Hydrochlorothiazide Degradation by UV-based Advanced Oxidation Processes
M Fernández Perales, M Sánchez Polo*, A M S Polo, M Rozalen and J Rivera Utrilla
Department of Inorganic Chemistry, University of Granada, Spain
Submission: April 15, 2019; Published: April 25, 2019
*Corresponding author: M Sánchez Polo Department of Inorganic Chemistry, Faculty of Science, University of Granada, 18071 Granada, Spain
How to cite this article: M Fernández P, M Sánchez P, A M S Polo, M Rozalen, J Rivera U. Hydrochlorothiazide Degradation by UV-based Advanced Oxidation Processes. Int J Environ Sci Nat Res. 2019; 18(5): 555997. DOI:10.19080/IJESNR.2019.18.555997
Keywords: Hydrochlorothiazide degradation; Oxidation processes; Pharmaceutical; Emergent pollutants; Conventional biological; Photodegradation; Toxicity
Introduction
Most of pharmaceutical products are considered as emergent pollutants. This represents a growing concern, especially in developed countries, where they are frequently used. Water waste treatment plants degrade partially these pollutants while others remain unaltered and are detected in the plant effluents, as it was reported in numerous studies [1,2]. Hydrochlorothiazide (HCTZ), a thiazide diuretic frequently used in the treatment of hypertension, appears as one of the twenty most detected and persistent compound in wastewater tributaries in Spain, Canada and Netherlands [3].
Conventional biological treatments have not demonstrated to be effective to remove this pollutant in wastewaters. The AOPS (Advanced Oxidation Processes) represent an innovative alternative technology to eliminate these resistant compounds. Moreover, the combination of direct photolysis (UV radiation) with oxidant species such as HO• or SO4•- radicals has been successfully applied to destruct organic micropollutants in different water matrices [4].
The main objective of this study was to analyse the efficiency of ultraviolet (UV) and solar radiation in the photodegradation of hydrochlorothiazide. For this reason, it is determined:
a) Quantum yield and photochemical kinetic parameters of the process were determined by UV photolysis of HTCZ with the influence of the different operational variables (initial concentration and pH),
b) Indirect photodegradation in presence of HO• radical promoter and
c) by-products toxicity.
Materials and Methods
UV photolysis experiments were conducted using a photochemical reactor equipped with a low-pressure mercury lamp which emitted monochromatic radiation at 254nm was used.
The influence of operational variables was investigating evaluating the effect of different initial pH (3,8.5 and 10) and the influence of concentration (25, 15 and 10ppm HCTZ). Additionally, in order to evaluate the effect of HO• radical, hydrogen peroxide was added at initial concentrations of 3, 5, 7 and 10 ppm. HCTZ concentrations were measured by HPLC (Novapak C18 column). The detection was performed at 310nm using a mobile phase of acetonitrile and formic acid (70:30 in volume), the elution flow rate was 0.35mL min−1 and the injection volume was 100uL in all samples.
By-products cytotoxicity were evaluated by using an MTS assay to determine the % viability of human embryo kidney cells (HEK-293) from the CIC cell bank of the University of Granada. Degradation kinetics were first studied in the presence of phosphate buffered saline(PBS), and 10,000 HEK-293 cells were then incubated for 24h, subsequently changing the medium and adding HCTZ by-products (10:100μL dilution);after incubation for a further 24h, 20μL MTS was added and its absorbance was measured at 2h using INFINITENANOQUA equipment, with a sample reading of 9 times at 490nm absorbance.
Results and Discussion
UV direct photolysis results showed a 90% reduction of the initial concentration of HCTZ after 30min, as it is observed in Figure 1. The values obtained are like those reported by other authors for other pharmaceutical compounds [5,6]. As it was expected, the obtained results in presence of HO• radical improved the treatment reaching a complete degradation after only 20min (Figure 1). However, the unique presence of radical promoters does not show any effect. The apparent reaction rate constant also increases with the addition of H2O2 (Table 1) due to the generation of HO• which attack HCTZ by degrading it into by-products of smaller molecular weight. The low molar absorption coefficient of H2O2 could be compensated by increasing the amount of reagent, however, HO• radicals generated can also react with H2O2, especially if it is in high concentrations. The reaction rate constant of HO• with HCTZ was determined to identify the influence of these recombination reactions, obtaining a value of kHCTZ/HO•=6.43x109 M-1s-1, higher than the reaction rate constant of HO• (3.3x107 M-1s-1). Therefore, degradation of HCTZ by HO• may be favoured until a given initial concentration of H2O2.
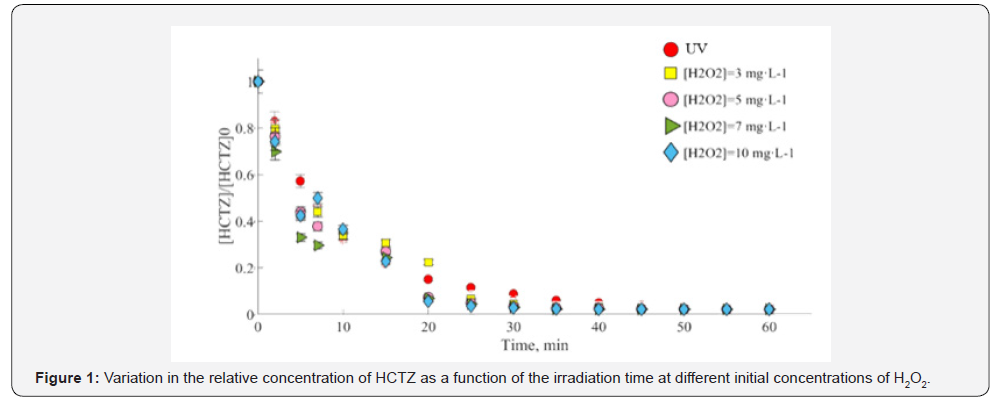
Operational variables studied showed the following results: Photodegradation rate decreases as HTCZ initial concentration increases due to the energy absorbed by each molecule. The effect of the initial pH pointed out an increase of degradation rate at acidic pHs, which is in accordance with the speciation of HCTZ.
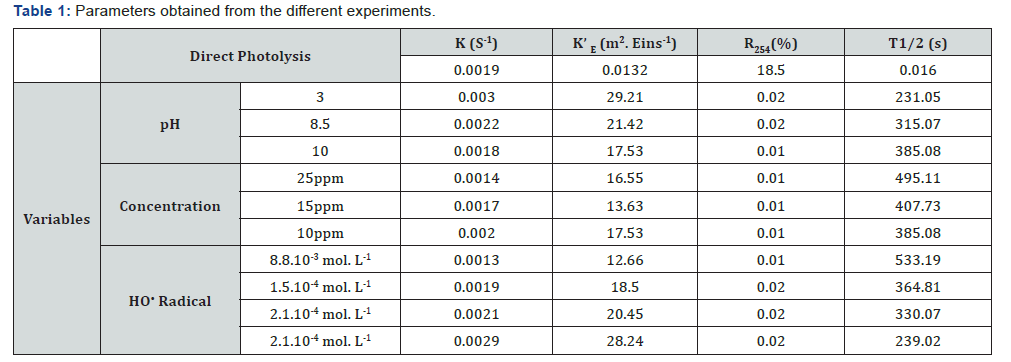
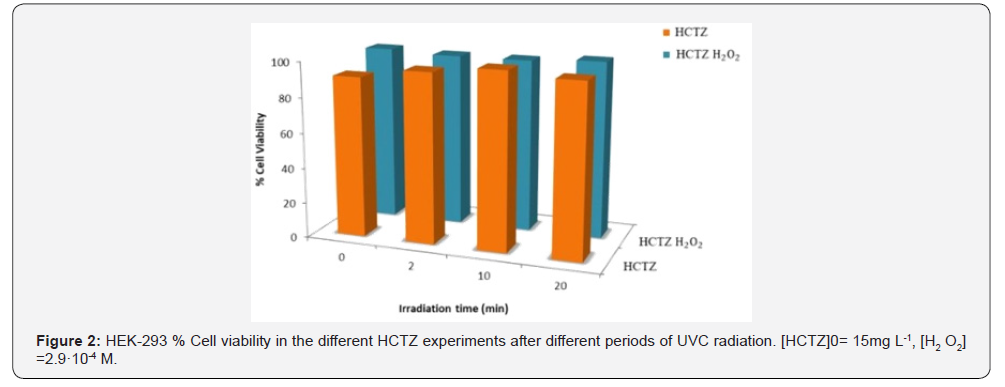
Table 1 depicts the obtained HCTZ quantum yield as a function of their concentration (25, 15, and 10mg·L-1 respectively), pointing out that HCTZ photodegradation rate decrease as concentration increases, due to the energy absorbed by each molecule. Hence, given that the radiation energy irradiated in the medium per unit of volume is constant, HCTZ molecules can accept more radiant energy at lower concentrations, explaining the behavior observed.
The comparison of the viability of by-product treated cell cultures with the viability of original product treated cell cultures (time 0) (Figure 2) showed a greater viability of the by-product treated cells, reason why these products are considered less toxic than the original product.
Conclusion
The results obtained in this study suggested that direct photolysis by UV photolysis is not sufficiently effective to reach the complete degradation of this drug. The operational variables play an important role in the design of the process, as well as, the addition of HO radical promoter, which in presence of UV radiation enhancing the degradation process. Moreover, the degradation by-products obtained are not toxic. Consequently, this procedure could be a promising technology for HCTZ degradation in waste waters.
References
- Tijani JO, Fatoba OO, Petrik LF (2013) A Review of Pharmaceuticals and Endocrine-Disrupting Compounds: Sources, Effects, Removal, and Detections. Water, Air, & Soil Pollution 224(11): 1770.
- Thanh Thuy HT, Nguyen TD (2013) The potential environmental risks of pharmaceuticals in Vietnamese aquatic systems: case study of antibiotics and synthetic hormones. Environ Sci Pollut Res Int 20(11): 8132-8140.
- Bueno MJ, Gomez MJ, Herrera S, Hernando MD, Agüera A, et al. (2012) Occurrence and persistence of organic emerging contaminants and priority pollutants in five sewage treatment plants of Spain: Two years pilot survey monitoring. Environ Pollut 164: 267-273.
- Acosta-Rangel A, Sánchez-Polo M, Polo AMS, Rivera-Utrilla J, Berber- Mendoza MS (2018) Sulfonamides degradation assisted by UV, UV/H2O2 and UV/K2S2O8: Efficiency, mechanism and byproducts cytotoxicity. J Environ Manage 225: 224-231.
- Canonica S, Meunier L, von Gunten U (2008) Phototransformation of selected pharmaceuticals during UV treatment of drinking water. Water Research 42(1-2): 121-128.
- Gómez-Pacheco V, Sánchez-Polo M, Rivera-Utrilla J, López-Peñalver JJ (2012) Tetracycline degradation in aqueous phase by ultraviolet radiation. Chemical Engineering Journal 187: 89-95.






























