Novel Fungal Endophyte Alternariaalternata Isolated from the Endangered Plant Rhusmysorensis in Sanganer Region of Rajasthan, India
Kartikeya Tiwari*
Microbial Biotechnology Laboratory, Management & Science University, Malaysia
Submission: December 13, 2017; Published: January 04, 2018
*Corresponding author: Kartikeya Tiwari, Microbial Biotechnology Laboratory, Management & Science University, Shah Alam, Selangor, 40100, Malaysia, Email: tiwarikartikl@gmail.com
How to cite this article: Kartikeya T. Novel Fungal Endophyte Alternariaalternata Isolated from the Endangered Plant Rhusmysorensis in Sanganer Region of Rajasthan, India. Int J Environ Sci Nat Res. 2017; 8(1): 555728. DOI: 10.19080/IJESNR.2018.08.555728.
Abstract
Endophytic association between plant and fungi are common. This symbiotic association between plant and fungi help each other to survive and multiply in adverse conditions. Sanganer region of Rajasthan witness temperature variation, water scarcity and extreme climate conditions. Therefore, the plants adapted and possess various unique mechanisms of survival in this region. Rhusmysorensis is endangered species of plant and growth and multiplication of this plant in extreme conditions of Sanganer region of Rajasthan is due to unique mechanism of survival. The present paper discusses the details of novel fungal isolate Alternariaalternata morphology and relation with the plant Rhusmysorensis.
Keywords: Endophytes; Mycelia Sterilia; Endangered Plant Species; Fungal Diversity
Introduction
Sanganer region of Rajasthan is one of twenty five hot spots of global biodiversity with approximately 5,000 species of flowering plants the mean temperature during the study period was 30± 2 °C. Some of the plants has long ancient history for the colonization in this region. The adaptation in adverse conditions and unique mode of survival, made these plants grow and flourish in seasonal variations. One of the important factor is endophytic fungal colonization in the roots of these plants. The relationship between endophytes and the host plants may represent a continuum of interaction, ranging from latent phyto- pathogenesis to mutualistic symbiosis [1-8].
Materials and Methods
Plant Selection
Rhusmysorensis L., (Darsan) have shown the maximum endophytic colonization, therefore this plant was selected for study and the samples were collected from the different sites of Sanganer region, during the months of July- November, 2010. Roots were collected randomly from Rhusmysorensis plant and were first washed with running water. The roots were cut into pieces (5x5mm) [1-8].
Surface Sterilization
All segments and pieces were successively surface-sterilized by dipping in 75% ethanol for 1 minute, 4 % sodium hypochlorite for 5 minutes followed by rinsing three times in sterilized distill water. In each petridish, a total of four-five processed root segments were evenly spaced onto the surface of potato dextrose agar (PDA) media supplemented with 200 |ig /ml tetracycline [18].
Isolation of Fungal Endophytes
All the inoculations were carried out in laminar air flow cabinet. The laminar air flow was swabbed with cotton dabbed in rectified spirit and then irradiated with ultra violet light for 1520 minutes before use. The stainless steel instruments and other items such as forceps, scalpel, scissors, coupling jar etc. were autoclaved before use. Petri plates, flasks containing distilled water, were also autoclaved prior to use. After keeping all the required material for inoculation except the root segments in the laminar air flow cabinet, an ultra violet irradiation was given for 10 minutes. The surface sterilized roots were then inoculated into the culture vessel containing potato dextrose agar medium supplemented with 200 |ig /ml tetracycline [1-8].
Incubation
After inoculation, cultures were incubated in the culture room which was provided with one air conditioner and temperature controller to regulate temperature and humidity of culture room at 26 ± 2°C and 55±5% respectively. Fluorescent tubes and incandescent bulbs were fitted in culture shelves to render constant high intensity of 2000-3000 lux. A photo period of 12 hours light and 12 hours of darkness was regulated with the help of a timer [1-8].
Observation
Daily observations were recorded and sporulating mycelia around the root segments were subsequently transferred in the potato dextrose agar media for further study [1-8].
Identification of Fungal Endophytes
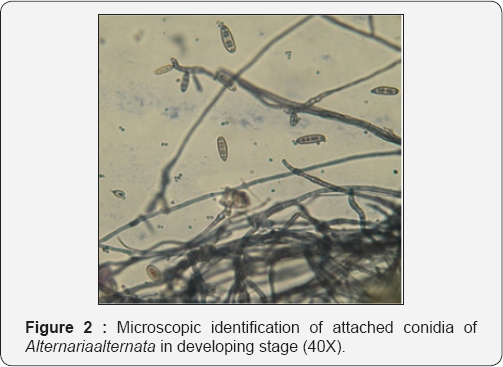
The identification of endophytic fungal strains was based on the morphology of the fungal culture colony or hyphae, the characteristics of the spores and reproductive structures if these features were discernible. Measurements of all fungal characters were made in water mounts, and the slides were subsequently mounted in lactophenol and sealed with nail vanish. All experiments and observations were repeated at least twice. Those cultures which failed to sporulate were named as mycelia sterilia, and divided into different morpho-species according to their cultural characteristics [1-8].
Preservation of Fungal Cultures
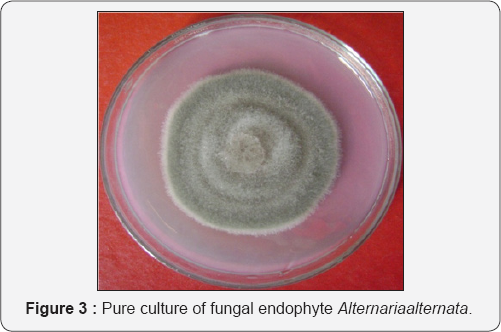
The fungi in pure culture were preserved on the slant at 40C with proper labelling; each tube was labelled with a code number of the host plant, batch number and full name of the fungi and date of storage. Several replica were made for each isolate and appropriate media was used according to the need of the fungi [1-8].
Results and Discussion
Conclusion
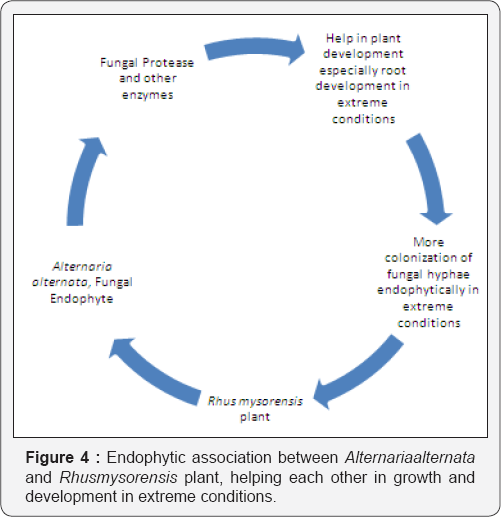
Various mechanisms for survival hired by the plants. These mechanisms include mycorrhiza, rhizosphere and plant growth promoter microorganisms. This research work confirms that endophytic fungal association of Alternariaalternata within roots of Rhusmysorensis plant; facilitate the growth and development in extreme conditions of Sanagner region of Rajasthan, India.
References
- Collado J, Platas G, Pelaez F (2001) Identification of an endophytic Nodulisporium sp. from Quercusilex in central Spain as the anamorph of Biscogniauxia mediterranea by rDNA sequence analysis and effect of different ecological factors on distribution of the fungus. Mycologia 93(5): 875-886.
- Frohlich J, Hyde KD, Petrini O (2000) Endophytic fungi associated with palms. Mycological Research 104(10): 1202-1212.
- Kumar DSS, Hyde KD (2004) Biodiversity and tissue-recurrence of endophytic fungi in Tripterygium wilfordii. Fungal Diversity 17: 69-90.?
- Photita W, Lumyong S, Lumyong P, Hyde KD (2001) Endophytic fungi of wild banana (Musa acuminata) at Doi Suthep Pui National Park, Thailand. Mycological Research 105: 1508-1513.
- Rodrigues KF (1994) The foliar fungal endophytes of the Amazonian palm Euterpeoleracea. Mycologia 86: 376-385.
- Schulz B, Wanke U, Draeger S, Aust HJ (1993) Endophytes from herbaceous plants and shrubs, effectiveness of surface sterilization methods. Mycological Research 97(12): 1447-1450.
- Taylor JE, Denman S, Crous PW (2001) Endophytes isolated from three species of Proteain a nature reserve in the Western Cape, South Africa. Sydowia 53: 247-260.
- Tiwari K, Chittora M (2013) Assessment of genetic diversity and distribution of endophytic fungal communities of Alternaria solani isolates associated with the dominant Karanja plants in Sanganer Region of Rajasthan. Springer plus 2(1): 300-313.






























