Distribution of Arsenic Species in Surface Water Using Flow Injection Hydride Generation Atomic Absorption Spectrometry and Furnace Method
Swapnila Roy*
West Bengal Pollution Control Board, Kolkata, India
Submission: May 25, 2018; Published: June 19, 2018
*Corresponding author: Swapnila Roy, West Bengal Pollution Control Board, Kolkata, India, Email: swapnilaroy@gmail.com
How to cite this article: Swapnila R. Distribution of Arsenic Species in Surface Water Using Flow Injection Hydride Generation Atomic Absorption Spectrometry and Furnace Method. Int J Environ Sci Nat Res. 2018; 12(4): 555843. DOI:10.19080/IJESNR.2018.12.555843.
Abstract
Arsenic has been considered detrimental to human health when accumulated in the body beyond the tolerance level. The toxicity level of certain species (like As3+) of Arsenic is higher than others, making the situation worse for human health with its presence. In this study, we used a combination of two principal atomic absorption spectroscopic methods, namely Flame-FIAS and the Furnace technique, to determine the distribution of inorganic Arsenic species in a synthetic sample. The Flame-FIAS technique was employed to determine the amount of toxic As3+ present in the samples and the Furnace method was used to measure the total Arsenic content. Following standardization of this experimental process, we used the technique to determine the distribution of inorganic Arsenic species in environmental samples with high total Arsenic content. Since the samples were collected from surface water systems, the conditions are supposed to be oxidizing. As per the natural geochemical distribution phenomena of Arsenic species, As5+ was found predominantly (around the range of 10-18 μg/l) in all samples, validating the process of species identification. In groundwater samples, conditions being reducing, the As3+ species is supposed to be predominant. These findings can guide future bioremediation strategies to be effectively designed, as per the distribution of Arsenic species in the surface water.
Keywords: As3+; As5+; FIAS; Furnace; Environmental Samples; Arsenic Speciation
Highlights
a) Standardization of Arsenic speciation in synthetic samples using FIAS (Flame Ionisation Atomic Absorption Spectrometry) and Graphite Furnace methods.
b) Application of Arsenic speciation in environmental samples with total arsenic content.
c) Determination of predominant inorganic Arsenic species in surface water system.
Introduction
Arsenic is a ubiquitous metalloid found in lithosphere, hydrosphere, atmosphere and biosphere [1]. Natural minerals are the key source of arsenic. The soil arsenic concentration ranges from 0.1 to 40 mg/kg around the world, while, in water, it ranges from <0.05 to 5000 μg/l [2,3]. Arsenic and its compounds are highly toxic. Studies have shown that consumption of water with high arsenic content can lead to arsenical skin lesions [4]. According to the World Health Organization (WHO), the recommended arsenic concentration in potable water is 10 μg/l. Prolonged exposure to arsenic can lead to skin cancer, neurological disorders, lung cancer, liver cancer and adverse obstetric effects [5-9]. Groundwater arsenic contamination has become an issue of great concern across the world. A number of countries have been affected by high arsenic concentration in groundwater, when it is a source of drinking water. Of them, Bangladesh and India (West Bengal) have been affected the worst [4,10,11]. The elevated presence of arsenic in the groundwater of the Bengal Delta Plain (BDP) has been termed as “the worst mass poisoning in human history” [12]. Other affected countries are Vietnam, Thailand, Cambodia, Taiwan, Mongolia, China (Xinjiang region), Chile, Argentina, Bolivia, Brazil, Mexico, Ghana, Germany, Greece, Spain, Canada and the United States [13]. In West Bengal, six districts are adversely affected by arsenic contamination in groundwater: North 24 Parganas, South 24 Parganas, Nadia, Burdwan, Murshidabad and Malda. The total affected area is around 34,000 km2 with a population of about 30 million (44.4% of the total population of West Bengal). About 800,000 people in this region drink arsenic contaminated water and about 175,000 people are suffering from arsenic related diseases [10]. Other Indian states such as Bihar, Jharkhand, Madhya Pradesh and Assam are also affected by the issue. Out of 64 districts in Bangladesh, 51 have been detected with arsenic contamination. The total area of these 51 districts is 121,145 km2 and population of about 113 million (87% of the total population of Bangladesh) [5]. Excessive use of hand tube wells for drinking water and other purposes has caused the major outbreak of arsenic contamination in these regions. The biogeochemical cycle of arsenic involves a number of physicochemical processes, such as redox reactions, adsorption, desorption, ion exchange, solid phase precipitation and dissolution. Microbiological processes play a crucial role in these processes [14]. Several factors like redox potential, pH, organic carbon and chemical speciation play important roles in these processes [14,15]. Arsenic occurs in four oxidation states: elemental arsenic (As0), arsenite (As3+), arsenate (As5+) and arsenide (As3-). Among these species, As3+ and As5+ are the most common ones found in aquatic environment [16]. Distribution and mobility of these arsenic species depend upon local physicochemical conditions as well as biological processes. The pKa values of arsenic acid (H3AsO4; contains arsenic in the form of As5+) are – pKa1 = 2.19, pKa2 = 6.94 and pKa3 = 11.5. Therefore, at low pH (i.e., below 6.9) and oxidizing condition, H2AsO4 - is the predominant form, whereas, at higher pH levels, HAsO4 2- is the predominant form. Arsenate, being negatively charged, gets adsorbed easily on the oxidized minerals [17]. The lowest pKa value for As3+ is 9.22. In most natural water with pH below 9.2 as well as reducing condition, As(OH)3 is the predominant form. Solubility of arsenic depends upon its speciation [18]. Elemental arsenic is not very common in the environment, and organic forms of arsenic are found only in extremely reducing conditions (within live biomass) [19].
Objectives of This Study
This study aims to identify the method of selective determination of As3+and the total Arsenic in a solution where Arsenic exists in both trivalent and pentavalent state, by combining two methods:
a) Hydride generation through Flame – FIAS technique. (PinAAcle 900H Atomic Absorption Spectrometer, Perkin Elmer)
b) Furnace method (PinAAcle 900H Atomic Absorption Spectrometer, Perkin Elmer)
Application of the method in determination of As species in environmental samples (river water samples).
Subtracting the result of trivalent arsenic from total Arsenic, pentavalent Arsenic concentration of the solution can be measured. Presence of other forms of Arsenic is assumed to be negligible.
Methodology and Methodology
Basic Reagent Preparation
a) As3+ standard 50 μg/l : From stock 1000mg/l NIST As3+ soln. 0.1 ml was added to 100 ml volumetric flask and volume made up to 100 ml with milli Q water resulting 1000 μg/L of As3+ solution. From it 5 ml was added to 100 ml volumetric flask yield to 50 μg/l of As3+ soln. by volume make up with milli Q water.
b) As5+ standard 50μg/l : From stock 1000mg/l NIST As5+ soln. 0.1 ml was added to 100 ml volumetric flask and volume made up to 100 ml with milli Q water resulting 1000 μg/L of As5+ solution. From it 5 ml was added to 100 ml volumetric flask yield to 50 μg/l of As3+ soln. by volume make up with milli Q water.
c) Calibration standard: from 1000 μg/L of As3+ solution 4,10,20,30,40 μg/L of calibration standards were prepared.
d) Tris - Buffer (2.5 M, pH 6.2) : 75.69 gm Tris Base was dissolved in 130 ml of milli Q water. Then Conc. HCl was added continuously by checking the pH in pH meter and final pH was maintained at 6.2. Then volume was made up to 250 ml with milli Q water in a 250 ml volumetric flask.
Specific Reagent for Flame-FIAS Method
Sodium Borohydride (Reductant): 6 gm of Sodium Borohydride was dissolved in 1000 ml vol. flask with 0.6 gm of NaOH with milli Q water
Carrier Acid (3% HCl): 30 ml Conc. HCl is dissolved in 1000 ml milli Q water in 1 L volumetric flask.
Specific Reagent For Furnace Method
Chemical modifier :1%(10g/L) Pd stock solution & 1%(10g/L) Mg stock solution were prepared. 3ml of Pd stock & 0.3 ml of Mg stock solution is added to 10 ml of MQ water.
Collection of Samples:
Sampling Sites: The sampling sites were selected based on the As contamination results obtained from routine monitoring procedure undertaken by the West Bengal Pollution Control Board. Typically, two surface water streams in the lower Gangetic delta exhibited high Arsenic contamination, namely Churni and Jalangi. Three sampling points were identified in River Churni and one point from River Jalangi. Both rivers are trans-boundary and are tributaries of the River Ganges. Sampling points on River Churni are located at Majdia (23° 23’ 60’’ N, 88° 42’ 0’’ E), Shantipur- Ranaghat (23° 10’ 12’’ N, 88° 32’ 60’’ E) and Mathabhanga Govindapur (23° 23’ 56.4’’ N, 89° 43’ 15.6’’ E) of the Nadia district. Sampling points on River Jalangi (23° 49’ 12’’ N, 88° 28’ 19.2’’ E) are located at Krishnanagar of Nadia District.
Sampling Procedure: The water samples were collected from the sampling points in sterile containers and were transported to laboratory on ice for further analysis. The samples were immediately transported to the laboratory in ice cold condition in heat insulated container. The samples were refrigerated at 4⁰C, if not processed immediately.
Experimental Procedure
1st Experiment
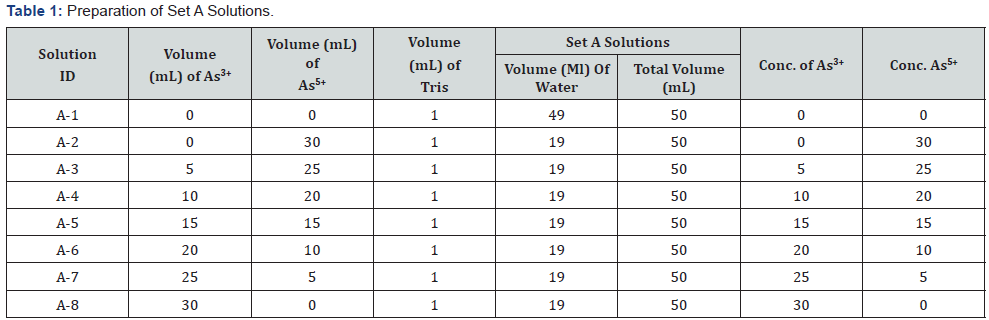
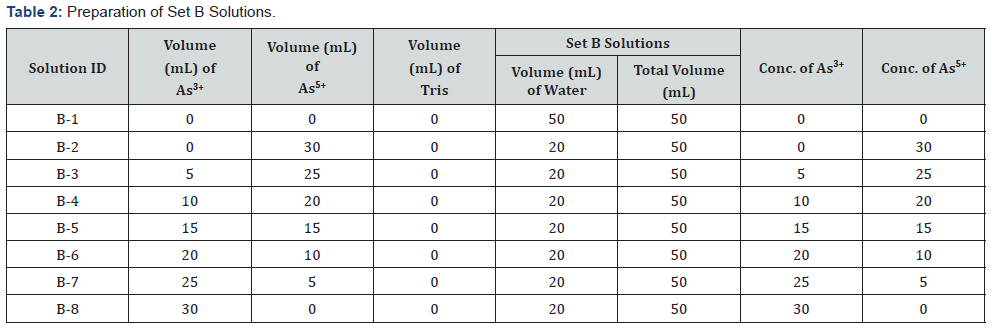
Two sets of mixed solutions were prepared according to the Table 1 – named as Set A (A1-A8) & Table 2 for Set B (B1-B8). 1 ml Tris buffer was added to each solution of Set A. The following experimental solutions are prepared from 50 μg/l of both As3+and As5+stock solutions.
2nd Experiment
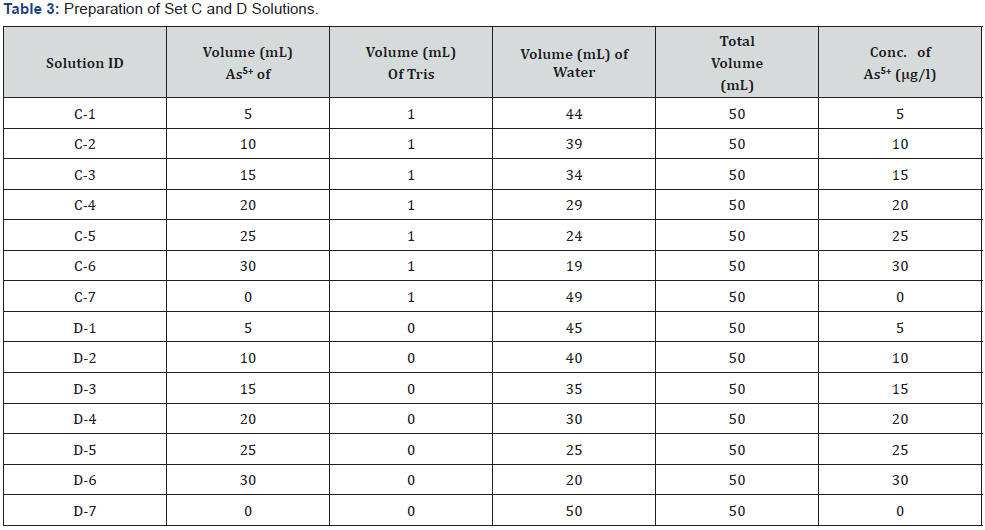
For checking interference, two sets of mixed solutions were prepared according to the Table 3 – named as Set C (C1-C7) & Set D (D1-D7). 1 ml Tris buffer was added to each solution of Set C. The concentrations of Set A, B, C & D solutions were measured by Flame FIAS method against the calibration curve.
3rd Experiment
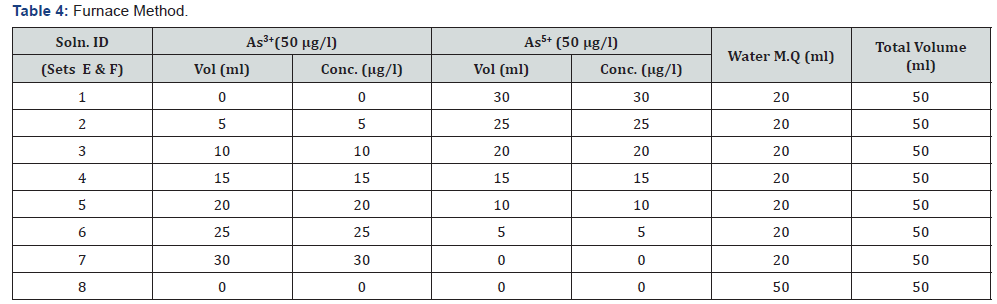
Two set of samples were prepared according to Table 4 & named as Set E (1-8) & F (1-8).Undigested synthetic samples were used in Set E. And in Set F, synthetic samples were digested with Conc. HNO3 by Microwave Digestion System (Anton PAAR). (Digestion Method: 15 ml sample + 0.75 ml conc. HNO3. Run Program- USER 004M) Then Set E & F, are analyzed by furnace method.
4th Experiment Applied on Environmental Samples
Eight environmental samples were taken for Arsenic speciation study. 50 ml aliquot of each sample was taken in 50 ml test tube. The pH of each sample was checked to neutral. Then samples were analyzed by Flame FIAS method to get As3+ concentration.
The same 8 environmental samples were subject to digestion. 15 ml of sample was digested in MDS with 0.75 ml conc. HNO3. Then samples were analyzed by Furnace method to get total Arsenic concentration. Subtracting As3+concentration from total Arsenic concentration, As5+ concentrations were obtained.
Results and Discussion
Several mixtures (As3+ + As5+) were analyzed both in the presence and absence of buffer. Reported values of As3+in both set A2 & B2 are 4.632μg/l & 4.804μg/l, respectively. However, the mixture (As3+/As5+ in sets A1 & B1) actually contains 0 μg/l As3+ & 30 μg/l As5+. Hence, it may be assumed that a positive interference (artifact) may be due to reduction of any As5+present or any impurities.
Whether the reported value of As3+in sets A2 and B2 (at actual value of 0 μg/l of As3+ in mixed solution As3+/As5+) depends on As5+ concentration or not, can be ascertained by analyzing different solutions with varying concentrations of As5+ against As3+ calibration curve in Flame-FIAS method. Blank concentrations in the presence and absence of buffer, i.e for A1 & B1 are reported as 0.006 μg/l & 0.096μg/l respectively
It can be presumed that a value due interference (obtained in case of seta A1 and B1) coming from As5+NIST Standard in mixed solutions of As3+/As5+are appeared. To check the interference, the solutions of As5+ of different concentrations are may be measured against As3+ standard curve by Flame FIAS method.
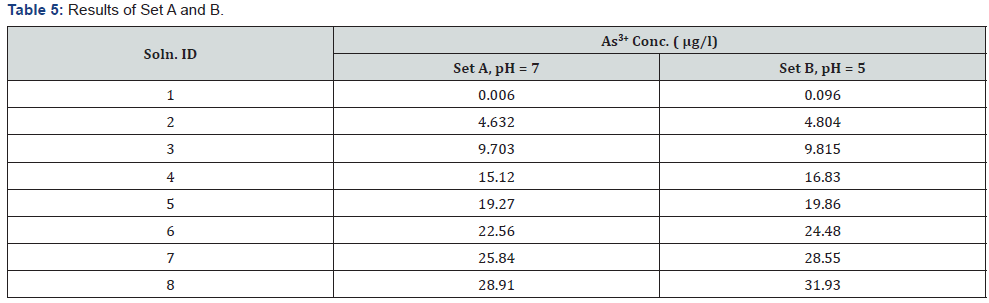
From the above experimental data (Table 5) it was observed that the interference value due to As5+ was not increasing proportionately & also reported below our lowest calibration standard value. Hence, the quantification of interference value due to As5+ is not possible at pH 6-7.
Chemistry Behind This Phenomenon
In the hydride generation method through Flame-FIAS, the total As (As3+ and As5+) was reduced to As3+ (followed by simultaneous conversion to arsine, AsH3 gas) using a reducing agent (Sodium borohydride). If the pH was maintained at near neutral range (6- 7), the reduction (As5+ to As3+) did not occur, and only the portion of the As present in trivalent form was selectively converted to AsH3. Using TRIS buffer, the pH of the solution was maintained at 6.2, where As5+ was expected not to get reduced to As3+, and only the fraction of As present in trivalent form gets converted to AsH3, As3+ (and not total As) was selectively determined by Flame – FIAS method. In this study, several mixtures (As3+ + As5+) are analysed both in the presence and absence of buffer to understand the effect of pH on the reduction of As5+ to As3+.
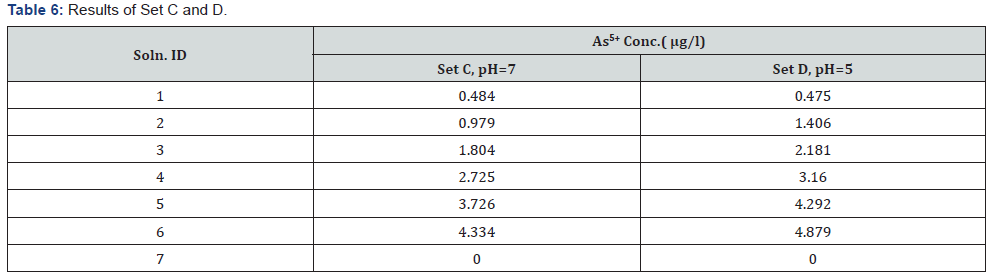
Chemical Reaction behind this conversion may be:
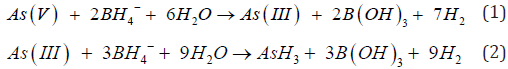
It was observed (as indicated in Figure 1) that on increasing the concentration of As3+ in the mixtures, the same was reflected in the spectrometric data, for both the sets (A & B), i.e. As3+ fraction in the solution gets converted to AsH3. It was also observed (as indicated in Figure 2) increasing pH (in the experimental range from pH 5 to pH 7) the interference of As 5+ decreased in arsenic solution in Flame FIAS method. From the Table 6, concentration of total Arsenic (given 30 μg/l) was varying from 23.25 to 24.6 μg/l in the undigested samples (set E) even at pH=2, due to unknown chemical interferences. But in case of digested samples at same pH (Set F) the concentration of Total As was around 30μg/l. In case of environmental samples the presence of total Arsenic went above the range of WHO standards (=10μg/l) but the more toxic species of Arsenic (i.e., As3+) varied from 1.66 to 3.94 μg/l following natural chemical phenomenon (Tables 7 & 8).
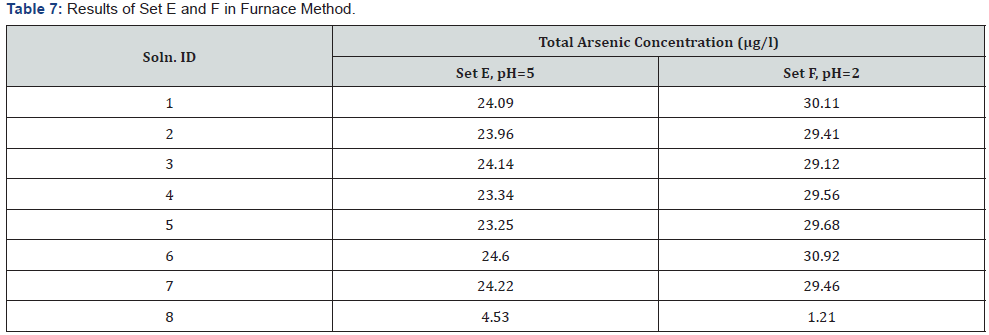

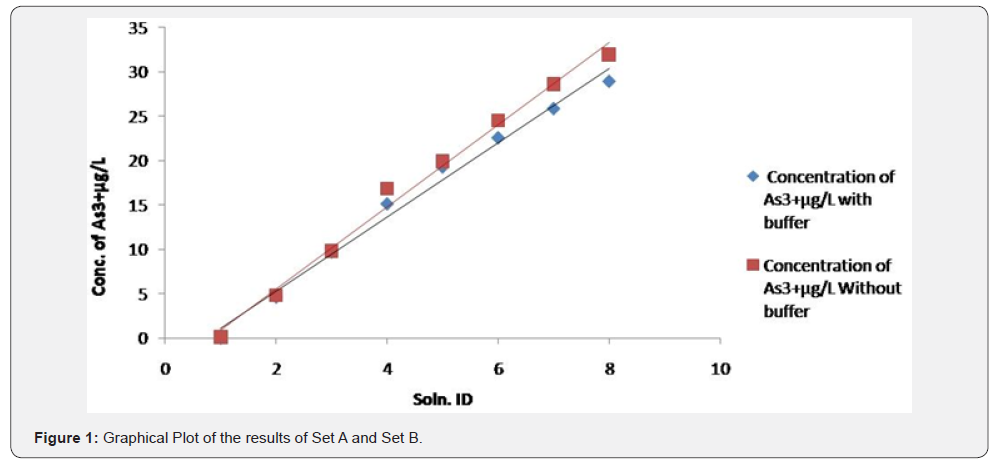
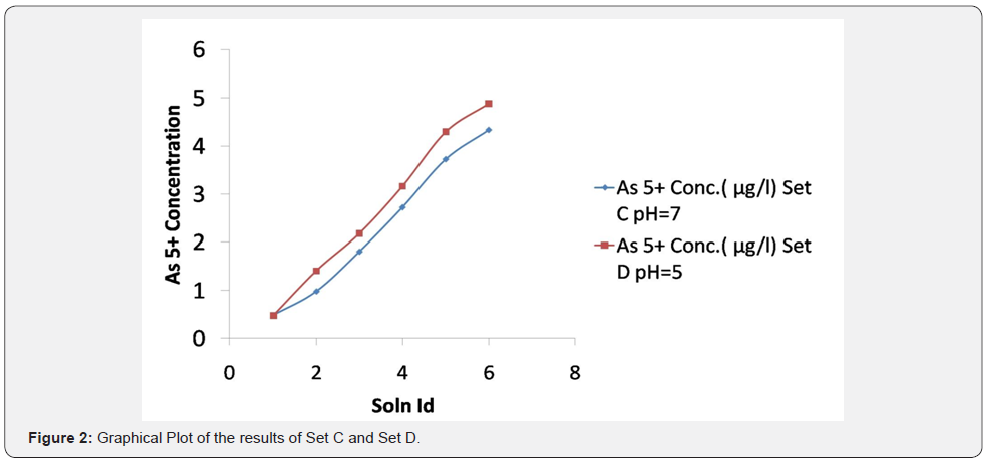
Therefore, we can apply this method for speciation of Arsenic in this kind of environmental samples.
Conclusion
We conclude that the FLAME-FIAS method can be used to determine As3+ in the mixed solution of As3+/As5+ at neutral pH as Arsenic speciation technique by plotting As3+ NIST standard calibration curve. An interfering value, due to transformation of As5+ to As3+ to some extent, is reflecting in the above experiment which cannot be nullified in this experimental condition. Additionally, in the case of the Furnace method, undigested synthetic samples have a certain amount of interference in comparison to the digested ones. Through application of this speciation study in environmental samples following standardized protocol, the concentration of As3+ is found to be low. Therefore, future remediation processes in this region should be planned according to the distribution of inorganic species reported here
Acknowledgment
Authors would like to acknowledge the Department of Environmental Science, University of Calcutta, India and West Bengal Pollution Control Board, India for their support and service.
References
- D Lièvremont PN, Bertin MC Lett (2009) Arsenic in contaminated waters: Biochemical cycle, microbial metabolism and biotreatment processes. Biochimie 91: 1229-1237.
- WJ Fitz, WW Wenzel (2002) Arsenic transformations in soilrhizosphere- plant system: Fundamentals and potential application to phyteremediation. J Biotechnol 99(3): 259-278.
- AK Sharma, JC Tjell, JJ Sloth, PE Holm (2014) Review of arsenic contamination, exposure through water and food and low cost mitigation options for rural areas. Appl Geochem 41: 11-33.
- D Chakraborti, MM Rahman, KPaul, UKChowdhury, MK Sengupta, et al. (2002) Arsenic calamity in the Indian subcontinent what lessons have been learned? Talanta 58(1): 3-22.
- D Chakraborti, B Das, B Nayak, A Pal, MK Sengupta, et al. (2008) Groundwater arsenic contamination in Ganga-Meghna-Brahmaputra plain, its health effects and approach for mitigation. Environ Earth Sci 70(5): 1993 -2008.
- MM Rahman, UK Chowdhury, SC Mukherjee, BK Mondal, K Paul, et al. (2001) Chronic arsenic toxicity in Bangladesh and West Bengal India A review and commentary. J Toxic 39(7): 683-700.
- SC Mukherjee, MM Rahman, UK Chowdhury, MK Sengupta, D Lodh (2003) Neuropathy in arsenic toxicity from groundwater arsenic contamination in West Bengal-India. J Environ Sci Health 38(1): 165- 183.
- D Chakraborti, SC Mukherjee, S Pati, MK Sengupta, MM Rahman (2003) Arsenic groundwater contamination in Middle Ganga Plain, Bihar, India: A future danger. Environ Health Perspec 111(9): 1194-1201.
- D Chakraborti, MK Sengupta, MM Rahman, S Ahamed, UK Chowdhury, et al. (2004) Groundwater arsenic contamination and its health effects in the Ganga Meghna Brahmaputra plain. J Environ Monit 6(6): 74-83.
- D Das, G Samanta, BK Mandal, T Roy Chowdhury, CR Chanda (1996) Arsenic in groundwater in six districts of West Bengal, India. Environ Geochem Health 18(1): 5-15.
- RK Dhar, BK Biswas, G Samanta, BK Mandal, D Chakraborti, et al. (1997) Groundwater arsenic calamity in Bangladesh. Curr Sci 73(1): 48-59.
- NM Smith, R Lee, DT Heitkemper, K deNicola Cafferky, et al. (2006) Inorganic arsenic in cooked rice and vegetables from Bangladeshi households. Sci Total Environ 370(2-3): 294-301.
- DK Nordstrom (2002) Worldwide occurrences of arsenic in ground water. Science 296(5576): 2143-2145.
- S Kar, JP Maity, JS Jean, CC Liu, B Nath, et al. (2010) Arsenic-enriched aquifers: occurrences and mobilization of arsenic in groundwater of Ganges Delta Plain, Barasat, West Bengal, India. Appl Geochem 25: 1805-1814.
- FS Islam, AG Gault, C Boothman, DA Polya, JM Charnock, et al. (2004) Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430: 68-71.
- K Kudo, N Yamaguchi, T Makino, T Ohtsuka, K Kimura, et al. (2013) Arsenic release from soil by a novel dissimilatory arsenate-reducing bacterium Anaeromyxobacter sp. PSR-1. Appl Environ Microbiol 79: 4635-4642.
- PA O Day, D Vlassopoulos, R Root, N Rivera (2004) The influence of sulphur and iron on dissolved arsenic concentrations in the shallow subsurface under changing redox conditions. Proc Nation Acad Sci 101: 13703-13708.
- L Drewniak, A Sklodowska (2013) Arsenic-transforming microbes and their role in biomining processes. Environ Sci Poll Res 20: 7728-7739.
- E Dopp, AD Kligerman, RA Diaz Bone (2010) Organoarsenicals: Uptake, metabolism and toxicity. Met Ions Life Sci 7: 231-265.






























