Characterization, Classification and Evaluation of Groundwater in and around the Open Cast Mining of Clay Deposits of Thonnakkal, South India
*Joji VS
Scientist- D, Central Ground Water Board, India
Submission: July 24, 2017; Published: August 09, 2017
*Corresponding author: Joji VS, Scientist- D, Central Ground Water Board, Kerala, Region, Kesavadaspuram, Thiruvananthapuram, Kerala, PIN- 695004, Kerala, India, & National Resource Person (Recognized Trainer) in Direct Trainer Skill (DTS), DoPT, Govt. of India, Tel: 09446361319; Email: jojivsdh@yahoo.com
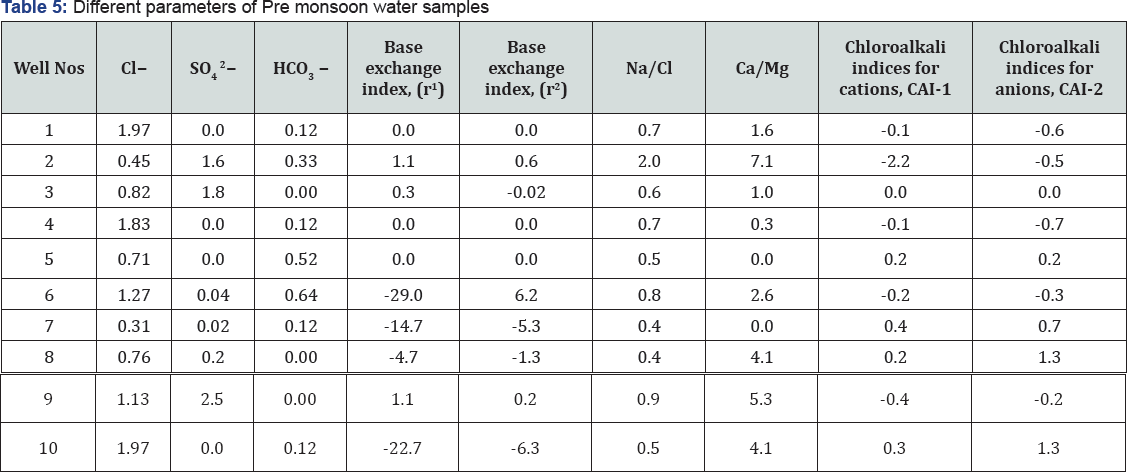
How to cite this article: Joji V.Characterization, Classification and Evaluation of Groundwater in and around the Open Cast Mining of Clay Deposits of Thonnakkal, South India. Int J Environ Sci Nat Res. 2017;4(1): 555630. DOI: 10.19080/IJESNR.2017.04.555630
Abstract
Characterization, classification, and evaluation of groundwater in and around the open cast mining of clay deposits of Thonnakkal, South India have been studied. The impact studied include changes happened to the qualitative and quantitative aspects related to the ground water. It is found that changes occurred in depth to the water level (decline of water level), lowering pH of ground water in the area, high sulfate and trace metals in the ground water. The groundwater of the area comes under Na+-HCO3- type and shallow meteoric percolation type and the scatter diagram of Ca2+ +Mg2+ vs HCO3 - + SO42- revealed that majority of samples in the season and different geological terrain fall below the equiline, indicating that silicate weathering was the primary process involved in the evolution of groundwater. The rock -water interaction played a major role in the evolution of water chemistry, which was partly by an evaporation process. The qualitative analysis of ground-water samples found that many samples are unfit for both domestic and irrigational purposes.
Keywords: Hydrochemistry; pH; Electrical conductivity; Suitability; Calcium concentration; Total hardness
Introduction
Population explosion coupled with industrialization over the past many years has increased a tremendous pressure on our natural resources resulting in rapid exploitation of our mineral resources. Local residents of the study area alleging that the mining continues to contaminate water resources and leads to an increase in the number of cancer patients in the locality. Detailed hydrogeological survey was carried out to evaluate the ground water scenario of the clay mining areas located in Azhoor, Mangalapuram and Andoorkonam Panchayaths of Thiruvananthapuram district to know the general ground water scenario of ground water reservoirs both quantitative and qualitative aspects viz. pre and post monsoon depth to water level and its fluctuation, flow direction, quality deterioration of sub-surface water, EIA of open cast clay mining on ground water conditions.
Mining hydrogeological studies were carried out by many authors viz. Stoertz et al. [1] on long-term water quality trends at a sealed, partially flooded underground mine, Alexander [2] on environmental impact of deep opencast limestone mine in Laegerdorf, Northern Germany, Suraj et al. [3] on GIS approaches for sustainable mining of tile and brick clay, Parappukara Panchayath, Thrissur district, Kerala, Anon [4] on environmental impact of clay mining for tile and brick industries in Thrissur District, Kerala using Remote Sensing and GIS and many others. The purpose of this investigation is to examine the various changes occurred in the hydrogeological ecosystem in and around Thonnakkal, Thiruvananthapuram District, South India due to of open cast mining of clay deposits.
Study Area
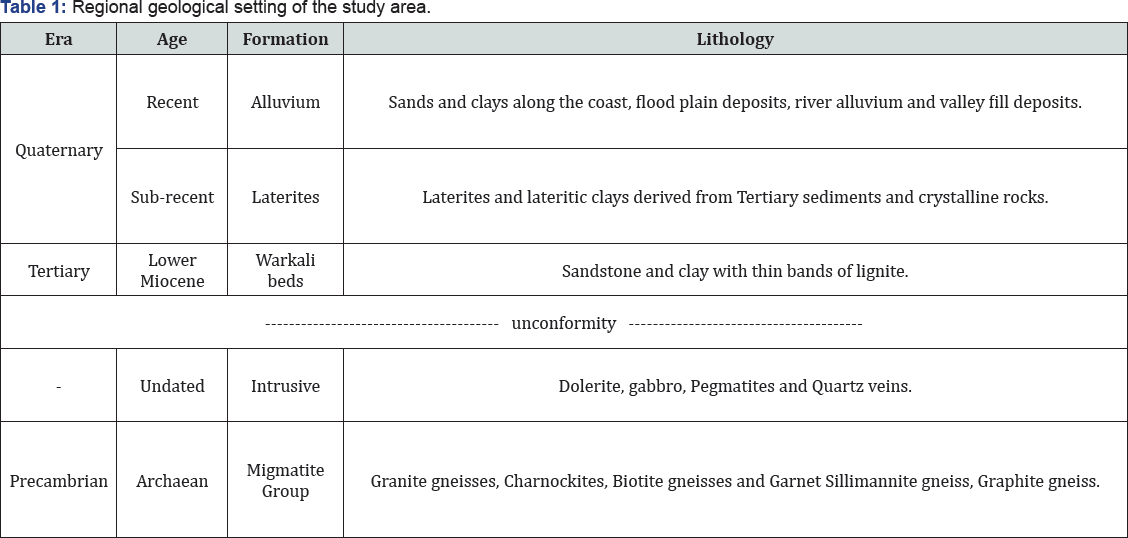
The Thonnakkal belt of the Kaolinised china clay is situated at Melthonnackal (8o38”05”:76°51’22”), Azhoor (8°38’31”:76°49’29”), Pallippuram (8°35’30”: 76° 51’30”), Chilampil (8°37’00”:76°50’00”) and Sasthavattam (8°38’45”:76°49’30”) areas. The English Indian Clays Ltd., Thiruvananthapuram operates a China clay mine since 1966 around Thiruvananthapuram, where the processing plant produces several grades of refined Kaolin, Metakaolin and Calcined Kaolin (Clays) for the paper, paint, rubber, plastic, fiberglass, cement and ultra-marine industries and the plant has a capacity of 190, 000 metric tons per annum and is the largest in South East Asia. The area lies between 76o 43' 21” and 77 o 12' North latitudes and 8 o 32' East and 8 o 53’34” longitudes and falls in parts of Survey of India Toposheet D/14 (Figure 1). Physiographically the study area falls mainly in the lowland (elevation between msl and 7.6 magl) and midland (elevation between 7.6 and 76 magl) areas of Trivandrum district of Kerala. The main drainages in the study area are the Vamanapuram River and its tributaries. The coastal alluvial soil, riverine alluvial soil and lateritic soil are the soils of the study area. The land use of the area includes open scrub, mixed crops, rubber plantations, paddy fields and water bodies. The study area is generally occupied by Precambrian crystalline rock overlain unconformably by a thick sequence of the Warkali Formation of Miocene age. The regional geological setting of the area is compiled (Table 1).
Materials and Methods
The Survey of India Topographical Map (Scale 1:50,000 of 1967-1968), Geo-coded imageries of Indian Remote Sensing Satellite (IRS -IC LISS III False Color Composite - FCC of 7.02.1997 of 1:50,000 scale) and Aerial photographs (of 1990 of 1:15,000 scale) are used for the preparation of base map of the study area. Major ground control points (GCPs) were identified and verified during field truth, which resulted in modifying the units and their boundaries. Map Info 6.5 has been used in GIS studies. The scanned maps were digitized and edited using Map Info 6.5. During editing the segment checking like intersection, self-overlap and dead-end corrections were carried out. Projection and polygonization of units followed the editing. After polygonization, annotations were given for different polygons and the maps were subjected for further analysis.
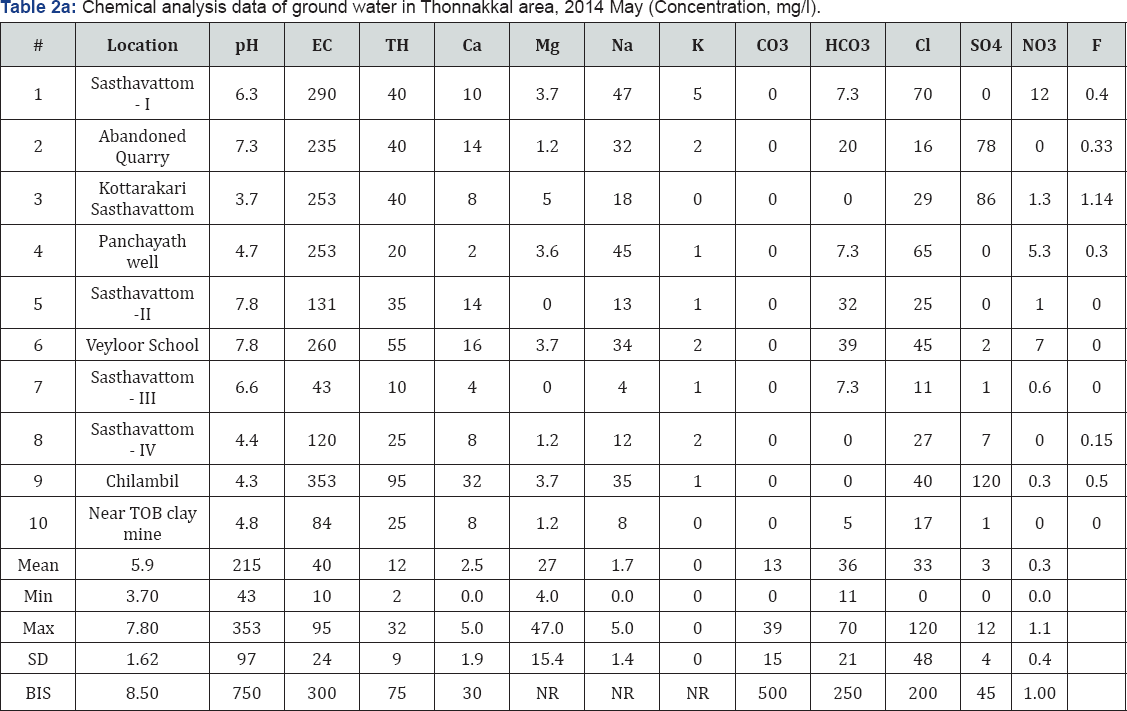
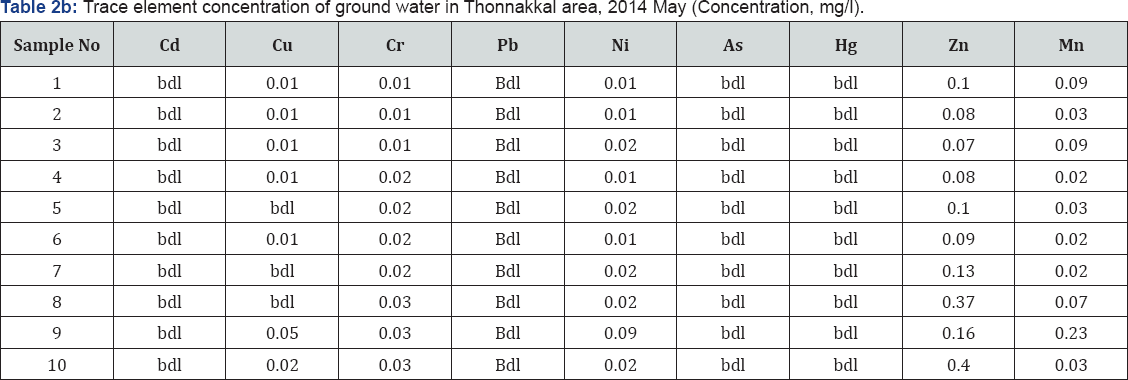
There were 10 groundwater samples collected from open dug wells. The pre monsoon samples (First week of May, 2014) were collected in polyethylene bottles in 2014 and analysed for pH, EC, F-, Cl-, NO3-, HCO3-, SO42-, Ca2+, Mg2+, Na+, and K+ as per standard procedures (APHA, 1995) and the in situ measurement of electrical conductivity and pH carried out by using EC and pH meters. The total dissolved solids were estimated by ionic calculation methods. The F-, Cl- and NO3- ions were determined by ion selective electrode; HCO3- by potentiometric titration; SO42- by modified titration method after Fritz and Yamamura (1955) and Haartz et al. [5] Ca2+ and Mg2+ in absorption mode while Na+ and K+ in emission mode of the atomic absorption spectrophotometer. Chemical standards and blanks were run and replicate analysis of each sample was done for each parameter and variations were ±5 - 10%. The analytical results are shown as Tables 2a & 2b.
Results and Discussion
Aquifer Systems
Groundwater occurs in all the geological formations ranging in age from Archean to the Recent and water bearing formations in the area are crystalline formations, Laterite, Tertiaries and Alluvium. The crystalline formations consist of Khondalites and Gneisses. The water bearing properties of crystalline formations which lack primary porosity depend on the extent of development of secondary inter granular porosity either through weathering or fracturing. Groundwater generally occurs under phreatic conditions in the weathered mantle and under semi confined conditions in the fissured and fractured zones at deeper levels. The thickness of weathered zone ranges less than 1m to more than 15m and the depth to the water level from 1015 mbgl.
Major portion of the study area is covered by laterites, the thickness of which increases from East to West. This includes the Tertiary laterites and the laterite occurring as highly weathered product of crystallines capping it. In crystalline formations the water bearing properties depend on the extent of development of secondary porosity either through weathering or fracturing. These aquifers are highly heterogeneous in nature due to variation in lithology and texture even within short distance. The in situ laterite development by the weathering of Khondalites in the study area is rich in clay content due to higher amount of feldspathic minerals in the parent rock. These aquifers mainly occur along the valleys and topographic lows and possess high porosity. The depth of the well in laterite ranges from 8 to 27 mbgl and depth to the water level is more than 10 m. By the onset of monsoon, the water level becomes shallow due to the porous nature of the aquifer. The thickness of weathered zones ranges from less than 1 m to more than 10 m. The yield of the well ranges from 0.5 to 6 m3/day and the recovery co-efficient ranges from 0.00096 to 0.025/min.
Groundwater occurs under phreatic condition in the shallow zone in Tertiary aquifers. Of the three tertiary formations (Warkali, Quilon and Vaikom), the Warkali beds are encountered in this area. Thick deposits of kaolin having an overall thickness of 25 to 45 m are encountered at Melthonnakkal and Pallipuram forming part of the Warkali Formation belonging to the Tertiary sequence in southern Kerala. The sedimentary clays are composed mainly of kaolinite, quartz and gibbsite. These sedimentary kaolins (hydrated aluminous silicate, Al2O3. SiO2.2H2O) are considered to have been formed by intense tropical weathering of the khondalites, and subsequently transported and deposited with high organic input into lakes near the weathering crust over the basement rock. Besides, the surficial parts of the sedimentary deposits are extensively lateralised with the formation of Goethite and Hematite by tropical weathering processes.
The thickness of the clay bed varies from 25 to 45 mbgl and is encountered in almost all the bore wells drilled in the area. The aquifer properties of clay include Grain size (< 0.002), Porosity (33-60), Specific Yield (2 to 5%) and Permeability (10-9 to 10-6 K cm/s). These properties of clay suggest that it is a poor aquifer and by large an aquitard with very low groundwater potential. The field survey in the area reveals that groundwater occurs under phreatic conditions in these Tertiary sediments. The premonsoon depth to water level ranges from 3.1 mbgl to 27.75 m bgl.
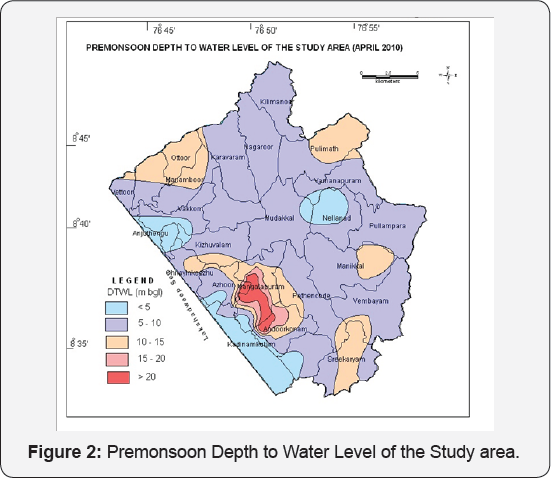
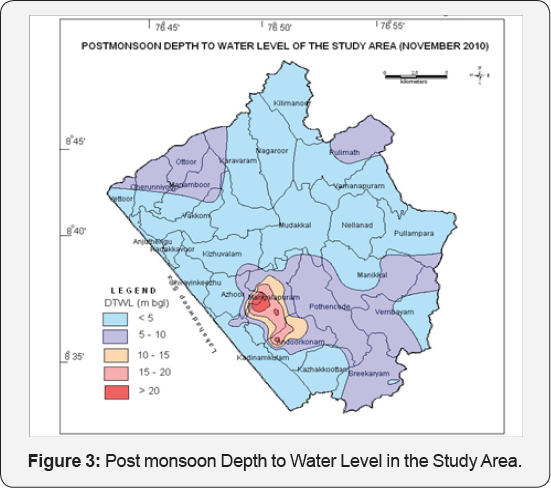
Maps depicting the depth to water level in the study area during April 2010 (Premonsoon) and November 2010 (Post monsoon) based on the water level data of the key wells established and the observation wells of CGWB are shown in Figures 2 & 3 respectively. During April 2010, the depth to water levels in wells of the study area ranged from 3.1 mbgl to 27.75 mbgl and the depth of well ranges from 6.0 to 29 mbgl. The deepest water levels were observed in Mangalapuram, Sasthanagar, Chilambil, Sasthavattom, Pallipuram, Karamoode areas. During November 2010, the depth to water levels in wells of the study area ranged from 3.04 to 25.9 mbgl. The deepest water levels were observed in Mangalapuram, Sasthanagar, Chilambil, Sasthavattom, Pallipuram, Karamoode areas. The yield of wells ranges from 0.5m3/day to 10m3/day and the recovery co-efficient ranges from .00056 to 0.0087/min.
Alluvium is the most potential phreatic aquifer in the study area and is extensively developed by dug wells and filter point wells. The depth to water level in this formation ranges from 1.89 to 3.04 m. The depth of wells ranges from 3.66 to 7.55 mbgl. The yield of the shallow dug wells ranges from 15 to 50 m3/day and the recovery co-efficient varies between 0.0093 and 0.094/ min.
Water Table Elevation
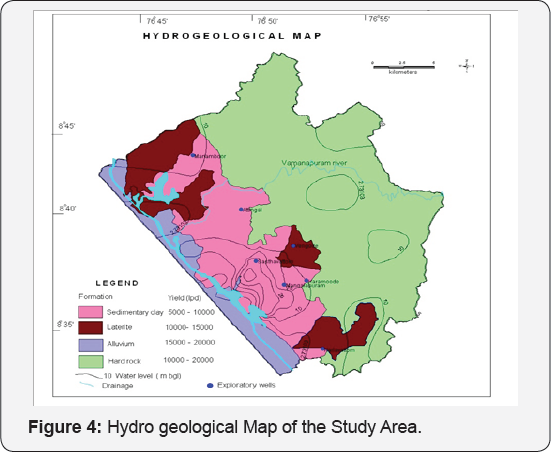
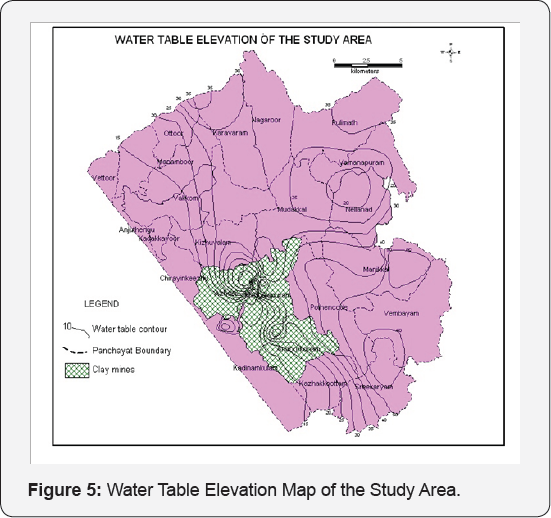
The elevation of ground water table during the period ranged from 7.75 above msl to 63.44 above msl. The flow lines from east to west indicate that the general hydrogeological regime is undisturbed except around the clay mines. A ground water trough has been formed around the clay mines indicating a reversal of hydraulic gradient. The deeper water level in the vicinity of the clay mines is the localized effect of the mines. The general hydraulic gradient is towards west in general. The closely spaced contours around the clay mines is indicative of a potential recharge zone underlying clay formation which is an aquitard inhibits any sort of recharge except for the construction of injection wells to penetrate the weathered zone encountered at a depth below 40 metres. The hydrogeological map and water table elevation contour maps of the area have been prepared (Figures 4 & 5).
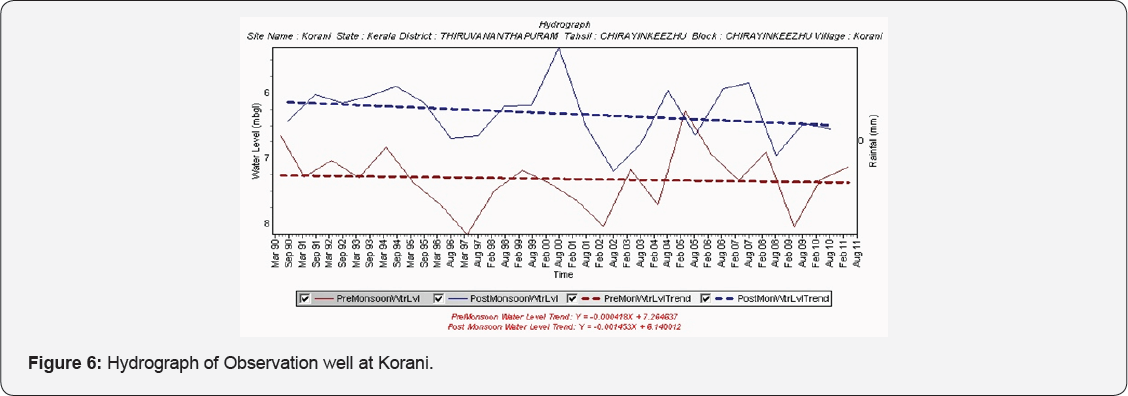
Analysis of Hydrograph
An analysis of depth to the water level has been carried out to understand the influence of lithology on water level. The hydrograph of the well at Korani tapping the Laterites show a decline trend in the premonsoon and post monsoon periods (Figure 6).
Hydrochemistry and Hydro Geochemical processes of Ground Water
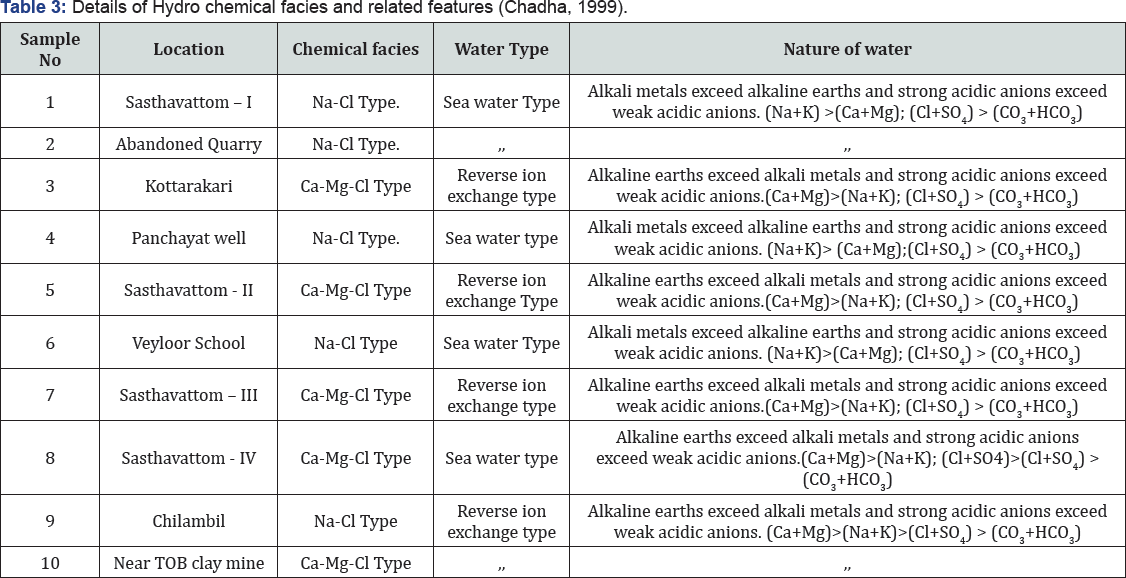
The groundwater samples of different geological terrains can be classified on the basis of ionic strength of Cl- , SO42-, and HCO3- and the water is Normal Chloride type if Cl- is <15 meq/l, Normal Sulphate type if SO4 2- is <6 meq/l and Normal Bicarbonate type if HCO3- varies between 2 and 7 meq/l. The ground water samples of the study area are of Normal Chloride type and Normal Sulphate type and concentration of salts in natural waters depends on the geology, environment, and movement of water Raghunath [6], Gopinath Seralathan [7](Tables 3 & 4).
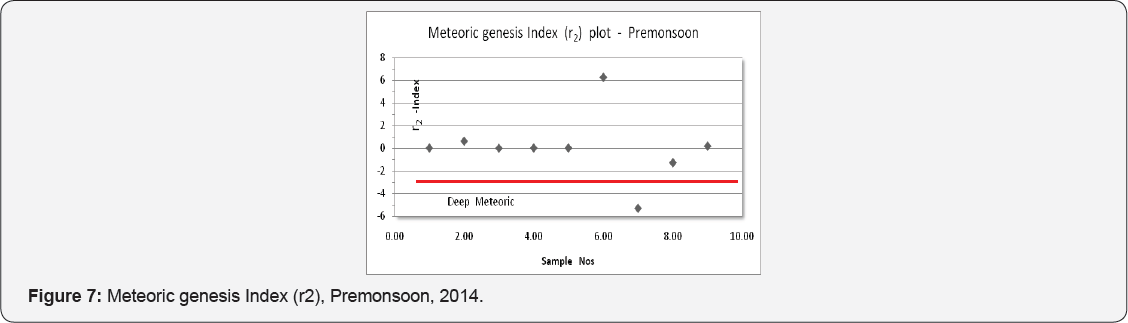
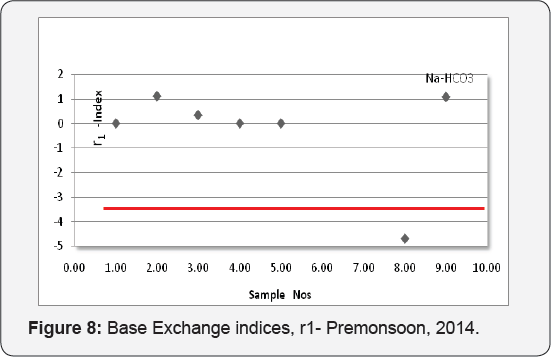
The Base Exchange indices, r1 (r1 = Na+ - Cl-/ SO42- meq/ l) and meteoric genesis index (r2) (r2 = K+ + Na+ - Cl- SO42-, meq/l) can be used for classifying groundwater Soltan [8]. The groundwater can be grouped as Na+ - HCO3- type if r1 > 1 and Na+- SO4 - type with r1 < 1; r2 < 1- groundwater is of deep meteoric percolation type and >1, shallow meteoric percolation type. The groundwater samples of the Thonnakkal area comes under Na+-HCO3- type and shallow meteoric percolation type except one which is deep meteoric percolation type and the details are plotted (Figures 7 & 8).
Red -Good correlation (r=> 0.6)
Yellow - Poor correlation (r = x003;0)
Hydro geochemical Evaluation of ground water
The Na+ / Cl- molar ratio will be 1 if halite dissolution is responsible for sodium dominance in groundwater and >1 if Na+ is released from silicate weathering process Meybeck [8,9]. As the Na+ / Cl- molar ratio is <1 in all samples of the season except at sample location No.2 (Abandoned Quarry), reveals that halite dissolution was the primary process responsible for the release of Na+ into the groundwater.
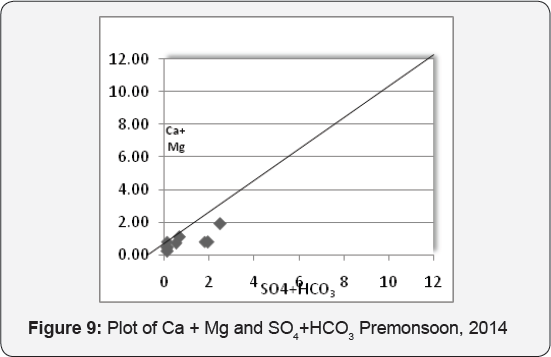
The study of Ca2+ / Mg2+ ratio reveals Ca2+ - Mg2+ contribution in the groundwater. The Ca2+ / Mg2+ ratio of 1 indicates dissolution of dolomite and of >2 an effect of silicate minerals May & Loucks [10]. Majority of the samples with Ca2+ / Mg2+ ratio between 1 and <2, indicating dolomite dissolution responsible for Ca2+ - Mg2+ contribution. The scatter diagram of Ca2+ + Mg2+ vs. HCO3- + SO42- (Figure 9) shows that majority of samples fall below the equiline, indicating that silicate weathering played primary role in the evolution of groundwater Datta & Tyagi [11]. If bicarbonate and sulphate are dominating than calcium and magnesium, it reflects silicate weathering was dominating and, therefore, was responsible for the increase in the concentration of HCO3 - in groundwater, Elango et al. [12], Elango & Kannan [13].
Evolution of Groundwater and Hydro Chemical Facies
(# Concentration, meq/l)
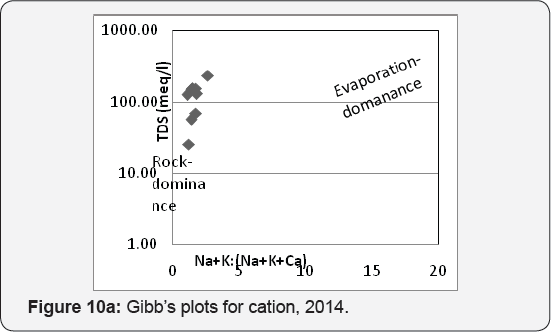
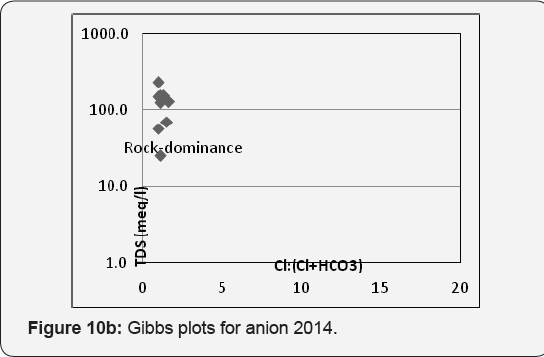
The evolution process of the groundwater tested by plotting TDS vs. Na+ /(Na+ +Ca2+ ) for cations and TDS vs. Cl- /(Cl- + HCO3- ) for anion Gibbs [14] and revealed that rock - water interaction played significant role in the hydrochemistry, which was partly influenced by evaporation process (Figures10a & 10b). It is also to be noted that geological location is another factor controlling quality variation Beck et al. [15]. The evolution of groundwater chemical composition further tested by calculating chloroalkali indices for cations (CAI-1- Cl- - (Na+ + K+) Cl-) and anions (CAI-2 - Cl- - (Na+ + K+)/(SO42- + HCO3- + CO3 - + NO-) after Schoeller [16]. The chloroalkali indices are negative indicating the predominance of ion exchange between Na+ -K+ in water and Ca2+ -Mg2+ in rocks McIntosh & Walter [17]. A close perusal of hydro chemical facies and water type study Chadha (1999) is compiled (Table 5).
Statistical analysis - correlation coefficient
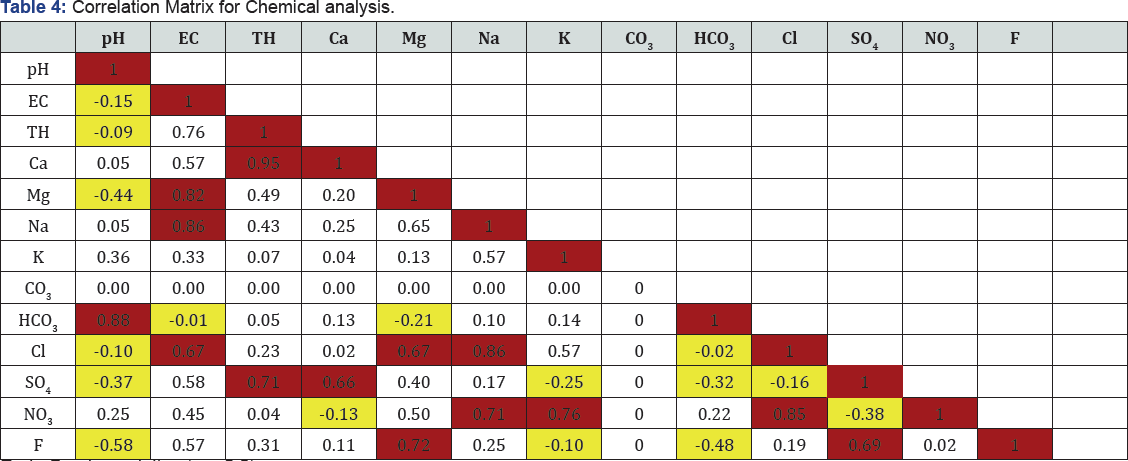
The relationship between two variables is the correlation coefficient which shows how one variable predicts the other. Associated with correlation coefficient r, the multiple correlations, which are the percentages of variance in the dependent variable, explained collectively by all of the independent variables. A high correlation coefficient (near 1 or -1) means a good relationship between two variables, and a correlation coefficient around zero means no relationship. Positive values of r indicate a positive relationship while negative values indicate an inverse relationship and are compiled (Table 6). The correlation coefficient is a measure of the extent to which two measurement variables vary together. Unlike the covariance, the correlation coefficient is scaled so that its value is independent of the units in which the two measurement variables are expressed. The value of any correlation coefficient must be between -1 and +1 inclusive. The parameters having good and poor correlations have been attempted.
Suitability of ground water for Irrigation purposes
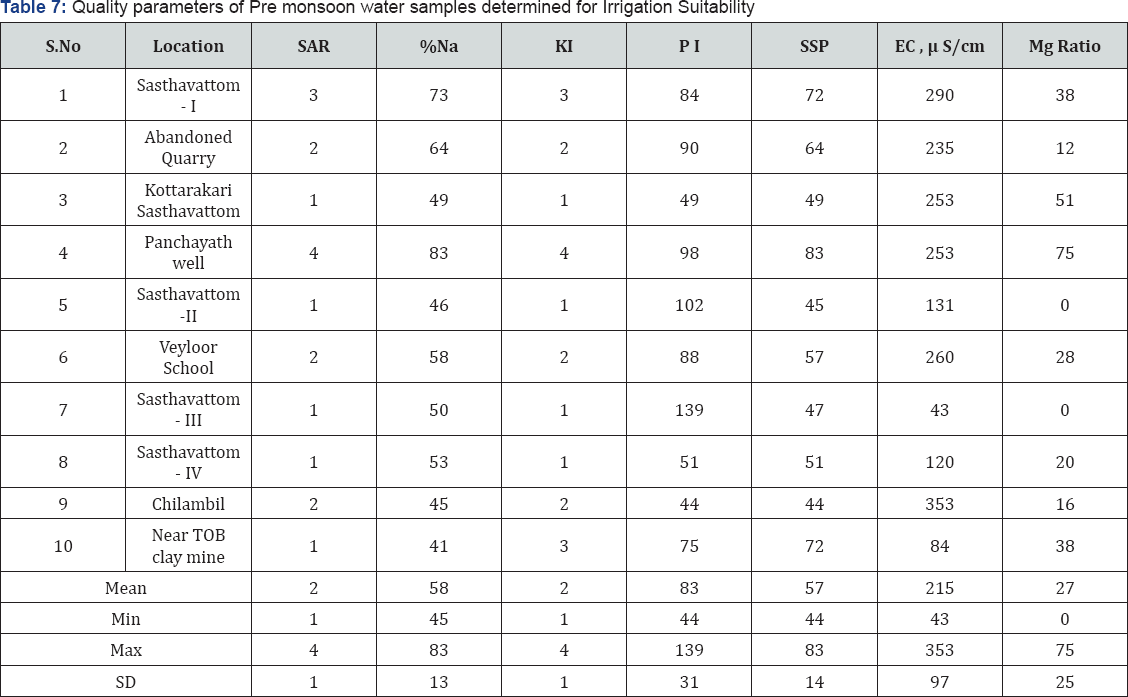
The irrigation suitability of ground water has been tested by using various methods (Table 5), methodology and analytical results are compiled (Tables 6 & 7). The electrical conductivity (EC) is a measure of salinity hazard to crops and there are of five major types for irrigational purposes (Raghunath (1987) and the samples in the area varies from excellent (50%) to good categories. The Sodium Absorption Ratio, SAR is an indicator of sodium hazard in irrigation water Gholami & Srikantaswamy [18] and all the water samples are excellent category Richard [19]. The per cent sodium (% Na) in irrigation water may increase the exchange of sodium content and affect soil permeability, structure and create toxic condition for plants Bangar et al. [20], Durfer & Backer [21] and Todd [22]. The % Na study revealed that samples come under permissible (70%), doubtful (20%) and other unsuitable categories and cannot be used for irrigation on almost all types of soil. On the basis of permeability index, PI Doneen [23] irrigation water quality classes include class I, II and III and all the samples come under Class II and suitable for irrigation in all types of soil.
The water is suitable for irrigation if Kelley's Index, KI value is <1; water with KI value of >1 is considered as of poor quality for irrigation and >2 KI makes the water unsuitable for irrigation Kelley [24]. In the study area about 70% KI values are above 1, and the water is poor quality for irrigation and 30% unsuitable for irrigation in all types of soil. Water with less than or equal to 50 Soluble Sodium Percentage, SSP value is of good quality and more than 50 is not suitable for irrigation as permeability will be very low. In the study area 60% of the water samples with SSP values more than 50. As per Lloyd and Heathcoat [25] water with less than or equal to 50 Magnesium ratio, MR value is of good quality and >50 is considered unsuitable for irrigation and except two water samples others with MR value more than 50 and are unsuitable for irrigation.
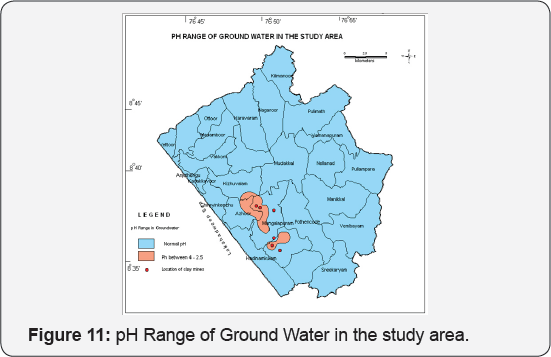
Variations in pH, Sulphate and Heavy Metal concentrations
The open cast mining in the area resulted rapid circulation of oxygen and water into the deep crust, where high concentrations of sulphides (Pyrite) exist. Thus, sulphides undergo the reaction.
2FeS2 + 2H2O + 7O2 = 2Fe2+ + 4SO42- + 4H+
This reaction developed low pH, high sulphate and trace metal concentrations. The chemical analysis of the ground water samples collected in the study area show low pH side indicating a mine environment similar to the above mentioned category [26-29]. The Pyrite present in the deep clay horizon gets exposed resulting in its oxidation and the end products being sulphate and hydrogen ions. The pyrite is present in the depth range of 35 to 40 mbgl. Hence ground water from deep wells puncturing this horizon generally has low pH value. The chemical data shows that the ground water collected from Mangalapuram, Chilambil, Sasthanagar and Valikonam have low pH [30-33]. It was also observed that the depth of these wells were also very deep (> 25 mbgl). The pH contours are depicted (Figure 11).
The general chemical reactions involved are
2FeS2 + 2H2O + 7O2 = 2Fe2+ + 4SO42- + 4H+
4Fe2+ + O2+ 4H+= 4Fe3+ + 2H2O
4Fe3+ + 12H2O = 4Fe (OH)3 + 12H+
FeS2 + 14Fe3+ + 8H2O = 15Fe2+ + 2SO42- + 16H+
The reactions indicate release of H+ which lowers the pH of the ground water of the area and produces sulphate ions. The field truth verification in the open cast mining areas of clay mining revealed the marked changes in the hydrogeological, agricultural and socio-economic environments of the area. The various impact include decline of depth to the water table in wells or changes in hydro geologic conditions in areas adjacent to the mining sites, thixotropism resulted land subsidence, sliding / slumping of walls of mines and well collapse, reversal of hydraulic gradient, inducing increased flow of ground water from the aquifer into the mines, causing deepening of water levels, drying up of shallow wells and reduction in the sustainability of ground water abstraction structures. Water quality deterioration resulted in the lowering of pH values and many areas water unsuitable for cultivation, reclamation and conversion of these wetlands to non-agricultural purposes has resulted transformation of this ecosystem, changes in landscape, land stability and soil loss especially areas of Mangalapuram, Andoorkonam, Azhoor and Sasthavattom and atmospheric pollution in the area.
Conclusion
Characterization, classification and evaluation of groundwater in and around the Open cast mining of clay deposits of Thonnakkal, South India have been examined. The general hydrogeological scenario has been identified during the study The opencast mining of clay deposits resulted lowering of depth to the water level, topographic changes and general changes in biodiversity. The groundwater of the area comes under Na+- HCO3- type and shallow meteoric percolation type except a few one which is deep meteoric percolation type. The rock water interaction played major role in the evolution of water chemistry, which was partly by evaporation process. The mining resulted low pH, high sulphate and trace metal concentrations in the ground water in and around the Thonnakkal clay belt. It is seen that the water quality is degrading very fast in the study area.
Acknowledgment
The author is grateful to Sh. V Kunhabu, Regional Director, Kerala Region, Central Ground Water Board, Govt. of India, Thiruvananthapuram for all the encouragement given during the course of the work. Thanks are also due to Kumari Himaganga Joji, daughter of the author for the data entry and editing. Thanks are also due to Smt. Mini Chandran and Sh. Rajan, Hydrogeologists in CGWB, Thiruvananthapuram and GWD, Thiruvananthapuram for providing some data input.
References
- Stoertz MW, Hughes ML, Wanner NS, Farley ME (2001) Long-term water quality trends at a sealed, partially flooded underground mine, Environmental and Engineering Geoscience 7(1): 51-65.
- Alexander I (2006) Environmental impact of deep opencast limestone mine in Laegerdorf, Northern Germany. Mine Water and the Environment 17(1): 52-61.
- Suraj R, Suresh F, Sumesh EV, Saritha VK, Manu V (2009) GIS Approaches for sustainable mining of tile and brick clay, Parappukara Panchayath, Thrissur district, Kerala. National seminar on Exploration Geology and Geoinformatics, Macmillan Advanced research Series, India, p. 87-97.
- Anon (2007) Report on environmental impact of clay mining for tile and brick industries in Thrissur District, Kerala using Remote Sensing and GIS. Kerala State Remote Sensing and Environment Centre (KSREC), India, pp. 103.
- Haartz JC, Eller PM, Hornung RW (1979) Critical parameters in barium per chlorate, Thorin Titration of Sulphate. Anal Chem 51(13): 22932295.
- Raghunath M (1982) Groundwater. New Delhi, India, pp. 456.
- Gopinath G, & Seralathan P (2006) Chemistry of ground water in the laterite formation of Muvatturpuzha river basin, Kerala. Journal of the Geological Society of India 68: 705-714.
- Soltan ME (1999) Evaluation of groundwater quality in Dakhla Oasis (Egyptian Western Desert), Environmental Monitoring and Assessment 57(2): 157-168.
- Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved leads, American Journal of Science287: 401-428.
- May A, Loucks MD (1995) Solute and isotope geochemistry and groundwater flow in the Central Wasatch Range, Utah. Journal of Hydrology 170: 795-840.
- Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: Chemical weathering processes and groundwater flow regime. Journal of Geological Society of India 47: 179-188.
- Elango L, Kannan R, Senthil Kumar M (2003) Major ion chemistry and identification of Hydro geochemical processes of groundwater in a part of Kancheepuram district, Tamil Nadu, Environmental Geosciences 1(4): 157-166.
- Elango L, Kannan R (2007) Chap 11 Rock-water interaction and its control on chemical Composition of Groundwater. Developments in Environmental Science, pp. 229-243.
- Gibbs RJ (1970) Mechanisms controlling World's Water chemistry. Science 170(3962): 1088-1090.
- Beck BF, Asmussen L, Leonard RS (1985) Relationship of geology physiography, agricultural land use and groundwater quality in southwest Georgia. Groundwater GRWAAP 23(5): 627-634.
- Schoeller H (1967) Qualitative evaluation of groundwater resources, In Methods and Techniques of Groundwater investigation and development, Water Research, Series 33, UNESCO, India, p. 44-52.
- McIntosh JC, Walter LM (2006) Palaeo water in Silurian-Devonian carbonate aquifers: Geochemical evolution of groundwater in the Great Lakes region since Late Pleistocene. Geochimica Cosmochimica Acta 70: 2454-2479.
- Gholami S, Srikantaswamy S (2009) Analysis of agricultural impact on the Cauvery River water around KRS dam. World Appl Sci J 6(8): 11571169.
- Richards LA (1954) Diagnosis and improvement of saline and alkali soils, US Laboratory Staff, US Department of Agriculture, Agricultural Handbook 60, Washington DC, USA.
- Bangar KS, Tiwari SC, Vermaandu SK, Khandkar UR (2008) Quality of groundwater used for irrigation in Ujjain district of Madhya Pradesh, India, Journal of Environmental Science Engineering. 50(3): 179-186.
- Durfer CM, Backer E (1964) Public water supplies of the three largest cities in the U.S, USGS Water supply paper No. 1812, USA, pp. 364.
- Todd DK (2005) Groundwater Hydrology, John Wiley, New York, USA, pp. 636.
- Doneen LD (1964) Notes on water quality in agriculture, Published as a water sciences and Engineering, Department of Water Sciences and Engineering, paper 4001, University of California, USA.
- Kelley WP (1940) Permissible composition and concentration of irrigation water, In Proceedings of the American Society of Civil Engineering 66: 607-613.
- Lloyd JW, Heathcote JA (1985) Natural inorganic hydrochemistry in relation to groundwater. An Introduction. Clarendon Press, Oxford, England, UK.
- American Public Health Association (1995) Standard methods for the examination of water and waste water, (19th edn), Washington DC, USA.
- Fritz JS, Yamamura SS (1955) Rapid micro titration of sulphate. Anal Chem 27(9): 1461-1464.
- Gosselin CD, Edwin HF, Flowerday C (2003) The complex Dakota aquifer: Managing groundwater in Nebraska, USA.
- Johnson RA, Wichern D (2001) Applied Multivariate Statistical Analysis, Prentice Hall of India, New Delhi, India.
- Sastry JCV (1994) Groundwater chemical quality in river basins, hydrogeochemical facies and hydrogeochemical modeling, in Lecture notes-refresher course conducted by School of Earth Sciences, Tamil Nadu, Bharathidasan University, Thiruchirapall, India.
- Soltan ME (1998) Characterization, classification and evaluation of some groundwater samples in Upper Egypt. Chemos 37(4): 735-747.
- UNESCO (1991) Hydrology and Water Resources of Small Island- A Practical Guide, Studies and Reports on Hydrology. No.49, UNESCO, Paris.
- Venkateswara Rao (1998) Sustainability measures for water resources in the context of Indian village system. Journal of Indian Water Resources Society 18(4): 42-44.






























