Statistical Modelling of Indigenous Chicken with Body Weight and Linear Body Measurements in Bench Maji Zone, South Western Ethiopia
Abiyu Tadele*
Department of Animal Science, Bonga University, Ethiopia
Submission: October 04, 2019; Published: October 30, 2019
*Corresponding author: Abiyu Tadele, Department of Animal Science, College of Agriculture and Natural Resources, Bonga University, P.O. Box 334, Ethiopia
How to cite this article: Abiyu Tadele. Statistical Modelling of Indigenous Chicken with Body Weight and Linear Body Measurements in Bench Maji Zone,South Western Ethiopia. Int J Environ Sci Nat Res. 2019; 22(2): 556083. DOI: 0.19080/IJESNR.2019.22.556083
Abstract
Human activities have been at the centre in land use and land cover changes. Activities such as farming, new settlement, land reclamation, industrialization together with urbanization have been seen to influence land use and land cover changes. This study examined how establishment of Rongo University as an institution of higher learning has influenced land use and land cover changes in Rongo Sub-County, Migori County, Kenya. The study adopted the use of GIS and Remote sensing techniques to acquire, analyze and determine changes in Landsat images for 2003, 2010, 2013, 2013, 2014, 2015, 2016, 2017 and 2018. Before and after the University was established. The land use and land cover classes that were used included tree cover, open land, plantation agriculture, settlement patterns, and grassland vegetation. Supervised classification was used to analyze land-use/cover change date generated from GIS and remote sensing which made it possible to define training data set/signature that indicated the pixels to be selected and the type of software to be used for land-cover categories. The study established that there is a significant influence on land use and land cover changes as a result of establishing Rongo University as an institution of higher learning. As they come with a number of activities in the areas of establishment. The study noted that new settlements have emerged and replaced the initial land cover with attendant effects on ecosystem and biodiversity. Further, the study recorded considerable transformation of farmlands into built up environment. The open land also reduces significantly, and university triggered informal settlement or slums also emerged.
Keywords: Institutions of higher learning University establishment Land use/land cover changes
Introduction
Over the years, many countries have focused on promoting development of learning institutions for the prosperity of their citizens. As much as development of learning institutions is seen as positive development, however, they have also brought with them significant negative impacts on the environment. Education is key in almost all our nations and it makes people gain knowledge and skills used in the development of all sectors. Education also enables citizens of particular nations to improve in their competencies and capabilities in competing with the changing world market in terms of securing jobs and being able to perform particular tasks requiring higher technology. It has always been known that establishment of institutions of higher in a given place not only brings ease in accessing education but also creates employment opportunities in the region. Their establishment also result in the development of business services and improve transport and communication infrastructure in the region. In many occasions, environment has been degraded knowingly and unknowingly in the process of establishing these institutions of higher learning. Our natural resources come from the environment and anything that disrupts the well-being of our environment, disrupts the very survival of these natural resources. Majority of the rural poor in developing countries depends on wood fuel and the surface water together with farming. They also depend on immediate pasture that they can get around. As we may all know, environment is the mother of all resources. Vegetation for instance is a land cover and helps to purify the air since green plants are able to manufacture their own food through the process of photosynthesis which yields oxygen. In the process of photosynthesis, carbon dioxide which is a greenhouse gas is absorbed by the plants and for this reason, plants act as carbon sinks hence purify the air. Vegetation which is a land cover protects the soil from being eroded or degraded by the runoff and also control the rate of infiltration.
It has been noted that where many structures are coming up, land cover is lost. That is, natural environment is transformed into a built environment. The land use also changes as what was initially used as farmland is changed into settlement or serve a different purpose. Open lands are also built up. Grassland is also lost together with the forested lands. Some wetlands are reclaimed and therefore their ecological roles compromised. Establishment of these institutions of higher learning are always associated with development of slums. These are unplanned urban centres which come up as a result of people who are drawn from various places while seeking business or job opportunities. As a result of the need for housing services, and business enterprises, unplanned development is realized. Often, development of these slums is characterized by haphazard placement of structures which do not conform with their neighbourhoods. They are also associated with poor waste management which in most cases interferes with surface and ground water quality and the general hygiene of the public.
Materials and Methods
Description of the study area
Bench Maji is one of the Zones of the Ethiopian Southern Nations, Nationalities, and Peoples’ Region (SNNPR). Bench Maji is bordered on the south by the Ilemi Triangle, on the west by South Sudan, on the northwest by the Gambela Region, on the north by Sheka, on the northeast by Keffa, and on the east by Debub Omo. The Omo River defines much of its eastern border with Debub Omo. The administrative center of Bench Maji is Mizan Teferi; other towns include Maji. Bench Maji has 142 kilometers of dry-weather roads, for an average road density of 22 kilometers per 1000 square kilometers. The highest point in this Zone is Mount Guraferda (2494 meters). The Omo National Park is located on the western bank of the Omo River. The main food crops in this Zone include maize, godere (taro root), and enset, while sorghum, teff, wheat and barley are cultivated to a significant extent. Although cattle, shoats and poultry are produced in limited numbers, meat and milk are very much appreciated. Cash crops include fruits (bananas, pineapples, oranges) and spices (e.g. coriander and ginger); honey is also an important local source of income. However, coffee is the primary cash crop. Based on the 2007 Census conducted by the CSA, this Zone has a total population of 652,531, of whom 323,348 are men and 329,183 women; with an area of 19,252.00 square kilometers, Bench Maji has a population density of 33.89. While 75,241 or 11.53% are urban inhabitants, a further 398 or 0.06% are pastoralists. A total of 157,598 households were counted in this Zone, which results in an average of 4.14 persons to a household, and 151,940 housing units.
Sampling technique and methods of data collection
The study areas were purposively selected based on their potential for chicken population, accessibility, presence of indigenous chicken production and agro ecology. Before the main survey was commenced, a preliminary assessment was made to identify whether there is pure exotic and/or their crosses in the study areas. From eleven districts of Bench Maji Zone, three districts were selected based on agro ecology. From each districts four rural kebeles were randomly sampled. Then, a total of 120 households, 10 households from each rural kebeles, who possess a minimum of five matured indigenous chickens were randomly selected. Closely adjacent households were also skipped to avoid the risk of sampling chickens sharing the same cock. From each household, 2 matured female chickens were sampled for body weight and linear body measurement traits with a total number of 240 chickens.
Measurements of quantitative traits
A total of 240 indigenous adult female chickens were randomly sampled from each household and was determined by “recalling methods” of the interviewed farmers. Quantitative measurements of linear traits and body weight were taken on sampled indigenous female chickens using a textile measuringtape (cm) and a hanging spring balance (kg). Data on body weight, chest width (the circumference of the breast region), body length (the distance from the tip of the beak to cauda / tail, without feathers), shank length (length of the shank from the top of the hock joint to the bottom of the footpad), shank circumference (measured around the midway of the shank), keel bone length (obtained from sternum to bottom of the keel), back length (length from insertion of the neck into the body to the saddle) and wing length (length between the base of the neck and the uropygial gland gland) were taken from female chicken following FAO’s descriptor for the characterization of chicken genetic resources [9].
Statistical Analysis
Data collected on quantitative traits of indigenous chicken populations were coded and entered into a computer using Microsoft Office Excel 2007. Body weight and linear body measurement traits were analyzed using the General Linear Model (GLM) procedures of Statistical Analysis System (SPSS, ver. 20). The model was fitted to main effects of district and sex on body weight and linear body measurements of chickens. Stepwise simple and multiple regression procedure was employed to regress body weight for female chicken to determine the best-fitting regression equations for the prediction of live body weight.
The following model were used for the statistical analysis:
Model
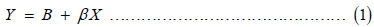
Simple regression model
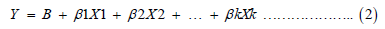
Multiple regression model
Where Y = dependent variable (body weight)
Xi = independent variables (BL; CC; KBL; SHL; SHC; BKL; WL)
β0 = the intercept
βi = the slopes
Results
Body weight and body linear parameters
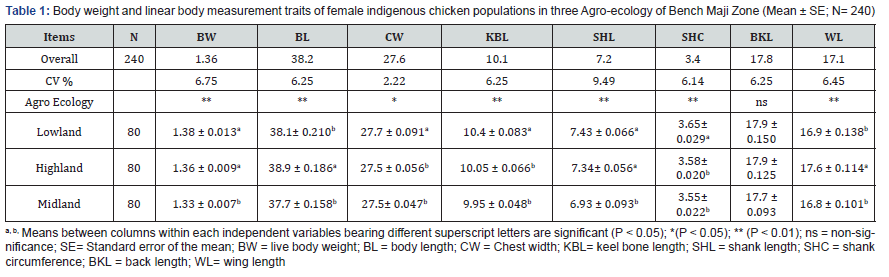
In the present study, Agro ecology had significant effect on body weight and all quantitative traits except back length (Table 1). The overall average body weight of female chicken was 1.36kg. The results for keel bone and wing length, body length and width, shank length and circumference were highly different across all the studied agro ecology. The value of keel bone for chickens reared in low altitude was significantly higher (P < 0.01) than, mid and high altitudes. However, there was no significance difference (P > 0.05) in keel bone of chickens reared in mid and high altitudes. Wing and body length of chickens reared in thestudy areas were significantly (P < 0.01) different. Wing and body length in high altitude chickens had higher value than mid and low altitudes. The value of back length of chickens reared in all the study agro ecology were not statistically significant (P > 0.05). The value of chest width in chickens reared in low altitude was higher (P < 0.01) than those chickens reared in mid and high altitudes whereas mid and high-altitude chickens had comparable values. Shank length values of chickens measured in high and low altitude had significantly higher values (P < 0.01) than those observed in mid altitude. The shank circumference of chickens reared in low altitude had significantly higher (P < 0.01) values than those reared mid and high altitudes.
Correlations between body weight and linear body measurement
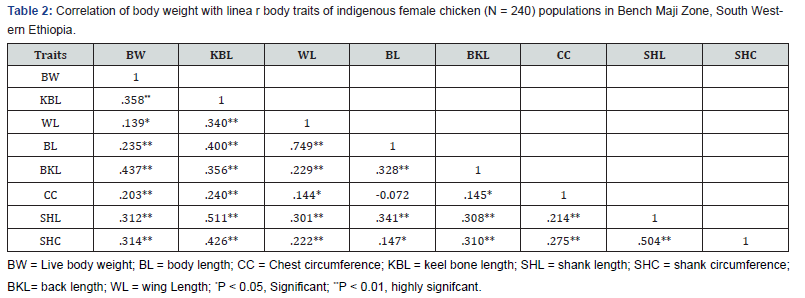
As presented in Table 2, all linear body measurements of chickens in the studied areas were highly correlated with body weight. The correlations of body weight with body length, chest width, keel bone length, shank length, shank circumference, back length and wing length were 0.235, 0.203, 0.358, 0.312, 0.314, 0.437 and 0.139, respectively.
Prediction of body weight using body linear parameters
Predictive equation relating to body weight of indigenous chickens to linear body measurements in the study area was shown in Table 3. Body weight and linear body measurements had significant (P < 0.01) associations.
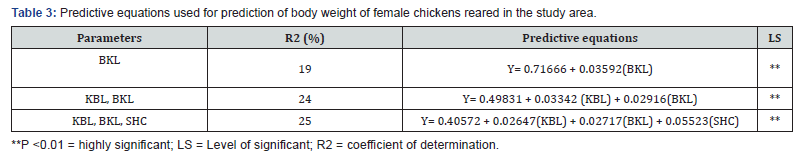
Y = predicted live body weight; KBL = keel bone length; SHC = shank circumference; BKL= back length.
Discussion
Body weight and body linear parameters
The average values of live weight of females observed in the current study was comparable with the findings of Addis et al. ([10]. However lower values of body weight were reported by various scholars in the country [8,11].
The results on linear body measurement traits in the current study was comparable with most of the observations in different parts of the country. Accordingly, the average value of BL in the present study was in close agreement with the reports of Eskindir et al. [11] and Addis et al. [10]. However, it was higher than those reported by Deneke et al. [8] and Emebet et al. [12]. These variations in body length might be due to the age of animals, agro-climatic conditions and status of nutrition of chickens when the data were collected by various scholars.
The average value of CW in the current study was in close agreement with the reports of Eskindir et al. [11] and Deneke et al. [8]. Chest width (chest girth) is an indicator of fleshing of a chicken. The average value of KBL in the present study was also comparable with the report of Deneke et al. [8]. However, higher values of KBL were reported by Eskindir et al. [11] from Horro and Jarso districts of Oromia Zone, which may reflect better frame size in the latter chicken ecotypes Length of keel and shank are also regarded as good indicators of skeletal development of a bird, which is related to the amount of meat a chicken can carry. The results obtained for average SHL in the present study were comparable with the reports of Addisu et al. (2013) and Eskindir et al. [11]. However, it was higher than those reported by Deneke et al. [8] in chickens reared in Southeastern Oromia Regional State of Ethiopia. These variations might be explained by the availability of scavengable feed resources both in quality and quantity in those different study locations.
The present average result pertaining to shank circumference was in close agreement with the reports of different scholars in Ethiopia [8,10]. The average values relating to back length were comparable with those reported by Eskindir et al. [11]. The observed significant effects of age on body weight and linear body measurement traits of chickens in the present study was in line with the reports of Semakula et al. [13] and Ojedapo et al. [14] who noted that, body weight and linear body measurements increases with the advancing age of chickens.
Correlations between body weight and linear body measurements
In the current study, positive and significant (P < 0.01) correlations was observed between body weight and linear body measurement traits and are in good agreement with the reports of Addis et al. [10] and Deneke et al. [8]. These positiveand significant correlations of body weight with linear body measurements observed in the present study and elsewhere suggest that measuring one of these quantitative traits enables to predict the body weight of local chickens in rural farming society. The results of the present study and findings of other scholars therefore suggests that, selection for any of these linear body measurable traits will cause direct improvement in body weight of indigenous chicken populations [7,15].
In the present study, the R2 values ranged from low (0.19) to relatively high (0.25) indicating that the calculated equations could be used to predict the body weight of chickens. Predictive equations provide a readily available tool in body weight estimation. This is particularly true in rural areas or areas where weighing scales are not available as suggested by Alabi et al. [16], Addis et al. [10] and Liyanage et al. [17,18].
Conclusion
Variations in linear body measurement traits were observed indicating the existence of genetic differences in major performance traits which makes selection between indigenous chicken populations a viable option to improve their genetic potentials. Moreover, authors recommend an in-depth molecular assessment to concretely validate the level of genetic variations and relationship existing among indigenous chicken populations of the study areas.
Acknowledgement
The author would like to acknowledge Bench Maji Zone, Livestock and Fishery development offices for their kind support and collaborative work. I am also grateful for those farmers and development agents who participated in this study.
References
- Kondombo SR (2005) Improvement of village chicken production in a mixed (chicken–ram) farming system in Burkina Faso. PhD thesis. Wageningen Institute of Animal Sciences, Animal Nutrition Group, Wageningen University, The Netherlands, p. 208.
- Alewi M, Melesse A (2013) Evaluating the growth performance of local Kei chickens and their F1-Crosses with Rhode Island Red and Fayoumi breeds in watershed areas of Guraghe Administrative Zone, Southern Ethiopia. Tropical and Subtropical Agro-ecosystems 16(1): 39-50.
- Melesse A (2014) Significance of scavenging chicken production in the rural community of Africa for enhanced food security. World’s Poultry Science Journal 70(3): 593-606.
- Central Statistical Agency (CSA) (2017) Agricultural Sample Survey Statistical Bulletin Addis Ababa, Ethiopia, p. 188.
- Halima H, Neser FWC, van Marle-Koster E, deKock A (2007) Phenotypic variation of indigenous chicken populations in northwest Ethiopia. Trop Anim Health Product 39(7): 507-
- Moges F, Melesse A, Dessie T (2010) Assessment of village chicken production system and evaluation of the productive and reproductive performance of local chicken ecotype in Bure district, North West Ethiopia. African Journal of Agricultural Research 5(13): 1739-1748.
- Ukwu HO, Okoro VMO, Nosike RJ (2014) Statistical Modelling of Body Weight and Linear Body Measurements in Nigerian Indigenous Chicken. IOSR Journal of Agriculture and Veterinary Science 7(1): 27-30.
- Negassa D, Melesse A, Banerjee S (2014) Phenotypic characterization of indigenous chicken populations in Southeastern Oromia Regional State of Ethiopia. Animal Genetic Resources 55: 101-
- FAO (Food and Agriculture Organization of the United Nations) (2012) Phenotypic characterization of animal genetic resources. FAO Animal Production and Health Guidelines, No. 11. Rome.
- Getu a, Alemayehu K, Wuletaw Z (2014) Phenotypic Characterization of Indigenous Chicken Ecotypes in North Gondar Zone, Ethiopia. Global Veterinarian 12(3): 361-368.
- Aklilu E, Kebede K, Dessie T, Banerjee AK (2013) Phenotypic Characterization of Indigenous Chicken Population in Ethiopia. International Journal of Interdisciplinary and Multidisciplinary Studies (1)1: 24-32.
- Moreda E, Singh H, Sisaye T, Johansson AM (2014) Phenotypic Characterization of Indigenous Chicken Population in South West and South Part of Ethiopia. British Journal of Poultry Sciences 3(1): 15-19.
- Semakula J, Lusembo P, Kugonza DR, Mutetikka D, Ssennyonjo J, et al. (2011) Estimation of live body weight using zoometrical measurements for improved marketing of indigenous chicken in the Lake Victoria basin of Uganda. Livestock Research for Rural Development 23(8).
- Ojedapo LO, Amao SR, Ameen SA, Adedeji TA, Ogundipe RI, et al. (2012) Prediction of body weight and other linear body measurement of two commercial layer strain chickens. Asian Journal of Animal Sciences 6(1): 13-22.
- Tabassum F, Hoque MA, Islam F, Ritchil CH, Faruque MO, et al. (2014) Phenotypic and Morphometric characterization of indigenous chickens at jhenaigati upazila of sherpur district in bangladesh. Saarc journal of Agriculture 12(2): 154-169.
- Alabi OJ, Ngambi JW, Norris D, Egena SSA (2012) Comparative study of three indigenous chicken breeds of South Africa: Body weight and linear body measurements. Agricultural Journal 7(3): 220-225.
- Liyanage RP, Dematawewal CMB, Silva GLLP (2015) Comparative Study on Morphological and Morphometric Features of Village Chicken in Sri Lanka. Tropical Agricultural Research 26(2): 261-273.
- Melesse A, Maak S, Schmidt R, von Lengerken G (2011) Effect of long-term heat stress.






























