Modifications of the Flora Zambeziaca in the Zambezi Basin by Environmental Antecedent Factors: Termites, Fire and Elephant
Clifford Tafangenyasha*, Patmore Ngorima, Stanely Musungwa and Blessing Kavhu
Department of National Parks and Wildlife Management Authority, Harare, Zimbabwe
Submission: June 05, 2018; Published: June 19, 2018
*Corresponding author: Department of National Parks and Wildlife Management Authority, Harare, Zimbabwe, Email: cliffordtafa@gmail.com
How to cite this article: Clifford T, Patmore N, Stanely M Blessing K. Modifications of the Flora Zambeziaca in the Zambezi Basin by Environmental Antecedent Factors: Termites, Fire and Elephant. Int J Environ Sci Nat Res. 2018; 12(3): 555840. DOI:10.19080/IJESNR.2018.12.555840.
Abstract
The study throws light on factors modifying flora Zambeziaca in the Zambezi Valley and investigates the phenomenon of tree mortality. The paper adds to the growing literature that suggests that harvester termites Isoptera: Termitidae, Macrotermitinae, elephant (Loxodonta Africana Blumenbach L) and fire reduce forest cover over wide swathes of land. To assess the impact of the hyper-abundant termites, fire and elephant in Zambezi Valley 18 belt transects were randomly placed in the study area in Sengwa Wildlife Research Area (SWRA). The 18 belt transects were characterised into nine paired plots that were considered degraded and Undegraded. The study formed part of a vegetation condition assessment of the SWRA. Spearman rank order test (rs) and Student T tests showed significant tests in elephant, termite and fire damage patterns. Principal Component Analysis (PCA) were used to bring out strong patterns (p<.05) in the dataset. The study results are critical for biodiversity management strategies in the Zambezi Valley, Zimbabwe.
Keywords: Termites; Elephant; Fire; Flora Zambesiaca; Vegetation Modification; Savanna Systems
Introduction
The Flora Zambeziaca in the Zambezi Basin is a keystone and flagship resource to a wide range of freely grazing and browsing wildlife species [1-3]. The Zambezi Basin presents interesting Savana and management styles available in the menu to rangeland ecologists Bignell [4] Walker [5], Cumming [6], Ben-Shar [7] Holdo [8]. Several studies Chafota and Owen-Smith [9], Nangendo [10], Nellis and Bussing [11], Tafangenyaha [12], Osborne [13] investigated vegetation change in Savana rangelands without concluding actual drivers of change. Zambezi Basin vegetation has been aptly described by Wild, Barbosa and Fernandez, Drummond [14], Timberlake among other workers. The original vegetation from the time of pioneer explorer David Livingstone (19 March 1813-1 May 1873), outback hunter Frederick Selous (1875-1917) and Wild et al (1967) was intact but has recently come under pressure from increasing mega herbivore populations and more recently termites (Isoptera: Termitidae, Macrotermitinae). Most recently there has been compression of the elephant population following land pressure from settlement and cultivation after tsetse fly eradication. The result has been massive reduction with upper canopy trees. A number of authors Dangerfield [15,16] Eggleton and Tayasu [17], Timberlake [18] attribute endogenous and exogenous factors as key to woody plant mortality and recruitment. The impact of natural forces such as herbivore and fire is ever changing in intensity and scale and the need to study its influence on structure and composition. Tafangenyasha noted that scale and intensity of disturbance in open rangelands need exhaustion after long-term studies in varied habitats created by mega herbivores and antecedent environmental factors. In addition, herbivore has been underscored in the present study as initiating tree damage with drought, changes to structure and composition of the forests. Termites are detrivores that eat dead and decaying organic matter. Symbiotic bacteria in the termite gut transforms decomposing cellulose into carbohydrates and sugar, whereas gut protozoa in turn relyon symbiotic bacteria embedded on their surfaces to produce some of the necessary digestive enzymes Eggleton and Tayasu. Bacteria form an important link in the cycling of nutrients in the forests by breaking down wood and organic residues on the ground which would otherwise take years to decay by microbes. In dry environments litter degradation and soil mixing is mainly performed by termites Dangerfield. In 1961, most (81%) termite mounds were not full size in the Zambezi Valley, which suggested that they were recent additions to the area. There was little change in mound density between 1970 and 1981, but between 1981 and 1984 and between 1984 and 1987, there were major increases in mound. Dangerfield contends that with one exception, where new mounds appeared after 1981 were areas where the abundance of the tall, tufted grass, Vetiveria nigritana, declined during the same period. Tree damage and loss may be a composite phenomenon that sets in forest gaps that may be colonised by noxious invasive species such as Lantana camara L Tafangenyasha. Maintaining functionality and sustainability of dry land systems requires sustainable land management over large areas such as Zambezi Valley. This research is critical in developing biodiversity management strategies in protected areas.
Study Area
In Zimbabwe changes in woodlands caused by elephants and other factors were studied in the Zambezi Valleyat Sengwa Wildlife Research Area with a long history of documented elephant (Loxodonta Africana Blumenbach 1797) population build up to 1965 followed by 24 years of sustained culling and 26 years of vegetation regeneration in aftermath of elephant culling. Sengwa Wildlife Research Area (SWRA) is situated at the southern end of Chirisa Safari Area (18o 10’ S, 28o 14’ E) in Gokwe South District, north-western Zimbabwe (Figure 1). Covering an area of about 373 km2, the area was set aside in the late 1960s for longterm wildlife and ecological research. There are a series of blackand- white aerial photographs from as far back as 1964. Three sets of detailed colour photography at scales of 1:10 000 and some at 1:5 000 were examined. The surface geology comprises of Lower and Upper Karoo age. The study area constituted by the Lower Karoo is represented by the Madumabisa mudstone formation weathering grey carbonaceous shale with Endothiodon spp fossil wood and Lamellibranchs-Pellaedonodonta spp and Ostracods- Darwinuda spp Selibas [19]. The Upper Karoo which overlies the mudstones gives rise to geologically and ecologically significant colluvial deposits with carbonaceous and siliceous matrices Figure 1.
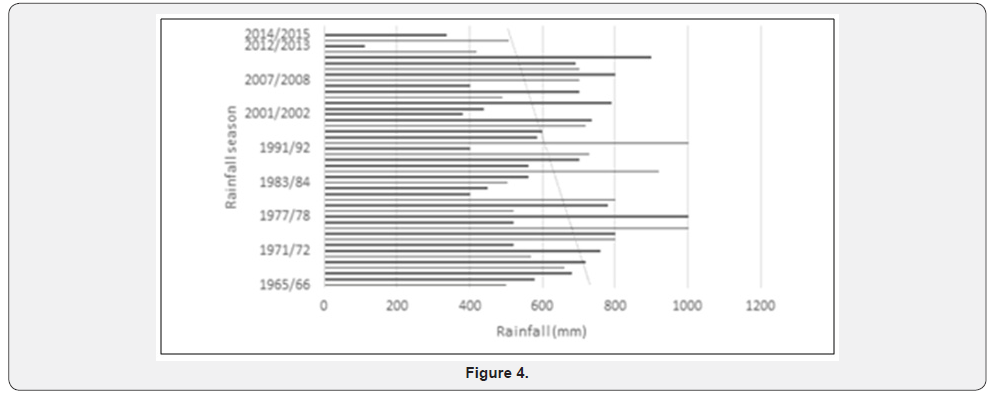
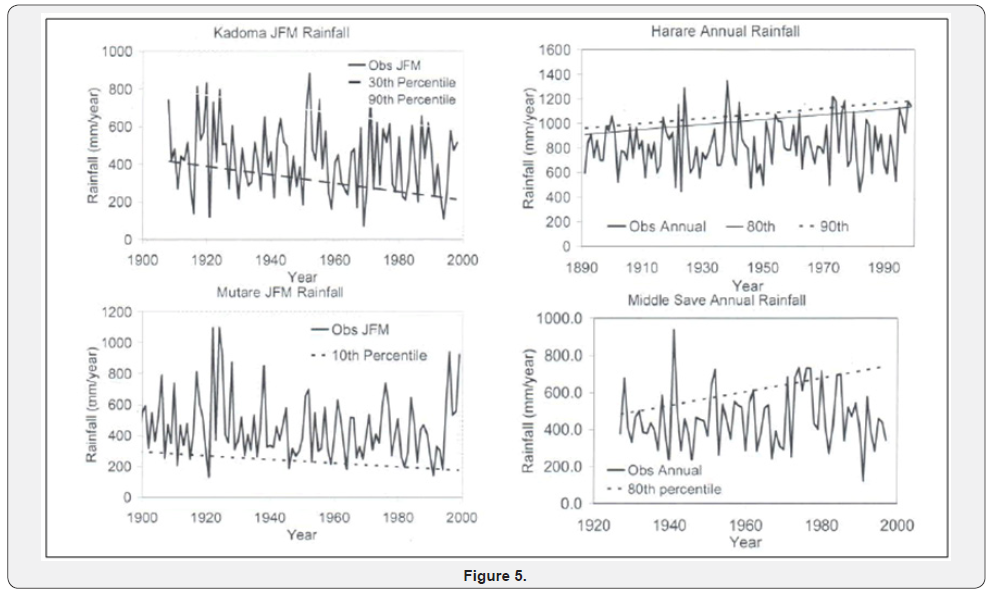
The geological formations have been dissected by the north flowing rivers to give rise to soil and vegetation types (Figure 1) that are an important source of water for the animals. SWRA is semi-arid ecosystem with low and irregular rainfall, high evapo transpiration and cyclical droughts. High evapo transpiration means soils dry up quickly reducing amount of water available for plant uptake. (Figures 2-4) suggest that severe droughts are a long term feature in the study area and region. Several topographic and vegetation maps of the area were used to make possible the study on homogeneous Madumabisa mudstones. Data on animal numbers and seasonal movements are available from counts done during organized patrols, along permanent transects and aerial surveys since the late 1960s. There is a wide variety of vegetation types, 26types having been described and mapped by Craig (1982). A wide range of research projects have been done in the SWRA since its establishment to establish diet preferences of herbivores and forage availability. The elephant (Loxodonta Africana Blumenbach L.) ranks as one most common and dominant herbivore in SWRA Cumming [20,21]. The wasteful feeding behaviour was militated against by a sustained culling programme terminated in 1989. Few studies have been conducted to understand the response of woody vegetation in the aftermath of culling.
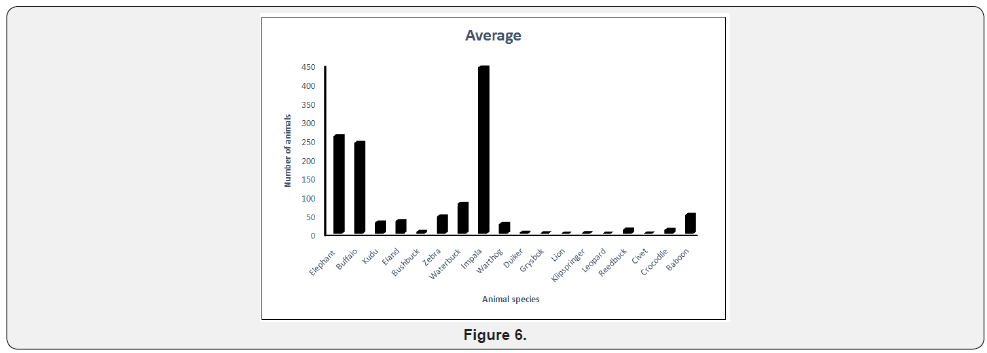
The SWRA has a diverse large animal mammal community of 7 species of large carnivores and 18 species of large herbivores. The area is home to diverse communities of birds, small mammals and reptiles. Some common wild animals in the SWRA are given in Figure 5. When an elephant range area reserve is fenced and supplied with water from permanent rivers, elephant population irruptions take place, necessitating culling. A comparison can be made between patches formerly degraded and undegraded in the aftermath of culling. Two assumptions were made about the history of the wood lands examined in this study. The first is that there is no difference in the woodland structure and composition of vegetation in over utilized and undegraded plots after culling had ceased. The second is that changes to the vegetation in SWRA were caused by elephants and fire. The 2014 elephant aerial survey of the Sebungwe Region showed a major decline in standing elephant populations from 13000 to 3500 Dunham [22]. But prior to establishment of national parks there were about 5000 elephant in Zimbabwe in the middle of the 19th century. The extent of gap colonization by non-native plant species were observed in the study area. The information obtained in the study should influence management decisions on appropriate park ecosystem health and understanding of changes in vegetation composition and structure and habitat preferences.
Materials and Methods
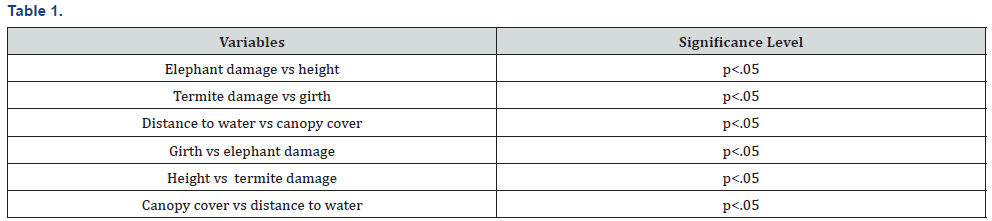
To assess the impacts of hyper-abundant termites, fire and elephant in Zambezi Valley 18 belt transects were randomly placed in study area in the SWRA in the dominant woodland types occurring in the study area. The 18 belt transects were located by pairing nine plots that were considered degraded and undegraded. A mosaic of patchy disturbance by elephant feeding habits occur in SWRA. The study formed part of a vegetation condition assessment of the SWRA. The woody vegetation types delineated by Craig [23] (Figure 6) were used as a guide to vegetation condition assessment (Figures 7-12). Spearman’ rank correlation (rs) coefficient is a non-parametric measure of rank correlation between rankings of two variables (Table 1). It measures the strength and direction of association between two ranked variables. PCA is a technique used to emphasise variation and bring out strong patterns in a dataset. The analyses highlight current understanding for improved Savana conservation. To obtain pattern from the data regression analysis was used.

Results
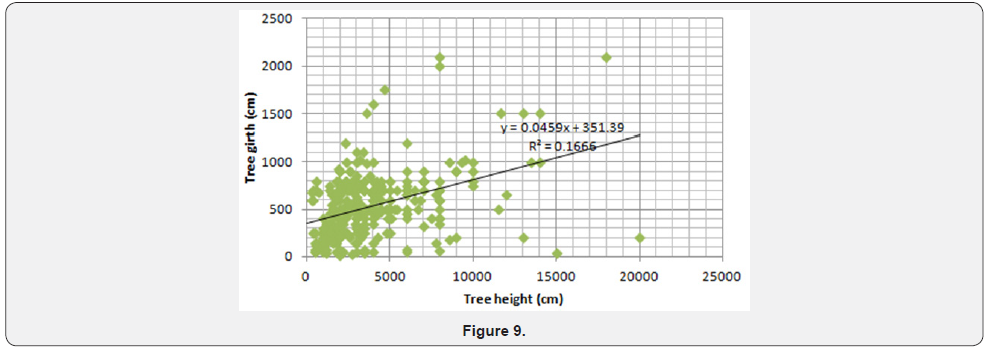
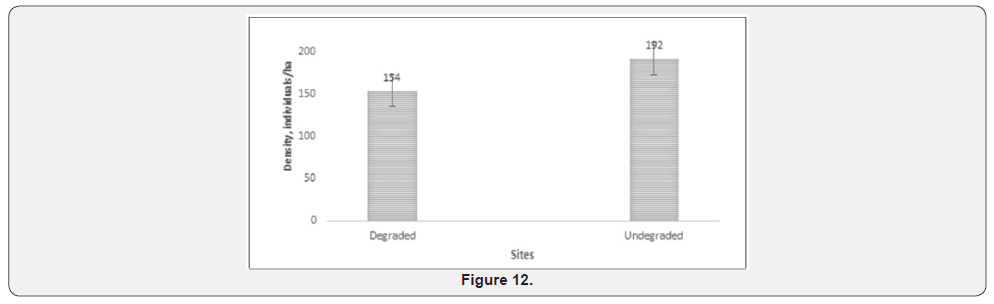
Spearman rank correlation showed strong positive relationship between elephant damage and termite damage (Table 1). There was a strong positive relationship between fire damage and elephant damage using Spearman rank correlation (Table 1). The metrics collected between degraded and undegraded plots showed variations that are summarised in Table 2. Degraded plots measured smaller mean girth (12, 2 cm) and mean height (446,7cm) when compared to undegraded plots (13,6 cm and 460, 2 cm, respectively, Table 2). Mean percentage canopy cover, elephant damage and termite damage was higher on undegraded plots compared to degraded plots (Table 3). Drought damage on woody plants was about the same between degraded (0,1%) and undegraded plots (0%) (Table 3). Degraded plots measured the highest mean distance (1026,8 cm) to water compared to undegraded plots (885,4 cm) (Table 3).
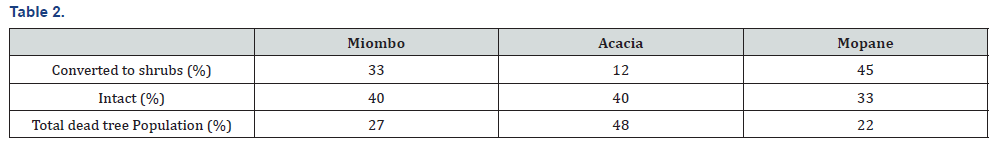
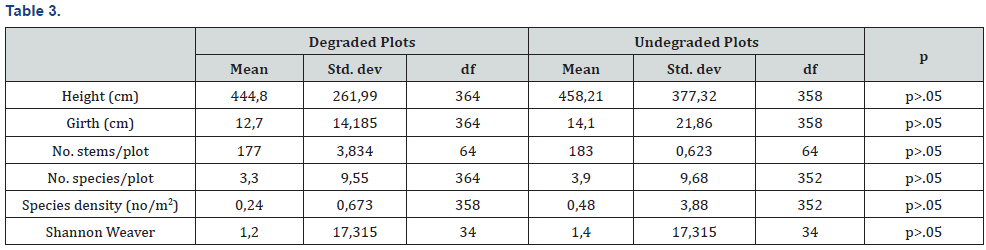
Formerly over utilized patches showed no significant differences (Student t test p>.05) in height, girth and number of stems per plot (Table 3). Table 3 showed no significant differences in number of stems per plot, species density (number per m2) and Shannon Weaver diversity indices. The results presented in Table 3 are surprising considering the recorded negative effects of elephant on Savanna wooded vegetation. The results suggest that the historically high densities of elephants altered forest structures and the vegetation may not be showing strong differences between formerly heavily utilised and relatively unutilised patches after repeated culling events. The general findings are outlined below:
A general recovery of woody vegetation in previously damaged sites is shown by counts of stems with few signs of fresh damage.
a) No significant differences in numbers of stems/trees between degraded and undegraded plots.
b) Low incidences of burn marks
c) Low damage levels by elephant.
d) High levels of termite activity on woody stems and litter cannot be explained at this stage.
Species richness is estimated with the total number of observed species. Species richness (usually notated S) of a dataset is the number of different species in the corresponding species list. Richness is a simple measure. The Shannon Diversity Index is calculated by multiplying a species proportional abundance by the natural log of that number:
ln i i H = −Σρ ρ
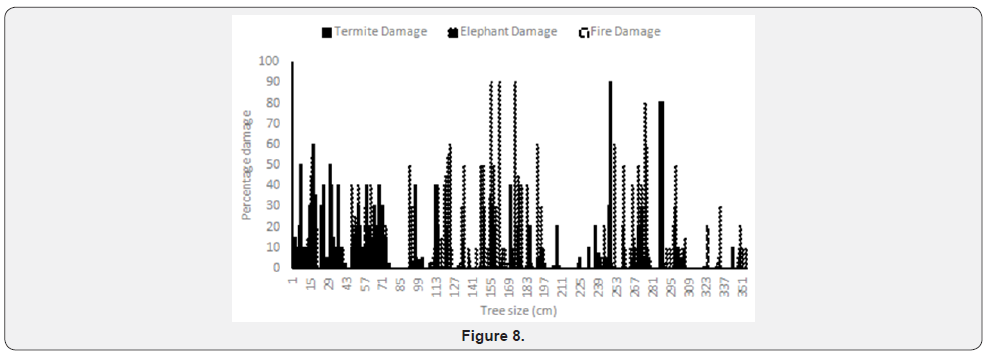
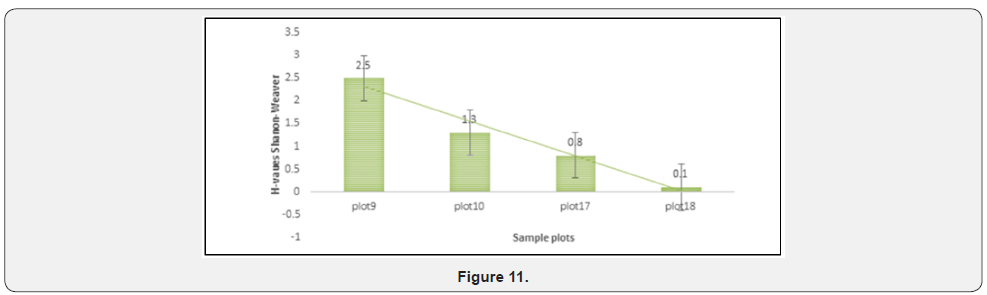
Where pi is the proportion of individuals found in the species “i”. This index assumes that individuals are sampled randomly from an infinite or very large population. Similarly, it supposes that all species are represented in the sample (Figures 7 & 8).
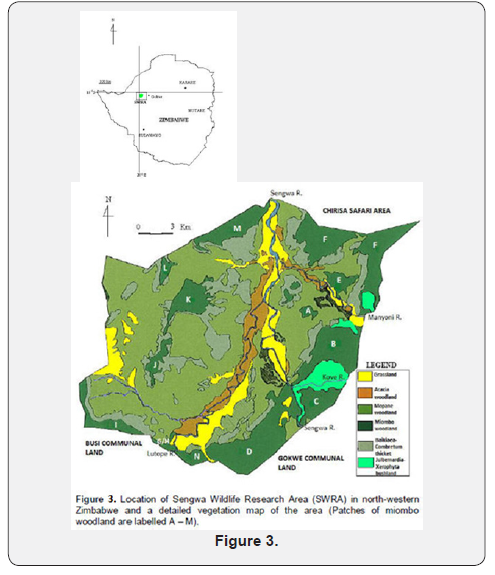
Factors important in vegetation change at Sengwa wildlife area (Figure 3):
a) Small plants common in the recruitment cycle of vegetation regeneration.
b) Large tree sizes have high levels of termite damage probably due to an abundance of cellulose and biomass for metabolic energetic.
c) Young trees not burdened by termite attack.
d) Elephant damage more pronounced on young mature plants.
e) Elephant damage and termite damage have greater incidence on young trees than on mature tree sizes.
f) Elephant damage is a major factor in the Savana vegetation affecting all three sizes.
g) Elephant culled/translocated to reduce populations, termites not.
h) Elephant damage a problem in all age classes.
i) Elephant population suppression likely to give recruitment of trees a chance of survival to maturity
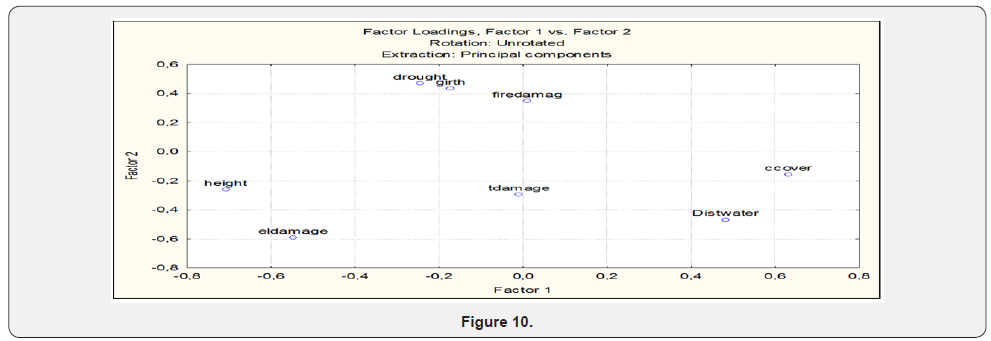
Tafangenyasha noted the importance of elephant and fire suppression on woodlands. Termite damage emerged as an important factor during dry periods. Fire and hyper-abundant termite damage have even importance (Figure 7) but with high fire damage loading scores. Elephant damage may have a role in controlling height of trees. Drought damage may affect large trees (Figure 7) Distance to water effect is distributed across the large stretches of water courses (Figure 7) SWRA has virtual reliance on surface water in the water courses.
Discussion
The data in this study seems to be in agreement with observations by Remmert, Dublin [24], Nangendo and O’Connor [25] and suggests that woodland dynamics may be changing all the time due to complex interactions of fire, elephant and termites. Termites have long been known to be voracious feeders of lignin and cellulose diet Becker their main sources of energy but their impacts on integrity of woodlands has always been sketchy. Elephant damage may have greater landscape change through felling and breakage of branches and stems. With growing aridity as predicted by IPCC agents of vegetation change may be considered de-novo. The consequences for woodland dynamics as recorded in this study depend largely on the size classes of the trees affected, as well as on how the disturbance is concentrated in time and space Chafota and Owen Smith, Timberlake and Mapaure Mortality of canopy trees has probably a much greater and longer-lasting impact than losses among the regenerating stages of these trees Tafangenyasha [26], Tafangenyasha. However, the consequences may be less adverse for ecosystem function and biodiversity if the disturbing effects are locally concentrated, generating a patch mosaic of stands at different stages of regeneration Campbell [27] Luoga [28] Remmert. In the study area, majority of trees were young trees suggesting that in a free ranging elephant country mature trees are damaged or eaten. Young trees have low cellulose, low lignin content, low fibre, low chemical and mechanical defensive structures and high crude protein content Midgley [29]. The consequences for woodland dynamics depend on the size classes of the trees affected, as well as on how the disturbance is concentrated in time and space Chafota and Owen-Smith, Timberlake and Mapaure. Chafota and Owen suggest that elephants can have a major transforming effect on Savana structure through felling, debarking or uprooting trees. However, it is difficult to separate their influence from that of other causes of tree mortality, including wind storms Spinage and Guinness [30] drought Lewis, van de Vijver [31] fire Higgins [32] and in some situations frost Childes & Walker [33] Holdo [34] especially when interactions among them may occur de Beer [35] Laws [36] Pienaar [37]. The size selection of trees by trees and the ubiquitous termite leave lasting scars on the landscapes Cizek noted that patches of miombo also occur somewhere in the empty polygons of northern Kruger National Park (see Wild, Fernandez and Barbosa. For example, importantly, there was a catastrophic decline of canopy of Brachystegia torrei across the Chihunja Platform in the last decades the last century Tafangenyasha, but patches still remain Clegg [38]. Which woody species selected by elephant are at risk of local extirpation may be based on an understanding of elephant digestive physiology, foraging ecology, attributes of individual plants and populations, and historical changes in ecosystems.
Elephant select items rich in cell solubles relative to availability for achieving maximum through put per unit time on account of their large energy requirement, hindgut fermentation with limited cell wall digestion, high passage rate, and inefficient recycling of microbial protein Midgley. Accordingly, diet is predominantly green grass and herbs in the wet season, green browse in the late wet and dry seasons, and bark and roots following leaf fall Holdo. Increased consumption of woody material indicates nutritional stress. Bulls graze more than cows and impact woody plants more when grazing deteriorates to create patches Roxburgh. A patch is defined as an area differing in appearance from its surroundings Roxburgh [39]. Landscape patches in the SWRA of the Zambezi Valley owe their existence to large herbivore feeding patterns. Examples of remnant patches are areas of vegetation which have escaped the effects of a widespread fire, and the patches of natural vegetation which can be found in many rangelands. Patches may also be transient, meaning that they may only exist for short periods of time such as heavy grazing/browsing pressure. With increasing mega faunal disturbance patches become numerous and smaller, varying in size and shape.
Vegetation on termite mounds usually differs highly from vegetation in the surrounding landscape Dangerfield, Eggleton and Tayanus. In African Savanas, Macrotermes mounds form ‘islands’ with high tree densities Wilson [40] Bignel contend that due to the digging of termites and due to their decomposition of plant material, the mound soils are generally more fertile than other soils. On top of that, mound soils have been found to contain more water than their surroundings, a clear advantage for plant growth in Savanas Bignel. The high tree densities on termite mounds attract high densities of browsing herbivores, due to the high nutrient contents in foliage from trees growing on mounds Dangerfield or perhaps due to the high quantities of food and shelter on mounds. Mound building termites contribute to Savana vegetation heterogeneity. Damage by termites is greater during dry periods or droughts than periods of regular rainfall Logan [41]. Damage by termites is greater during dry periods or droughts than periods of regular rainfall Logan [41]. The increases in termite damage could also be associated with climate change induced drought. In recent decades, drought linked to El Niño episodes has become more intense and widespread in southern Africa. It may follow that termite damage, elephant and fire damage creates a mosaic of patches on landscapes in the Zambezi Valley. Overall mortality of woody vegetation has been estimated at 29.0 % by Swanepoel Swanepoel [42] Damage and mortality rates varied annually possibly related to rainfall linked with elephant densities Swanepoel, Dunham [43]. The increases in termite damage could also be associated with climate change induced drought. In recent decades, drought linked to El Niño episodes has become more intense and widespread in southern Africa Harrington and Stork [44]. Termite mounds should clearly be considered a focus of conservation importance and their destruction and utilization for farming Nyamapfene detrimental to a diverse range of plant and animal species. The study suggests an interesting interaction of fire, elephant and termites in creation of Savana heterogeneity. Termites as ecosystem engineers drive Savana nutrient dynamics by being tertiary consumers. The hyper-abundant termitidae in the Zambezi Valley gives insight into forest dynamics using the gaps created by elephant, forest health and behaviour of mesoherbivores that is initiated by fire and elephant. The study opens insights into a complex ecosystem functioning in the Zambezi Valley where once intact miombo woodland is being fragmented [45-55].
Conclusion
Fire, elephant and termites, can have a major transformation and modification of the Zambezian vegetation through complex interactions. However, it is difficult to separate influence of each factor from that of other cause of tree mortality, including drought, fire and major herbivores such as elephant especially when interactions among them may co-occur. The consequences or woodland dynamics may depend on the size classes of the tree affected as well as how the disturbance is concentrated in time and space. It seems, the consequences of vegetation modifications by antecedent environmental factors may be more for an ecosystem function and biodiversity in the case that the disturbing effects are fire, elephant and termites. Fire impacts, termite attacks and concentrations of mega-herbivores are significantly concentrated along the Zambezi River, generating a patch mosaic of stands at different stages of regeneration. The paper presents interesting Savana ecosystem processes and management styles available in the menu to rangeland ecologists.
Acknowledgement
Grateful thanks are extended to many individuals who are too numerous to mention in the execution of the research. The National Botanic Gardens assisted with plant specimen identification and are gratefully acknowledged.
References
- Mapaure A, Timberlake J (2004) A checklist of Zimbabwean vascular plants Southern African Botanical Diversity Network Report No. 33 Sabonet. Pretoria and Harare p. 75.
- Timberlake N, Nobanda I Mapaure (1993) Vegetation survey of the communal lands-north and west Zimbabwe. Kirkia 142: 171-270.
- (2014) Ministry of environment, water and climate. National Biodiversity strategy and action plan. p. 76.
- Bignell DE, Roisin Y, Lo N (2010) Biology of Termites: a Modern Synthesis. (1st edn.), Dordrecht: Springer
- Walker B (1998) The art and science of wildlife management. Oecologia 155: 487-496.
- Cumming DHM (1981) The management of elephant and other large mammals in Zimbabwe. In Jewel PA and Holt S (Eds.), Problems in managing locally abundant wild animals, Academic Press, New York, USA, pp. 91-118.
- Ben Shahar R (1999) Elephants and their woodlands in northern Botswana. Pachyderm 27: 101-104.
- Holdo RM, Holt RD, Fryxell JM (2013) Herbivore vegetation feedbacks can expand the range of savanna persistence: insights from a simple theoretical model Oikos 122: 441- 453.
- Chafota J, owen-Smith (2009) Episodic severe damage to canopy trees by elephants: interactions with fire, frost and rain. Cambridge University Press, USA.
- Nangendo G, Stein A, Ter Steeg H, Bongers F (2005) Changes in woody plant composition of three vegetation types exposed to a similar fire regime for over 46 years. Forest Ecology and Management 217: 351- 364.
- Nellis MD, Bussing CE (1990) Spatial variation in elephant impact on the Zambezi teak forest in Chobe National Park, Botswana. Geocarto International 2: 55-57.
- Tafangenyasha C (1997) Should Benji Dam be dredged? A preliminary impact assessment to dredging a water reservoir in an African national park. Environmentalist 17: 191-195
- Osborne FV (2002) Elephant induced change in woody vegetation and its impact on elephant movements out of a protected area in Zimbabwe. Pachyderm 33: 50-57.
- Drummond RB (1975) A list of trees, shrubs and woody climbers indigenous or naturalised in Rhodesia. Kirkia 10(1): 248.
- Dangerfield JM (1990) The distribution and abundance of Cubitermes sankurensis (Wassmann) (Isoptera; Termitidae) within a miombo woodland site in Zimbabwe. Afr J Ecol 28: 15-20.
- Dangerfield JM, TS McCarthy, WN Ellery (1998) The mound building termite Macrotermes michaelseni as an ecosystem engineer. J Trop Ecol 14: 507-520.
- Eggleton P, Tayasu I (2001) Feeding groups, life types and the global ecology of termites. Ecol Res 16(5): 941-960.
- Timberlake JR (2004) AWF Four Corners TBNRM Project. Reviews of existing Biodiversity information. The Zambezi Society and Biodiversity Foundation.
- Selibas NJ (1972) Notes on the geology of the SWRA and a preliminary basin analysis. Unpublished BSc honours dissertation.
- Cumming DHM (1982) The influence of large herbivores on Savanna structure in Africa. Ecology of tropical savannas pp. 217-245.
- Cumming DHM (2008) Large scale conservation planning and priorities for the Kavango Zambezi Transfrontier Conservation Areas. Conservation International pp. 124.
- Dunham KM (2015) National summary of aerial survey results for elephant in Zimbabwe. Great elephant census: A Paul G, Allen Project, Harare, p. 45.
- Craig GC (1983) Vegetation survey of Sengwa. Bothalia14: 759-763.
- Dublin HT, Sinclair ARE, Mcglade J (1990) Elephants, fire as causes of multiple stable states in the Serengeti-Mara woodlands. Journal of Animal Ecology 59(3): 1147-1164.
- Connor TG, Goodman PS, Clegg B (2007) A functional hypothesis of local extirpation of woody plant species by elephant in Africa. Biological Conservation 136: 326-345.
- Tafangenyasha C (1997) Tree loss in the Gonarezhou National Park (Zimbabwe) between 1970 and 1983. Journal of Environmental Management 49: 355-366.
- Campbell BM, Frost P, Byron N (2006) Woodlands and their use: overview and key issues, in The Miombo in Transition: Woodlands and Welfare in Africa. Centre for International Forestry Research, Bogor, Indonesia.
- Luoga ETF, Witkowski TF, Balkwill K (2004) Regeneration by coppicing (resprouting) of miombo (African savanna) trees in relation to land use. Forest Ecology and Management 189: 1-3.
- Midgley JJ, Balfour D, Kerley GIH (2005) Why do elephants damage Savana trees? South African Journal of Science 101: 213-215.
- Spinage CA, Guinness FM (1971) Tree survival in the absence of elephants in the Akagera National Park, Rwanda. Journal of Applied Ecology 8: 723-728.
- Van de Vijver CADM, Foley CA, Olff H (1999) Changes in the woody component of an East African savanna during 25 years. Journal of Tropical Ecology 15: 545-564.
- Higgins SJ, Bond WJ, Trollope WSW (2000) Fire resprouting and variability: a recipe for grass-tree coexistence in savanna. Journal of Ecology 88: 213-229.
- Childes SL, Walker BH (1987) Ecology and dynamics of the woody vegetation on Kalahari sands in Hwange National Park. Vegetatio 72: 111-128.
- Holdo RM (2006) Elephant herbivory, frost damage and topkill in Kalahari sand woodland savanna trees. Journal of Vegetation Science 17: 509-518.
- De Beer Y, Killan W, Versveld W, Van Arde RJ (2006) Elephants and low rainfall alter woody vegetation in Etosha National Park, Namibia. Journal of Arid Environments 64: 412-421.
- Laws RM, Parker ISC, Johnstone RCB (1975) Elephants and their habitats. The ecology of elephants in North Bunyoro, Uganda. pp. 376.
- Piennar UDEV, Van WYK P, Fairall N (1966) An aerial census of elephant and buffalo in the Kruger National Park, and the implications thereof on intended management schemes. Koedoe 9: 40-107.
- Clegg B (2003) Vegetation of northern Gonarezhou National Park. Malilangwe Trust, Chiredzi, Zimbabwe.
- Roxburgh S (2001) Population dynamics and landscape ecology. John Wiley and Sons, New York, USA, pp. 1-26.
- Wilson EO (2014) A window on eternity: a biologist’s walk through Gorongosa National Park. Simon & Schuster.
- Logan MW, RH Cowie, TG Wood (1990) Termite (Isoptera) control in agriculture and forest by nonchemical methods a review. Bull Entomol Res 80: 309-330.
- Swanepoel CM (1993) Baobab damage in Mana Pools National Park. African Journal of Ecology 31 (3): 220-225.
- Dunham KMJ (1990) Fruit production by Acacia albida trees in Zambezi riverine woodlands. Tropical Ecology 6(4): 445- 457.
- Harrington R, Stork NE (1995) Insects in a changing environment. Academic Press, New York, USA.
- Ben Shahar R (1998) Changes in structure of Savanna woodlands in northern Botswana following the impacts of elephants and fire. Plant Ecol 136: 189-194.
- Guy PR (1981) The estimation of the above-ground biomass of the trees and shrubs in the Sengwa Wildlife Research Area, Zimbabwe. South African Journal of Wildlife Research 11: 135-142.
- Guy PR (1981) Changes in the biomass and productivity of woodlands in the Sengwa Wildlife Research Area, Zimbabwe. Journal of Applied Ecology 18: 507-519.
- Mazvimavi D (1998) Water availability and utilization in Zimbabwe. Geographical Journal of Zimbabwe 29: 23-36
- Mazvimavi D (2010) Hydrological changes over time of annual rainfall in Zimbabwe. Hydrological and Earth System Sciences 14: 2671-2679.
- Moyo G (2011) Monitoring climate in SWRA. A description of long term rainfall trends. Zimbabwe Department of National Parks and Wildlife Management Authority, Harare.
- Tafangenyasha C, Campbell BM (1998) Initiation and maintenance of degraded landscape in the Sinamatella area of Hwange National Park, Zimbabwe. Journal of Environmental Management 52: 69-78.
- Tafangenyasha C (1998) Phenology and mortality of common woody plants during and after a severe drought in South-eastern Zimbabwe. Transactions of the Zimbabwe Scientific Association 72: 1-6.
- Tafangenyasha C (2001) Decline of Brachystegiaglaucescens in the Gonarezhou National Park, southeast Zimbabwe. Journal of Environmental Management 63: 37-50.
- Tafangenyasha C, Mthembu AT, Chkoore H, Ndimande N, Xulu S, et al. (2011) The effects of soil density on the vegetation of the Umfolozi catchment in South Africa. Journal of Soil Science and Environmental Management p. 14-24.
- Tafangenyasha C, Mthembu AT, Chkoore H, Ndimande N, Xulu S, et al. (2011) Rangeland characteristics of a supercritical degraded landscape in the semi-arid area of South Africa. Journal of Soil Science and Environmental Management p. 80-87.
- Timberlake JR, Mapaure I (1992) Vegetation and its conservation in the eastern mid-Zambezi Valley, Zimbabwe. Transactions of the Zimbabwe Scientific Association 66: 1-14.






























