Evaluation of Water Melon Peels as Economic Adsorbents for Removal of High Levels of Iron from Different Water Sources
Hassouna MEM1*, Marzouk MA2, Elbably MA3 and El Maghrabi AH1
1Department of Chemistry, Beni-Suef University, Egypt
2Department of Botany & Microbiology, Beni-Suef University, Egypt
3Department of Hygiene, Management & Zoonoses, Beni-Suef University, Egypt
Submission: November 19, 2018; Published: March 08, 2019
*Corresponding author: Hassouna MEM, Department of Chemistry, Faculty of Science, Beni-Suef University, Egypt
How to cite this article: Hassouna MEM, Marzouk MA, Elbably MA, El Maghrabi AH. Evaluation of Water Melon Peels as Economic Adsorbents for Removal of High Levels of Iron from Different Water Sources. Int J Environ Sci Nat Res. 2019; 17(4): 555970. DOI:10.19080/IJESNR.2019.17.555970
Abstract
Optimum conditions for the adsorption of iron onto crude and modified (with lactic acid and tri sodium citrate) water melons peels (WMP) from aqueous solution are investigated as inexpensive and eco-friendly available adsorbents. Water samples are gathered from different sources (surface, tap and ground). The three WMP forms are characterized by FT-IR and SEM. Adsorption variables such as: adsorbent dose, stirring time and pH have been optimized. The removal efficiency of the unmodified WMP reaches its maximum uptake in the pH range 4-6, the other modified WMP powders in the range 5-7. The removal process is slow in the first 10min. then it is gradually increased till equilibrium after 20min in case of unmodified WMP, the two modified forms reach equilibrium after 15min. Kinetic studies indicated that adsorption follows the Langmuir and Freundlich adsorption isotherms. Desorption processes assured the possibility to regenerate and reuse the adsorbents..
Keywords: Heavy metals; Fe; Water melon peels; Economic sorbent; Water sources; Adsorption
Introduction
Heavy metals have become a question of global concern considering their hard consequences which requires ongoing evaluation and revision of water resource policy at all standard international levels down to individual aquifers and wells [1,2]. Their main sources include wastewater discharged from health facilities [3], other industries, metal plating and alloy manufacturing [4,5]. The technological advancement in electronic industry is posing a new environmental challenge in the form of electronic waste, the electronic devices after their disposal into the soil are not processed properly which result in the accumulation of the toxic metals in the soil [6]. Heavy metals present in the wastewater are persistent and non-degradable in nature [7]. Presence of these metals in waste stream and ground water is one of the most environmental concerns since these metal ions are toxic to various life forms especially if their concentration is more than the accepted limit [8,9]. For the elimination of dissolved heavy metal ions, literature [10] is full of techniques that have been performed such as solvent extraction, ion exchange, membrane process, electro dialysis, precipitation, phytoextraction, ultra-filtration, reverse osmosis and adsorption. These methods, generally, are of high cost with troubles such as incomplete metal removal, high reagent consumption, energy requirements and generation of toxic sludge or other waste products that need further disposal or treatment [11]. Iron is commonly found in rocks and soil. Under suitable conditions, iron will leach into water resources. Iron concentration greater than 0.3mg/L causes water staining that negatively affects plumbing fixtures dishware and clothes and produce a yellow to reddish color in water appearance. These levels may also change the taste and odor of drinking water. This led many investigators to search for inexpensive substitutes such as zeolites, silica gel, chitosan, clay materials and agricultural wastes [12,13]. The adsorption technique remains the more favorable method because of its high capacity and low cost. During the past few years, several research articles were published reporting the successful use of different kinds of agricultural wastes for the removal of metal ions from aqueous solutions [14-17]. Such as Acacia leucocephala bark powder [18], Moringa oleifera bark (MOB) [19], cork waste biomass [20,21] rice straw [22], sugarcane bagasse waste [23,24], garden grass (GG) [25], castor leaf powder [26], green bean husk (GBH) [27], ficus carcia leaves [28], Avenafatua biomass [29]. Fruit peels have been extensively used [30-34] for the same purpose including banana (Musa paradisiaca), lemon (Citrus limonum) and orange (Citrus sinensis) peels [35]. The present study has been carried out to investigate the possibility of the use of peels of water melons (Citrullus vulgaris) (WMP) powder as an effective and efficient agricultural solid waste byproduct for the removal of iron ions from aqueous solutions.
Materials and Methods
Chemicals
Ferrous ammonium sulphate (Aldrich), Hydrochloric acid (BDH), 1, 10 Phenanthroline (Aldrich) (0.2%) solution of phenanthroline hydrochloride or hydrate (phen.) in 0.1M HCl, Tri sodium citrate (Aldrich) and Hydroxylamine hydrochloride (Aldrich).
Reagents
a) Standard Iron (II) solution (1000 ppm) Fe (II) stock solution was prepared by dissolving 0.7016g of ammonium ferrous sulphate (NH4)2SO4. FeSO4.6H2O, (Aldrich, USA) in DDW containing 5mL conc. H2SO4 and accurately dilute to volume in 100mL volumetric flask.
b) 1, 10 Phenanthroline (0.2%) Phenanthroline hydrochloride or hydrate (phen), in 100mL volumetric flask 0.2gm of phenanthroline are dissolved in doubly distilled water (DDW) and diluted to the mark with 0.1M HCl.
c) Tri sodium citrate (10 %) solution in 100mL volumetric flask, 10g of tri sodium citrate are dissolved in doubly distilled water (DDW) and dilute to the mark.
d) Hydroxylamine hydrochloride (10 %): 10g of hydroxylamine- HCl are dissolved in DDW and diluted to 100mL.
Preparation of the WMP powder and its modified forms
Preparation of the WMP powder
Water melon peels (WMP) are collected from the agricultural Egyptian fields. The white part of peels is cut, washed, air dried and then are finely powdered in a mixer till being near the nano size. The final product is applied as the crude peels powder for the removal of iron from water samples according to the proposed procedure.
Lactic Acid Modified (WMP) powder
100g of the crude (WMP) powder are refluxed with 500mL of 0.5M lactic acid solution over a boiling water bath for 6h. The produced precipitate is separated, repeatedly washed with DDW till free from acid then dried in an oven at 60oC for two hrs. After cooling in a desiccator to room temperature, it is finely grinded once again.
Tri sodium citrate Modified WMP powder
100g of the crude (WMP) are, similarly, refluxed with 500mL of 0.5M tri sodium citrate solution over a boiling water bath for 6h. The produced precipitate is, similarly, separated, washed, dried and grinded.
Instruments
UV/Vis. Spectrophotometer (Shimadzu UV/Vis. Perkin Elemer Lambada 3B Spectrophotometer using 1cm Quartz cell is applied to determine the concentration of the residual iron ions in the effluents after the application of the adsorption processes); Flame Atomic Absorption Spectrophotometer AA 240FS, Agilent Technologies, applied for the rapid and confirmation determination of the concentration of iron ions; pH meter (The pH measurements are carried by using the microprocessor pH meter BT 500 BOECO, Germany, which is calibrated versus two standard buffer solutions at pH4 & 9 and Mechanical Shaker (with up to 200rpm with speed control was used). The morphologies of the prepared samples and composites are examined using Scanning Electron Microscopy (SEM), FTIR spectroscopy Fourier transform infrared spectra of crude and chemically modified (WMP) powder forms are recorded with a Shimadzu FTIR spectrometer (resolution 4cm−1), equipped with highly sensitive pyroelectric detector (DLATGS). The IR shows the structure morphology, nature of surface hydroxyl groups, and adsorbed H2O.
Procedure
Spectrophotometric Determination of Iron
The remaining iron in the solution is determined spectrophotometrically after reduction to the Fe (II). In a 25mL volumetric flask, add 0.5mL of the 10% hydroxylamine solution, 2mL 10% tri sodium citrate solution and 5mL of the standard Fe (II) solution. The pH is adjusted in the range 3-4. Add 2.5mL of 0.2% 1, 10 phenanthroline solution, dilute to the mark with DDW and mix thoroughly. After 5min the absorbance of the solution is measured at 512nm against a blank.
FAA spectrometric method: Flame Atomic Absorption Spectrophotometer is used for the rapid and confirmation determination of the residual concentrations of iron ions after carrying out both the adsorption or the desorption procedures after making appropriate dilutions. The solution is directly measured at λmax equals 372.0nm with detection limit of 50μg/L using a mixture of Acetylene - Nitrous Oxide flame.
Optimization of the Factors Affecting the Adsorption of Iron from Standard Solutions
Optimization of the pH
To investigate the effect of pH on the uptake % (adsorption) of iron from aqueous media by the crude (WMP) powder, aliquots of 25 mL containing 20 ppm of the metal ion are transferred to a group of 100 mL conical flasks each containing 0.1 g of the crude adsorbent. Adjust the pH for each flask in the order ranging from 2-10, respectively by using 0.1M NaOH and HCl solutions and stir for 1hr. Centrifuge the contents of each flask and determine the remaining iron content in the supernatant solution. The sorption percentage of the metal ion by the WMP powder is calculated by equation (1):
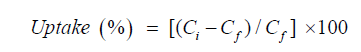
Where Ci and Cf are the initial and final concentrations of metal ion respectively. Biosorption capacity q (mg/g) is calculated by equation (2):
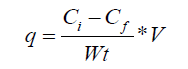
Where Ci and Cf are the initial and final concentrations of iron (ppm), Wt is the dose of sorbents (g) and V is the volume of solution (mL). The optimum pH is adjusted to be in the range 4-7. When the same procedure is parallel repeated using the sodium citrate and lactic acid modified powders, the optimum pH is in the range 5-6 in both cases.
Effect of temperature
The effect of temperature on the removal of iron ions by the three modified (WMP) powdered forms are studied at different temperatures between 30 and 70°C.
Sorbent dose
Aliquots of 25mL solution containing 20ppm iron are transferred to a group of 100mL conical flasks. Adjust the pH for each flask to the optimum value. Varying amounts of the crude (WLP) powder in the range 50-350mg are added to each flask, respectively. The mixtures are stirred for 1h. The remaining iron content in supernatant soln. separated by centrifugation is determined spectrophotometrically. The procedure is repeated with the other two (WLP) modified powders.
Contact time
A set of 100mL conical flasks, each of which is uploaded with 0.2g of the three (WMP) powder forms and aliquots of 25mL solution containing 20ppm of iron at the optimum pH and the shaking time is varied for different intervals of time (10-80min) for each flask in its turn, respectively.
Metal ion concentration range
Applying the optimum conditions of the weight of (WMP) powder, pH and stirring time in a group of flasks. Aliquots of 25mL solution containing varying concentrations of iron ions in the range 10-100ppm are added to each flask, respectively. The same procedure is applied, and the remaining iron content is determined from which the uptake percent is calculated.
Optimum sample volume
Different volumes of iron sample in the range from 10-100mL are tested.
Results and Discussion
Bio sorbent production may be an added value to the agrowastes and eventually reduces the agro-wastes management problems over the world. Agro-materials usually have their composition of lignin and cellulose as major constituents and may also include other polar functional groups of lignin, which include alcohols, aldehydes, ketones, carboxylic, phenolic and ether groups. These groups can bind to some extent heavy metals by donation of an electron pair from these groups to form complexes with the metal ions in solution [36]. During the past few years, several research articles were published reporting the successful use of different kinds of agricultural wastes in the removal of metal ions from aqueous solutions. The use of plant’s peels is reported in literature e.g., Ni (II) and Cd (II) removal from aqueous solution on modified plantain peels [8], biosorption of cadmium and nickel by grapefruit peels [34], biosorption of heavy metals present on polluted water by using different waste fruit cortex, banana (Musa paradisiaca), lemon (Citrus limonum) and orange (Citrus sinensis) peel [35], biosorption of aquatic cadmium (II) on unmodified rice straw [21], biosorption on peanut shell [22], equilibrium and kinetics of biosorption of cadmium(II) , copper(II) ions by wheat straw [37] and iron by green clover leaves [38]. Watermelon’s botanical name, Citrullus vulgaris, comes from the diminutive form of citrus, referring to the color and shape of the fruit, and vulgaris meaning common or ordinary fruit [39]. Our thinking was forwarded towards trying (WMP) fine powder as a low cost adsorbent for the treatment of a real local problem viz., the existence of iron (and manganese) in the ground water of some wells at El-Wasta, a town which lies 35 Km to the north of Beni-Suef Governorate, Egypt.
Factors that affect the Adsorption Process have been Studied in Details to Improve the Uptake % of Iron from the Aqueous Solutions
Optimum pH
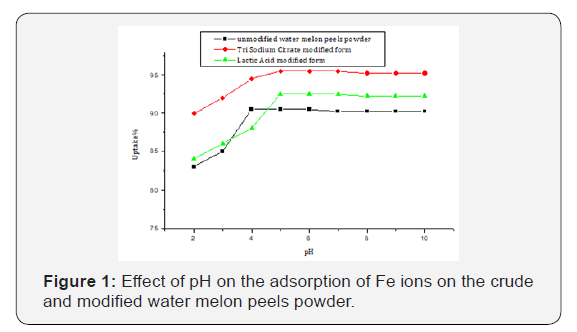
The pH is directly related to the competition ability of hydrogen ions with metal ions to active sites on the bio sorbent surface. The results indicated low sorption efficiency at low pH values (pH=2-3), this was attributed to the high concentration and high mobility of H+, which are preferentially adsorbed instead of metal ions. The removal efficiency of the sorbent is increased by increasing the pH value until reaches its maximum uptake in the range 4-6 by the unmodified (WMP). The sodium citrate and lactic acid modified (WMP) powders showed maximum adsorption at the pH range 5-7. Heavy metal biosorption on the specific and nonspecific bio sorbents is pH dependent; other researchers found that an increase in adsorption is a result of increasing the pH of the solution (Figure 1, Table 1).

Effect of temperature

Maximum adsorption recovery is obtained at 30 °C for the three adsorbent forms. The uptake percentage decreased with the increase in temperature suggesting that the adsorption process is exothermic [40]. As observed in (Figure 2, Table 2), tri sodium citrate modified form gave better adsorption performance than the crude and lactic acid forms. These results also proved that during the adsorption process, no permanent chemical bonds are formed [41].

Stirring time
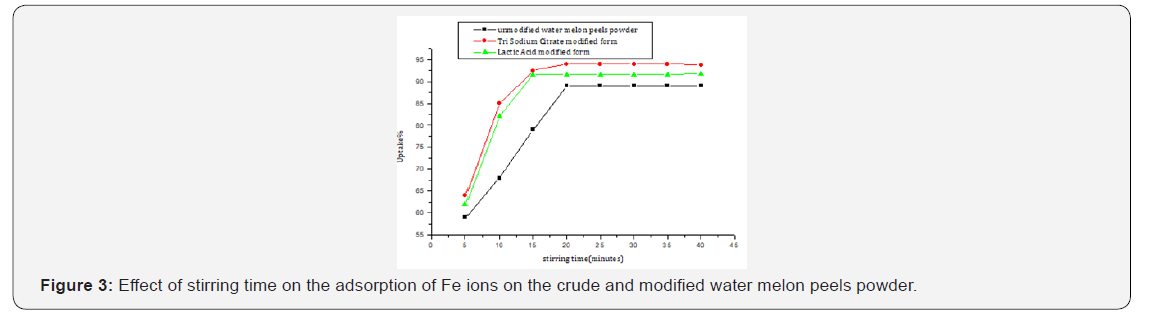
Equilibrium time is one of the important parameters for selecting a wastewater treatment system [37]. The results show that removal is slow in the first 10 min, then the adsorption % is gradually increased till equilibrium is reached after 20 min for the unmodified powder. While both the tri sodium citrate and the lactic acid modified forms attained equilibrium after 15 min. The rate of biosorption seems to pass through two steps, the first one is very rapid surface biosorption, while the second is slow intracellular diffusion (Figure 3, Table 3).

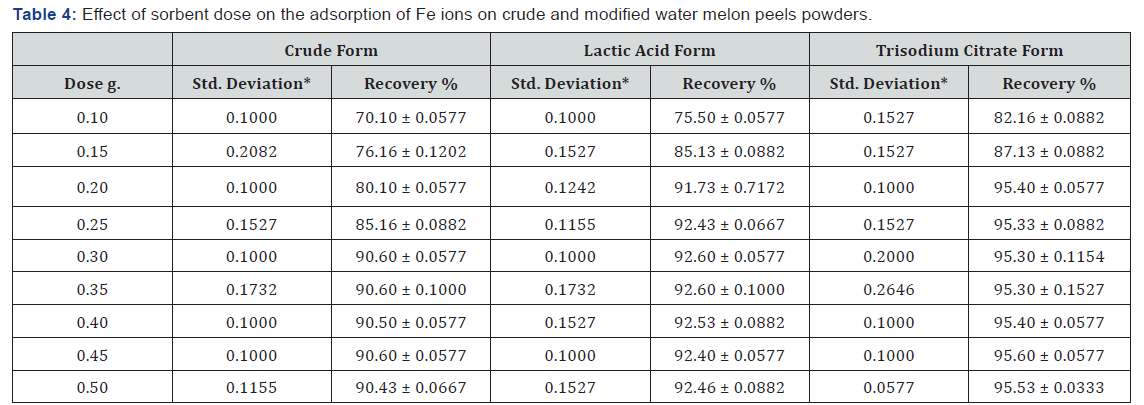
Sorbent dose
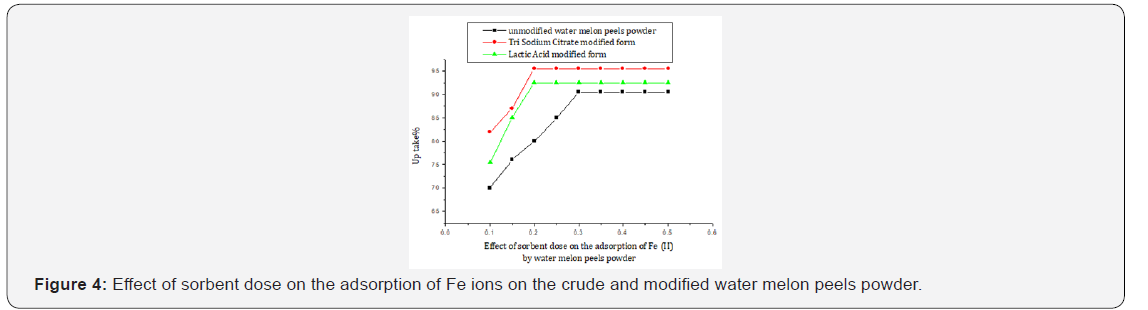
For a specific metal initial concentration, increasing the adsorbent dose provides greater surface area and availability of more active sites, thus leading to the enhancement of metal ion uptake till reach equilibrium [42]. For the unmodified powder, a dose of 0.3 g sorbent can achieve an uptake of 90.5% of iron at optimum pH conditions. While for the tri sodium citrate modified form, a dose of 0.2 g achieved better uptake % of iron of 95.5%. Correspondingly, 0.2 g of the lactic acid modified one achieved an intermediate iron removal % of 92. (Figure 4, Table 4).
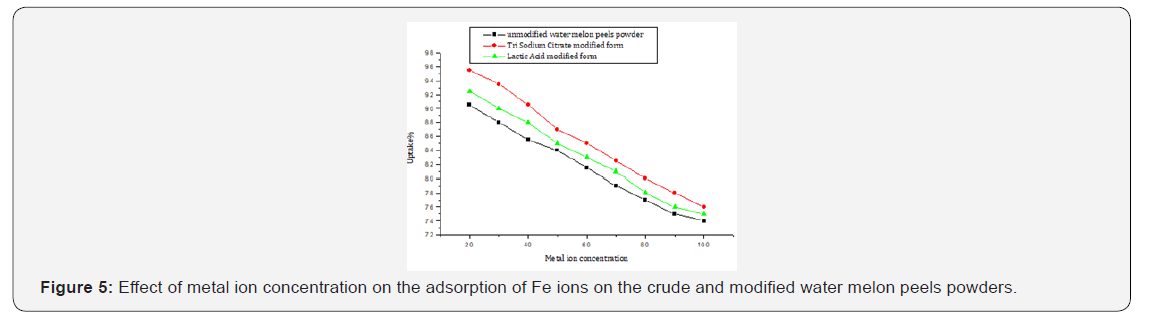
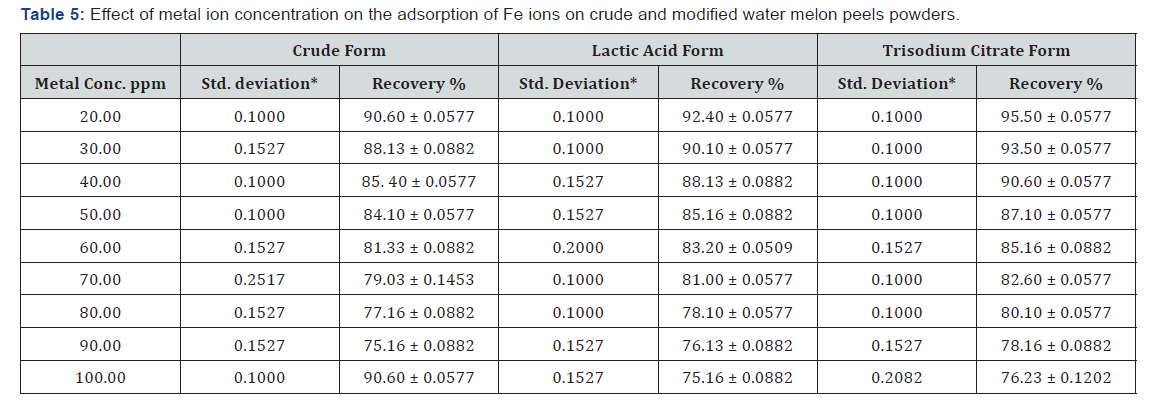
The initial metal ion concentration does a substantial force to overcome all mass transfer resistances of the metal ions between aqueous and solid phase [43]. At lower concentrations the adsorption sites utilize the available metal ion more rapidly in comparison to higher concentrations where the metal ions need to diffuse to the sorbent surface by intra particle diffusion. The maximum metal uptake was 90.5 % in the case of the unmodified (WMP) powder, 92.5% for lactic acid modified one and 95.5% in the case of tri sodium citrate form at metal ion concentration of 20 ppm, (Figure 5, Table 5).
Sample volume

At optimum conditions the volume of 25 mL achieved the best adsorption percentage with all tested (WMP) powders. It is clear from the Table 6, it is clear that iron removal percentage decreases with the increase of the volume.
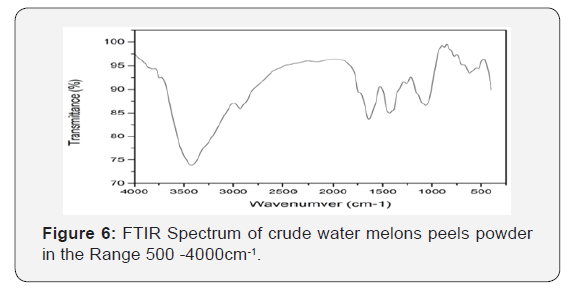
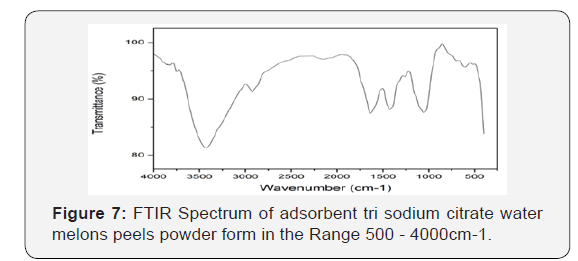
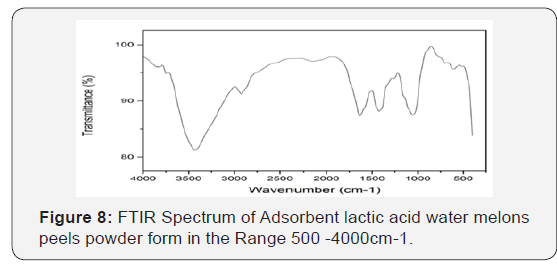
Characterization of used adsorbents
The composition and topography of the studied adsorbents have been characterized by FT-IR and SEM.
Infra-red spectroscopy: Figure 9 & 10 adepicts the FT-IR spectrum of the unmodified (WMP) powder form. The broad band in the region around 3425cm−1 is specific to the surface hydroxyl groups of bonded carboxylic acid. The O–H stretching vibrations occurred within a broad range of frequencies indicating the presence of free hydroxyl groups and bonded O–H bands of carboxylic acid, the -OH carbonyl and carboxylic groups have been reported as very important sorption sites for metal ions. The asymmetric C–H stretching of surface methyl groups usually present on the lignin structure is observed at 2900cm−1. The characteristic peaks because of the C-O group in carboxylic and alcoholic groups are present at 1053cm−1 [44]. The ionization of both the carboxylic acid and hydroxyl functional groups present in the structure of the adsorbent can be achieved by deprotonation that allowing it to interact with metals more easily and therefore provides the major biosorption sites for the removal of iron ions from solutions [44]. A broadening peak at around 1650cm−1 due to carbonyl group peak is observed. This indicates the involvement of both the hydroxyl and carbonyl groups in the adsorption of iron. Figure 7 the presence of COO− of the carboxylate can be attributed to the peak positions at 1452.30cm−1 and 1400.22cm−1. Alkyl chains around are observed at 2920-2850cm−1. The peaks in range 1000-1200cm−1 symbolize C-C and C-O stretching.


The Morphologies of the prepared adsorbents

This study revealed the micro porous structure of the adsorbent. The SEM micrographs of (WMP) are as shown in Figures 9-11. The micrographs showed that the bio sorbent has a smoothening and irregular surface (Figure 9). A significant change in the bio sorbent surface is observed after the modification (Figure 11&12). A rough effect is perceived in (Figure 12) It is observed as providing a large area for ion–surface interaction. Different surface shapes of the bio sorbent may be due to its modification with lactic acid and tri sodium citrate.
Selectivity of the adsorbent
Adsorption studies applying the three adsorbents under investigation, proved that they are nonselective towards different heavy metals that may be present in wastewaters as pollutants. However, the non-selectivity of such types of adsorbents is frequent in the literature; their tolerance is attributed to being low cost and available. Current work in our laboratory is devoted for the development and applications of selective and low-cost adsorbent.
Adsorption isotherm studies
For solid–liquid adsorption system, the adsorption behavior can well be described as adsorption isotherm model, the adsorption isotherm meaning the distribution of adsorbate molecules among the solid phase and the liquid phase at equilibrium. Equilibrium is said to be reached when the concentration of adsorbate in bulk solution is in dynamic balance with that on the liquid adsorbate interface. It is significant to study the adsorption behavior in order to describe adsorption process using appropriate adsorption isotherm model.
Langmuir adsorption isotherm
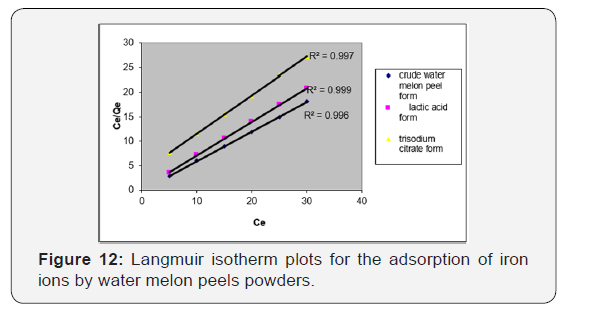
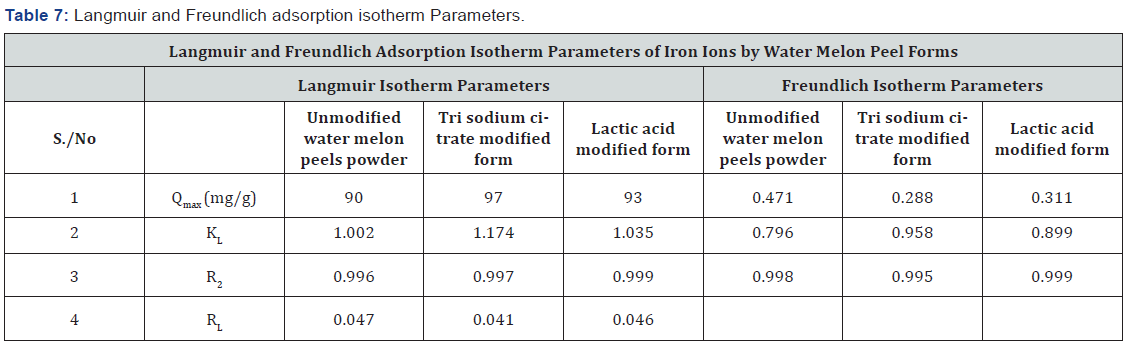
The Langmuir isotherm model assumes a surface with homogeneous binding sites, equivalent sorption energies, and no interaction between the sorbet species [45]. The equilibrium adsorption data for the concentrations of iron ions is fitted into the linear form of Langmuir’s isotherm equation, to determine the distribution of iron ions between the adsorbent and solution according to equation (3):
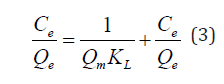
Where Ce is the concentration of the iron ions in solution (mg L−1), Qe is the equilibrium concentration of iron ions on (WMP) adsorbent (mg g−1), Qm and KL are Langmuir constants related to sorption capacity and the rate of adsorption respectively. Maximum adsorption capacity (Qm) is the monolayer capacity of the adsorbent (mg g−1) and KL is the Langmuir adsorption constant. A plot of Ce/Qe against Ce over the entire concentration range is a straight line with a slope of 1/Qm and the intercept of 1/Qm KL. The correlation coefficient (R2) values reported are very close to 1 indicating that the adsorption follows the Langmuir adsorption isotherm. The quality of Langmuir isotherm can be determined by the magnitude of a dimensionless constant RL known as the separation factor expressed in equation (4):
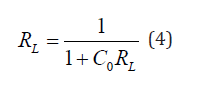
where Co is the initial concentration of the iron ions in mg L−1
and KL is the Langmuir constant described earlier. The adsorption
process is favorable within the range 0
Freundlich adsorption isotherm
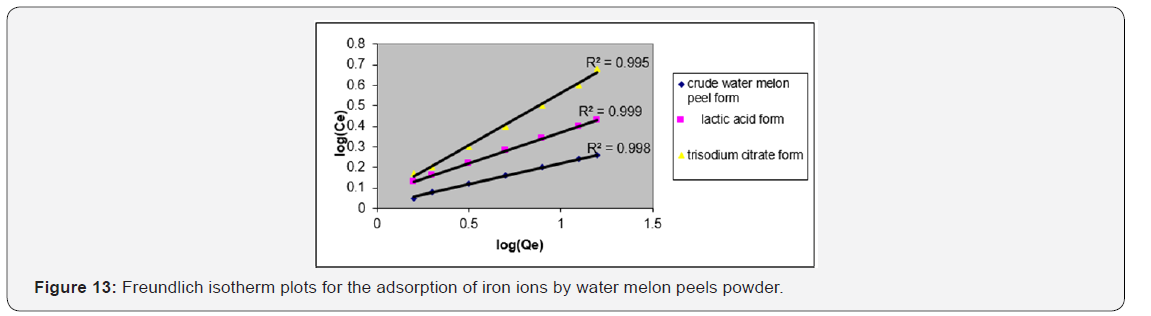
The Freundlich isotherm model applies to adsorption on heterogeneous surfaces with the interaction between adsorbed molecules, and the application of the Freundlich equation also suggests that sorption energy exponentially decreases on completion of the sorption centers of an adsorbent [28]. The linear form of the Freundlich adsorption Model equation (5):
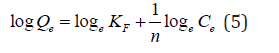
Where Qe is the amount of iron ions adsorbed at equilibrium per gram of the adsorbent (mg g−1), Ce is the equilibrium concentration of the iron ions in the solution (mg L−1), and Kf and n are the Freundlich adsorption model constants related to the adsorption capacity and adsorption intensity respectively. Loge Qe is plotted against loge Ce and a straight line obtained gave the intercept of log Kf and the slope of 1/n. The numerical value of 1/n reported is less than1, (Figure 13, Table 7).
Analysis of real samples
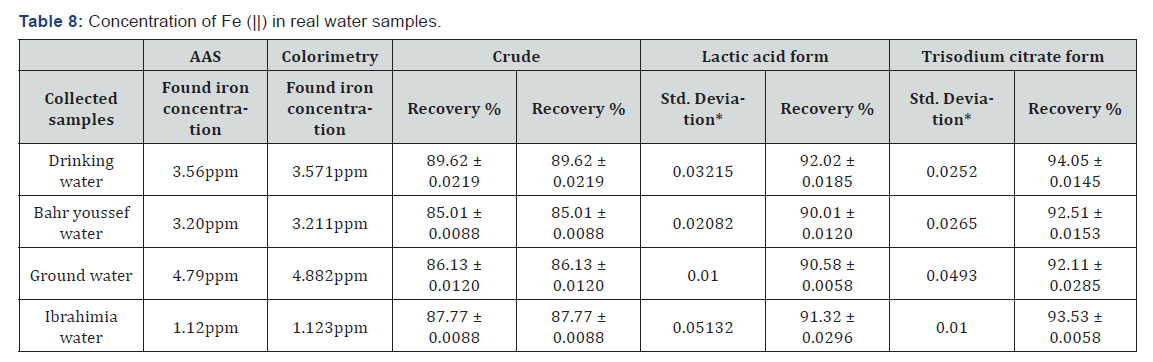
Water samples are collected from tap water, Bahr Youssef water, ground water and Ibrahemia (stream)water; samples are subjected to the adsorption procedure as explained previously and the residual iron is analyzed by two methods of finish viz., colorimetry and AAS (Table 8).
Desorption studies
Reusability of the adsorbent is tested by regenerating the spent adsorbent following a modified literature procedure [46]. The adsorbed iron ions onto the three WMP surfaces are treated with 25 mL 0.1M HCl and stirred for 1h. The amount of iron ions remained in the solution after filtration or centrifugation is measured using the mentioned spectrophotometric and/ the FAAS methods and the percentage desorption (Rb) was calculated relatively to the equation (6):
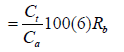
Where Ct is the experimental concentration in the solution at time t (ppm), Ca is the adsorbed concentration of sorbate onto the adsorbent surface.
The Parameters Affecting the Desorption Process are Studied as Illustrated Below
Effect of pH on desorption of iron
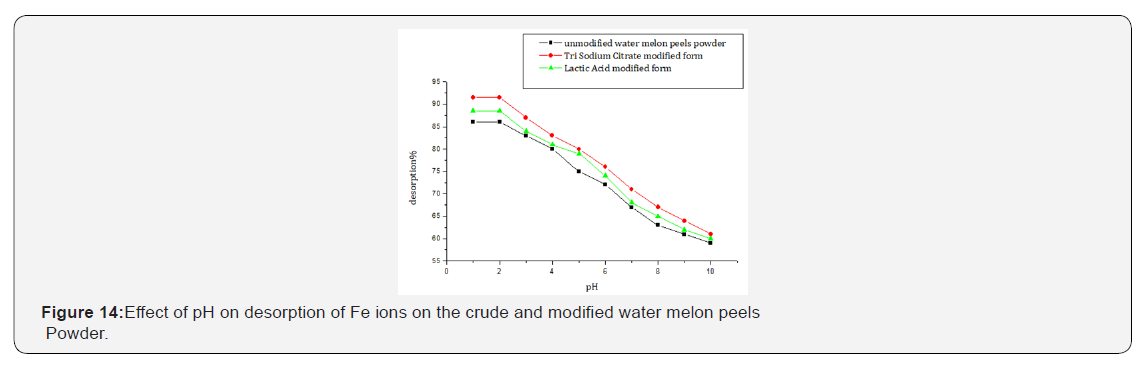
In strong acidic media at pH range (1.2-1.9) the three forms of the WMP powder showed high desorption percentages, on increasing the pH values desorption percentage decreases (Figure 11, Table 9).
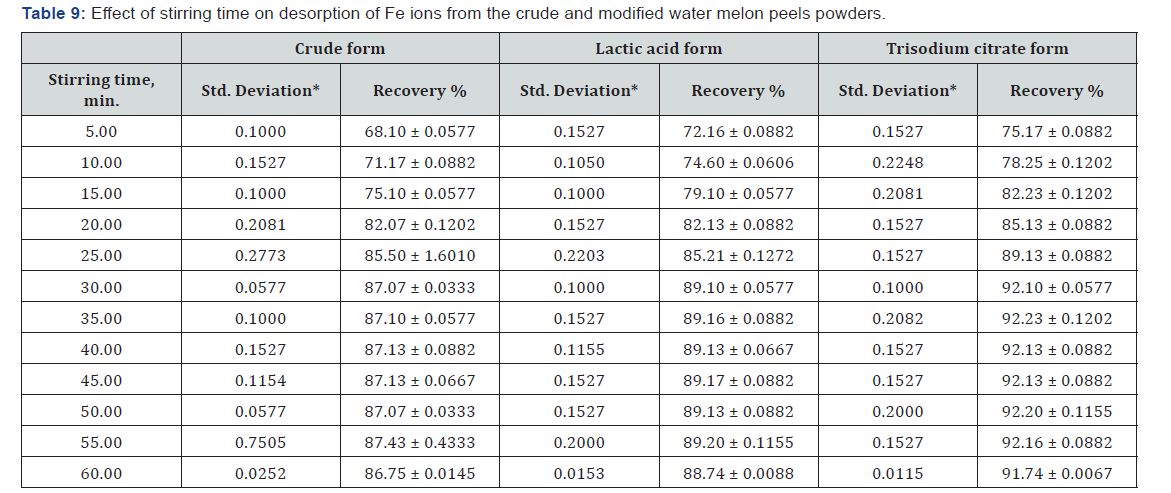
Stirring Time

The desorption percentages are gradually increased till equilibrium is reached after 25 min in case of the crude WMP powder, while the powders modified with lactic acid and tri sodium citrate show equilibrium after 30min. A desorption value of 87% has been recorded for the unmodified WMP powder, 92.5% for the tri sodium citrate modified form and 89% for lactic acid one (Figure 14, Table 9).
Conclusion
Water melon peels powder (WMP) and its modified forms proved to be potential bio sorbents for the removal of iron and other co present heavy metals from aqueous solutions, being available low-cost material. The proposed adsorbents were applied on real water samples. The adsorption process is best described by the Langmuir and Freundlich isotherm models. Results of desorption investigations also, assured the possibility to regenerate and reuse the bio sorbents once again.
References
- Adekola FA, Hodonou DSS, Adegoke HI (2016) Thermodynamic and kinetic studies of biosorption of iron and manganese from aqueous medium using rice husk ash. Appl Water Sci 6(4): 319-330.
- Wasiu MO, Ayodele OE, Ayodele TI, Oluremi OI, Ogundele KT, et al. (2016) Heavy metal contamination in stream water and sediments of gold mining areas of South Western Nigeria. African J Environ Sci Tech 10(5): 150-161.
- Verlicchi P (2018) Hospital Wastewaters, Characteristics, Management, Treatment and Environmental Risks. The Handbook of Environmental Chemistry p. 60.
- Krishnan KA, Sreejalekshmi KG, Baiju RS (2011) Nickel (II) adsorption onto biomass based activated carbon obtained from sugarcane bagasse pith. Bioresour Technol 102(22): 10239-10247.
- Kula I, Uğurlu M, Karaoğlu H, Celik A (2008) Adsorption of Cd (II) ions from aqueous solutions using activated carbon prepared from olive stone by ZnCl2 activation. Bioresour Technol 99(3): 492-501.
- Ramakrishna G (2017) Biosorption of lead by Bacillus licheniformis isolated from e-waste landfill, Hyderabad, Telangana, India. Inter J Bioassays 6: 5240-5244.
- Tripathi A, Ranjan MR (2015) Heavy Metal Removal from Wastewater Using Low Cost Adsorbents. J Bioremed Biodeg 6(6): 315-320.
- Garba ZN, Ugbaga NI, Abdullahi AK (2016) Evaluation of optimum adsorption conditions for Ni (II) and Cd (II) removal from aqueous solution by modified plantain peels (MPP). J Basic & Appl Sci 5(2): 170-1 7 9.
- Alslaibi TM, Abustan I, Ahmad MA, Abu Foul A (2013) Cadmium removal from aqueous solution using microwaved olive stone activated carbon. J Environ Chem Eng 1(3): 589-599.
- Fu F, Wang Q (2011) Removal of heavy metal ions from wastewater: A review. J Environm Manag 92(3): 407-418.
- Avalude S, Tella AC (2016) Removal of hexavalent chromium from aqueous solutions by adsorption on modified groundnut hull. J Basic & Appl Sci (BJBAS) 159: 377-388.
- Mekatel El H, Amokrane S, Aid A, Nibou D, Trari M (2015) Adsorption of methyl orange on nanoparticles of a synthetic zeolite. Comptes rendus - Chimie 18(3): 336-344.
- Shirzad Siboni M, Khataee A, Hassani A, Karaca S (2015) Preparation, characterization and application of a CTAB-modified nanoclay for the adsorption of an herbicide from aqueous solutions: kinetic and equilibrium studies. Comptes rendus - Chimie 18(2): 204-214.
- Acharya J, Kumar U, Rafi PM (2018) Removal of Heavy Metal Ions from Wastewater by Chemically Modified Agricultural Waste Material as Potential Adsorbent-A Review. Int J Curr Eng Techno 8(3): 526-530.
- Abdel Tawwab M, El-Sayed GO, Shady SHH (2017) Capability of some agricultural wastes for removing some heavy metals from polluted water stocked in combination with Nile tilapia, Oreochromis niloticus (L.). Int Aquat Res 9(2): 153-160.
- Negm N, Hefni HHH, Abd Elaal AA (2018) Assessment of Agricultural Wastes as Biosorbents for Heavy Metal Ions Removal from Wastewater. In book: Surfactants in Tribology 5: 466-487.
- Qu J, Meng X, Jiang X, You H, Wang P, et al. (2018) Enhanced removal of Cd (II) from water using sulfur-functionalized rice husk: Characterization, adsorptive performance and mechanism exploration. J of Cleaner Production 183: 880-886.
- Munagapati VS, Yarramuthi V, Nadavala SK, Alla SR, Abburi K (2010) Biosorption of Cu (II), Cd (II) and Pb (II) by Acacia leucocephala bark powder: Kinetics, equilibrium and thermodynamics. Chem Eng J 157(2-3): 357-365.
- Reddy DHK, Ramana DKV, Seshaiah K, Reddy AVR (2011) Biosorption of Ni (II) from aqueous phase by Moringa oleifera bark, a low cost biosorbent. Desalination 268: 150-157.
- López-Mesas M, Navarrete ER, Carrillo F, Palet C (2011) Bioseparation of Pb (II) and Cd (II) from aqueous solution using cork waste biomass. Modeling and optimization of the parameters of the biosorption step. Chem Eng J 174(1): 9-17.
- Ding Y, Jing D, Gong H, Zhou L, Yang X (2012) Biosorption of aquatic cadmium (II) by unmodified rice straw. Bioresour Technol 114: 20-25.
- Witek Krowiak A, Szafran RG, Modelski S (2011) Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination 265(1-3): 126-134.
- Alomá I, Martín Lara MA, Rodríguez IL, Blázquez G, Calero M (2012) Removal of nickel (II) ions from aqueous solutions by biosorption on sugarcane bagasse. J Taiwan Inst Chem Eng 43(2): 275-281.
- Witek Krowiak A (2012) Analysis of temperature-dependent biosorption of Cu2+ ions on sunflower hulls: Kinetics, equilibrium and mechanism of the process. Chem Eng J 192: 13-20.
- Hossain MA, Ngo HH, Guo WS, Setiadi T (2012) Adsorption and desorption of copper (II) ions onto garden grass. Bioresour Technol 121: 386-395.
- Martins AE, Pereira MS, Jorgetto AO, Martines MAU, Silva RIV, et al. (2013) The reactive surface of Castor leaf [Ricinuscommunis L.] powder as a green adsorbent for the removal of heavy metals from natural river water. Appl Surf Sci 276: 24- 30.
- Iqbal M, Saeed A, Edyvean RGJ (2013) Bioremoval of antimony (III) from contaminated water using several plant wastes: Optimization of batch and dynamic flow conditions for sorption by green bean husk (Vignaradiata). Chem Eng J 225: 192-201.
- Farhan AM, Al-Dujaili AH, Awwad AM (2013) Equilibrium and kinetic studies of cadmium (II) and lead (II) ions biosorption onto Ficuscarcia leaves. J Ind Chem 4: 24-31.
- Areco MM, Saleh-Medina L, Trinelli MA, Marco-Brown JL, Dos Santos Afonso M (2013) Adsorption of Cu (II), Zn (II), Cd (II) and Pb (II) by dead Avenafatua biomass and the effect of these metals on their growth. Colloids Surf. B. Biointerfaces 110: 305-312.
- Al-Azabi K, Al-Marog S, Abukrain A, Sulyman M (2018) Equilibrium, Isotherm Studies of Dye Adsorption onto Orange Peel Powder. Chem Res J 3(1): 45-59.
- Bharat S, Manchanda D (2017) Efficient bio adsorbents for removal of heavy metals from water: A review. Int J Chem Studies 5(4): 1691- 1694.
- Jain N (2015) Removal of heavy metal b using different fruit peels, vegetable peels and organic waste - A review. Int J Adv Res 3(11): 916- 920.
- Taralgatti PD (2016) Removal of copper from waste water by using potato and banana peels as bio-adsorbent. Int J Sci Eng & Techn Res (IJSETR) 5(10): 3038-3040.
- Torab Mostaedi M, Asadollahzadeh M, Hemmati A, Khosravi A (2013) Equilibrium, kinetic, and thermodynamic studies for biosorption of cadmium and nickel on grapefruit peel. J Taiwan Inst Chem Eng 44: 295-302.
- Kelly Vargas K, Cerro Lopez M, Reyna Tellez S, Bandala ER, Sanchez Salas JL (2012) Biosorption of heavy metals in polluted water, using different waste fruit cortex. J Phys Chem Earth 37: 26-29.
- Demirbas A (2008) Heavy metal adsorption onto agro-based waste materials: a review. J Hazard Mater 157(2-3): 220-229.
- Abdel Ghani N, Hefny M, El Chaghaby GAF (2007) Removal of lead from aqueous solution using low cost abundantly available adsorbents. J Environ Sci 4(1): 67-73.
- Hassouna MEM, Elbably MA, Marzouk MA, El Maghraby AH (2017) Evaluation of Green Clover Leaves as Green and Economic Sorbent for Removal of High Levels of Iron from Different Water Sources. Int J Environ Sci Nat Res 1(2): 555559.
- https://en.wikipedia.org/wiki/Watermelon
- Vidhyadevi TA, Murugesan A, Kalaivani SS, Premkumar MP, Vinoth kumar V, et al. (2014) Evaluation of equilibrium, kinetic, and thermodynamic parameters for adsorption of Cd2+ ion and methyl red dye onto amorphous poly(azomethinethioamide) resin. Desalination Water Treat 52(19-21): 3477-3488.
- Kalaivani SS, Vidhyadevi T, Murugesana A, Thiruvengadaravi KV, Anuradha D, et al. (2014) The use of new modified poly (acrylamide) chelating resin with pendent benzothiazole groups containing donor atoms in the removal of heavy metal ions from aqueous solutions. Water Resources and Industry 5: 21-35.
- Kumar D, Gaur JP (2011) Metal biosorption by two cyanobacterial mats in relation to pH, biomass concentration, pretreatment and reuse. Bioresour Technol 102(3): 2529-2535.
- Dang VBH, Doan HD, Dang Vu T, Lohi A (2009) Equilibrium and kinetics of biosorption of cadmium (II) and copper (II) ions by wheat straw. Bioresour Technol 100(1): 211-219.
- Krishnani KK, Xiaoguang M, Christodoulatos C, Boddu VM (2008) Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J Hazard Mater 153(3): 1222-1234.
- Gupta VK, Rastogi A, Nayak A (2010) Biosorption of nickel onto treated alga (Oedogoniumhatei): Application of isotherm and kinetic models. J Colloid Interface Sci 342(2): 533-539.
- Dehghani MH, Sanaei D, Ali I, Bhatnagar A (2016) Removal of chromium (VI) from aqueous solution using treated waste newspaper as a lowcost adsorbent: kinetic modeling and isotherm studies. J Mol Liquids 215: 671-679.






























