higher average Corg in deep (5-15cm) compared to surface (0-5cm) sediment, with similar trend for both treatments (Figure 2). However, error bars display a pronounced difference between surface (0-5cm) and deep (5-15cm) sediment in harvested plots, while in control plots error bars in surface and deep sediment are superposing.
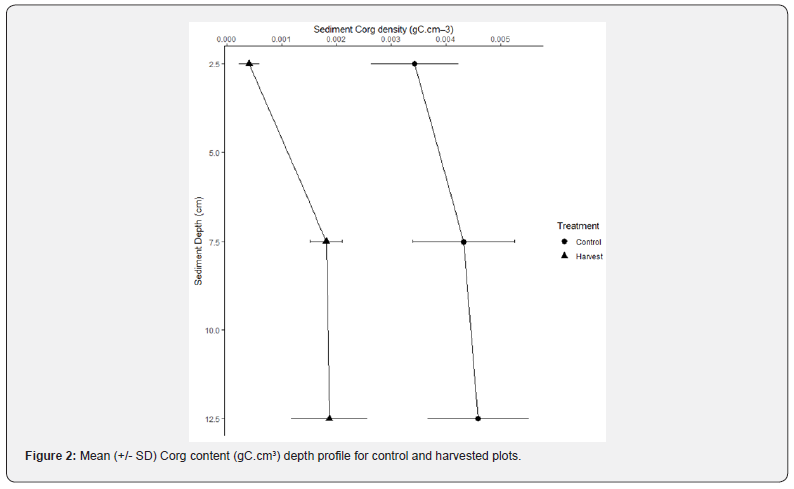
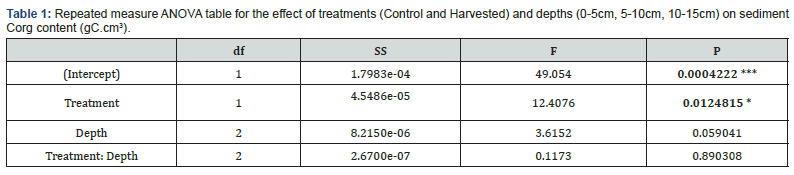
Harvested treatment induced significant differences on Corg content not only over the top 5cm (Wilcoxon : p = <0.05), but also over the top 15cm for every sediment layer tested as displayed by the ANOVA (Table 1). Depth was not a significant factor influencing Corg content, neither was the interaction term with treatment. Therefore, treatment was the main factor responsible for Corg loss in harvested compared to control plots.
An average of 948 ± 342 (SD) g.d-1 DW of sediment was collected per mound, ranging from 149.14g.d-1 to 1740g.d-1 (Table 2) [31]. Burrowing shrimps collected by the plastic tarp method belonged to the family Callianassidae (Appendix 2).


Discussion
The results of this study found that Corg content in harvested plots was significantly lower than that of control pots down to a depth of 15cm. Conversely to the experiments led by Macreadie et al. [17] and Dahl et al. [20], they attest of the effects of seagrass lost on their sediment Corg stocks, which was observed after natural loss of seagrass [9,17,18]. The 68% loss of Corg over the top 15cm of sediment in two years displayed in this study is similar to the 72% loss of Corg observed by Macreadie et al. [17] over the top 30cm, fifty years after the disturbance and without any seagrass recovery. Long scale and long-term experimental disturbance of seagrass are reflecting the observed effect of natural seagrass loss on their sediment Corg stocks. These results are complementary to those displayed by Githaiga et al. [21], which found significant lower Corg in harvested compared to control plots for the surface sediment. This highlights the importance of the impact of long-term seagrass disturbance on sub-surface sediment Corg content. Erosion led to the loss of 3cm of sediment 18 months after the start of the experiment, driving an additional loss of Corg [21]. We did not measure the sediment loss 6 months later, but assume that sediment kept undergoing erosion and that we are here underestimating the loss of Corg in harvested plots.
The fate of blue carbon loss after perturbation is still debated. It is likely that much of the Corg stored in soils under lost seagrass meadows is released back to the ocean-atmosphere CO2 pool, therefore fuelling climate change [4]. A range of emissions modelled by Lovelock et al. [32] estimated a release rate ranging initially from 65Mg CO2.ha.yr-1 and decreasing to 30Mg CO2.ha.yr-1 after 3 years of perturbations for the first meter of sediment of lost seagrass beds. Nonetheless, these estimates rely on the release of all the Corg content from the soil after perturbation, but very few studies have estimated in situ Corg content loss and CO2 emission rate resulting from seagrass loss [3,32]. It could also be moved and stored elsewhere, hence overestimating the impact of seagrass loss on blue carbon storage [33]. This gap needs to be addressed in the scope of the inclusion of seagrass blue carbon loss in offsetting schemes.
It has been hypothesized that significant changes to seagrass faunal communities may influence seagrass sediment carbon stocks, in particular bioturbators [11,12]. Burrowing shrimp of Gazi Bay, assumed to be part of the Callianassidae family, have an important bioturbation capacity, with a sediment turnover here estimated here to be around 948 ± 342 (SE)g.d-1 DW. This bioturbation activity seems to be in coherence with other studies, especially with the results of Kneer et al. [15] (Table 2). However, it is difficult to compare this study to others as methodologies and units between studies are inconsistent (Table 2). If we are to understand the influence of Callianassidae shrimp bioturbation activity on the blue carbon stored in seagrass ecosystems, we need to standardise the methods employed to assess their sediment turnover capacity in the field. Future studies on burrowing shrimp sediment turnover rates should use the bucket trap method employed in this study, as it is an easy to implement, cost-effective method using a consistent unit of measurement in g (dry).mound-1.d-1 [28].
Conclusion
This study demonstrated that long-term disturbance of tropical seagrass meadows can lead to sub-surface loss of Corg stored in their sediment. The presence of burrowing shrimps, co-existing naturally in seagrass meadows, might enhance Corg remineralisation due to their bioturbation activity. More studies are required to understand the impact of burrowing shrimps on seagrass Corg storage capacity. Furthermore, there is a need to understand the fate of the lost soil Corg to accurately estimate the impact of seagrass loss in blue carbon budget. Ecological interactions in tropical meadows are complex, and interdisciplinary research projects are required to understand their outcomes.
Declaration of Funding
This research was partly funded by the Edinburgh Napier University, the Agence Universitaire de la Francophonie and a scholarship from AquiMob.
Acknowledgement
I acknowledge the Kenya Marine and Fisheries Research Institute (KMFRI) for accommodating this research at the Gazi Bay substation. Especially, I would like to thank the substation director Dr. J. G. Kairo, Dr. M. N. Githaiga, the field assistants Laitani Suleiman and Tom Peter Kisiengo, alongside all members of this substation for their help during these experiments. I am specifically grateful to Pr. M. Huxham for his support and advice all along this study. Finally, a special thanks to Alex Pearse for his helpful reviews of this manuscript.
References
- Hemminga MA, Duarte CM (2000) Seagrass Ecology.
- Nellemann C, Corcoran E, Duarte CM, Valdés L, De Young C, et al. (2009) Blue Carbon - The Role of Healthy Oceans in Binding Carbon, Environment.
- Pendleton L, Donato DC, Murray BC, Crooks S, Jenkins WA, et al. (2012) Estimating Global “Blue Carbon” Emissions from Conversion and Degradation of Vegetated Coastal Ecosystems. PLoS One 7(9): e43542.
- Fourqurean JW, Duarte CM, Kennedy H, Marbà N, Holmer M, et al. (2012) Seagrass ecosystems as a globally significant carbon stock. Nat Geosci 5: 505-509.
- Kennedy H, Beggins J, Duarte CM, Fourqurean JW, Holmer M, et al. (2010) Seagrass sediments as a global carbon sink: Isotopic constraints. Global Biogeochem Cycles 24(4): 1-8.
- Duarte CM, Kennedy H, Marbà N, Hendriks I (2013) Assessing the capacity of seagrass meadows for carbon burial: Current limitations and future strategies. Ocean Coast Manag 83: 32-38.
- Waycott M, Duarte CM, Carruthers TJB, Orth RJ, Dennison WC, et al. (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci USA 106(30): 12377-12381.
- Orth RJ, Carruthers TJB, Dennison WC, Duarte CM, Fourqurean JW, et al. (2006) A Global Crisis for Seagrass Ecosystems. Bioscience 56(12): 987-996.
- Arias-Ortiz A, Serrano O, Masqué P, Lavery PS, Mueller U, et al. (2018) A marine heatwave drives massive losses from the world’s largest seagrass carbon stocks. Nat Clim Chang 8: 338-344.
- Spivak AC, Sanderman J, Bowen JL, Canuel EA, Hopkinson CS (2019) Global-change controls on soil-carbon accumulation and loss in coastal vegetated ecosystems. Nat Geosci 12: 685-692.
- Cadier C, Frouws A (2019) Experimental harvest in a tropical seagrass meadow leads to shift in associated benthic communities. Community Ecol 20(2): 138-148.
- Thomson ACG, Trevathan Tackett SM, Maher DT, Ralph PJ, Macreadie PI (2018) Bioturbator-stimulated loss of seagrass sediment carbon stocks. Limnol Oceanogr 64(1): 342-356.
- Castorani MCN, Hovel KA, Williams SL, Baskett ML (2014) Disturbance facilitates the coexistence of antagonistic ecosystem engineers in California estuaries. Ecology 95(8): 2277-2288.
- Suchanek TH (1983) Control of seagrass communities and sediment distribution by Callianassa ( Crustacea, Thalassinidea) bioturbation. J Mar Res 41: 281-298.
- Kneer D, Asmus H, Jompa J (2013) Do burrowing callianassid shrimp control the lower boundary of tropical seagrass beds? J Exp Mar Bio Ecol 446: 262-272.
- Kristensen E, Penha Lopes G, Delefosse M, Valdemarsen T, Quintana CO, et al. (2012) What is bioturbation? the need for a precise definition for fauna in aquatic sciences. Mar Ecol Prog Ser 446: 285-302.
- Macreadie PI, Trevathan Tackett SM, Skilbeck CG, Sanderman J, Curlevski N, et al. (2015) Losses and recovery of organic carbon from a seagrass ecosystem following disturbance. Proc R Soc B Biol Sci 282(1817).
- Marba N, Arias Ortiz A, Masque P, Kendrick GAA, Mazarrasa I, et al. (2015) Impact of seagrass loss and subsequent revegetation on carbon sequestration and stocks. J Ecol 103(2): 296-302.
- Macreadie PI, York PH, Sherman CDH, Keough MJ, Ross DJ, et al. (2014) No detectable impact of small-scale disturbances on ‘blue carbon’ within seagrass beds. Mar Biol 161: 2939-2944.
- Dahl M, Deyanova D, Lyimo LD, Näslund J, Samuelsson GS, et al. (2016) Effects of shading and simulated grazing on carbon sequestration in a tropical seagrass meadow. J Ecol 104(3): 654-664.
- Githaiga MN, Frouws AM, Kairo JG, Huxham M (2019) Seagrass removal leads to rapid changes in Fauna and loss of carbon. Front Ecol Evol 7: 1-12.
- Githaiga MN, Kairo JG, Gilpin L, Huxham M (2016) Carbon storage in the seagrass meadows of Gazi Bay, Kenya. PLoS One 12(5): 1-13.
- Harcourt WD, Briers RA, Huxham M (2018) The thin(ning) green line? Investigating changes in Kenya’s seagrass coverage. Biol Lett 14(11).
- Kitheka JU (1997) Coastal tidally-driven circulation and the role of water exchange in the linkage between tropical coastal ecosystems. Estuar Coast Shelf Sci 45(2): 177-187.
- Galllin E, Coppejans E, Beeckman H (1989) The mangovre vegetation of Gazi Bay (Kenya). Bull la Société R Bot Belgique.
- Bowden DA, Rowden AA, Attrill MJ (2001) Effect of patch size and in-patch location on the infaunal macroinvertebrate assemblages of Zostera marina seagrass beds. J Exp Mar Bio Ecol 259(2): 133-154.
- Berkenbusch K, Rowden AA, Myers TE (2007) Interactions between seagrasses and burrowing ghost shrimps and their influence on infaunal assemblages. J Exp Mar Bio Ecol 341(1): 70-84.
- Rowden AA, Jones MB (1993) Critical evaluation of sediment turnover estimates for Callianassidae (Decapoda: Thalassinidea). J Exp Mar Bio Ecol 173(2): 265-272.
- Priosambodo D, Kneer D, Asmus H, Zamani NP, Von Juterzenka K, et al. (2014) Community Analysis of Burrower Shrimp in Bonebatang Seagrass Bed South Sulawesi. Proc 1st Int Conf Sci 1-9.
- R Core Team (2014) R: A language and environment for statistical computing. R Found Stat Comput, Vienna, Austria.
- Suchanek TH, Colin PL (1986) Rates and effects of bioturbation by invertebrates and fishes at Enewetak and Bikini Atolls. Bull Mar Sci 38(1): 25-34.
- Lovelock CE, Fourqurean JW, Morris JT (2017) Modeled CO2 emissions from coastal wetland transitions to other land uses: Tidal marshes, mangrove forests, and seagrass beds. Front Mar Sci 4: 143.
- Johannessen SC, Macdonald RW (2016) Geoengineering with seagrasses: Is credit due where credit is given? Environ Res Lett 13(2).






























