Plant Microbiomes and Its Beneficial Multifunctional Plant Growth Promoting Attributes
Ajar Nath Yadav1*, Priyanka Verma2, Divjot Kour1, Kusam Lata Rana1, Vinod Kumar1, Bhanumati Singh3, Vinay Singh Chauahan3, TCK Sugitha4, Anil Kumar Saxena5 and Harcharan Singh Dhaliwal1
1Department of Biotechnology, Akal College of Agriculture, Eternal University, India
3Department of Biotechnology, Bundelkhand University, India
4Department of Agricultural Microbiology, Tamil Nadu Agricultural University, India
5ICAR-National Bureau of Agriculturally Important Microorganisms, India
Submission: June10, 2017; Published: June 26, 2017
*Corresponding author: Ajar Nath Yadav, Department of Biotechnology, Akal College of Agriculture, Eternal University, Sirmour, India, Tel no: +91-9882545085; Email: ajarbiotech@gmail.com
How to cite this article: Ajar N Y, Priyanka V, Divjot K, Kusam L R et . al. Plant Microbiomes and Its Beneficial Multifunctional Plant Growth Promoting Attributes. 05 Int J Environ Sci Nat Res. 2017;3(1): 555601. DOI: 10.19080/IJESNR.2017.03.555601
Abstract
Plant microbiome (Epiphytic, endophytic and rhizospheric) plays important role in plant growth, development, and soil health. Plant and rhizospheric soil are valuable natural resource harbouring hotspots of microbes, and it plays critical roles in the maintenance of global nutrient balance and ecosystem function. The diverse group of microbes is key components of soil-plant systems, where they are engaged in an intense network of interactions in the rhizosphere/phyllospheric/endophytic. The microbes with plant growth promoting (PGP) attributes have emerged as an important and promising tool for sustainable agriculture. PGP microbes promote plant growth and development directly or indirectly, either by releasing plant growth regulators/phytohormones; solubilization of phosphorus, potassium and zinc; biological nitrogen fixation or by producing siderophore, ammonia, HCN and other secondary metabolites which are antagonistic against pathogenic microbes. The PGP microbes belonged to different phylum of archaea (Euryarchaeota); bacteria (Acidobacteria, Actinobacteria, Bacteroidetes, Deinococcus-Thermus, Firmicutes and Proteobacteria) and fungi (Ascomycota and Basidiomycota), which include different genera namely Achromobacter, Acinetobacter, Agrobacterium, Alcaligenes, Arthrobacter, Aspergillus, Azoarcus, Azospirillum, Azotobacter, Bacillus, Beijerinckia, Brevibacterium, Burkholderia, Collimonas,Curtobacterium, Diplococcus, Enterobacter, Erwinia, Flavobacterium, Flexibacterium, Gluconoacetobacter, Haloarcula, Halobacterium, Halococcus, Haloferax, Herbaspirillum, Klebsiella, Methylobacterium, Microbiospora, Micrococcus, Micromomospora, Nocardioides, PaeniBacillus, Pantoea, Penicillium, Piriformospora, Planomonospora, Pseudomonas, Rhizobium, Serratia, Streptomyces, Thermomonospora and Xanthomonas.These PGP microbes could be used as biofertilizers/bioinoculants at place of chemical fertilizers for sustainable agriculture.
Keywords: Biodiversity; Endophytic; Epiphytic; Microbiome; Plant Growth Promotion; Rhizospheric; Sustainable Agriculture
Introduction
Plant-microbes interaction is a key for plant growth, development and soil health. An understanding of plant microbiome and their beneficial attributes could have multiple benefits towards sustainable agriculture. Recently, a great emphasis is given on decoding of microbial diversity associated with plants from diverse habitats. Microbial diversity is considered important for maintaining for the sustainability of agriculture production systems. In the 90s, the interaction of microbes with plants was simply thought of as being an effect, but today it is recognized as a process with a high level of complexity in which at least different type of microbes share information without sharing the same spaces from a cellular perspective. In general, there are three kinds of plant-microbes interactions are considered i.e. epiphytic, endophytic and rhizospheric.
The rhizosphere is the zone of soil influenced by roots through the release of substrates that affect microbial activity. It is characterized by greater microbiological activity depending on the distance away from plant roots and constitutes a system especially suitable for obtaining culturable beneficial microbes. The rhizospheric microbes have the ability to attach to the root surfaces allowing these to derive maximum benefit from root exudates. Several factors such as soil type, its moisture, pH and temperature and, age and conditions of plants are known to influence the types of rhizospheric microbes. A number of microbial species belonging to different genera Acinetobacter, Alcaligenes, Arthrobacter, Aspergillus, Azospirillum, Bacillus, Burkholderia, Enterobacter, Erwinia, Flavobacterium, Haloarcula, Halobacterium, Halococcus, Haloferax, Methylobacterium, PaeniBacillus, Penicillium, Piriformospora, Pseudomonas, Rhizobium and Serratia were revealed from rhizosphere of different crop plants [1-8].
The phyllosphere is a common niche for synergism between microbes and plant. The leaf surface has been termed as Phyllosphere and zone of leaves inhabited by microorganisms as phyllo sphere. The plant part, especially leaves are exposed to dust and air currents resulting in the establishments of typical flora on their surface aided by the cuticles, waxes and appendages, which help in the anchorage of microorganisms. The phyllospheric microbes may survive or proliferates on leaves depending on extent of influences of material in leaf diffuseness or exudates. The leaf diffuseness contains the principal nutrients factors (amino acids, glucose, fructose and sucrose), and such specialized habitats may provide niche for nitrogen fixation and secretions of substances capable of promoting the growth of plants. The phyllospheric microbes may performs an effective function in controlling the air borne pathogens inciting plant disease. Microbes on leaf surface are said to be extremophiles as they can tolerate low/high temperature (5-55°C) and UV radiation. Many microbes such as Achromobacter, Agrobacterium, Azotobacter, Bacillus, Beijerinckia, Brevibacterium, Burkholderia, Diplococcus, Flexibacterium, Methylobacterium, Microbiospora, Micrococcus, Micromomospora, Nocardioides, Pantoea, Penicillium, Planomonospora, Pseudomonas, Rhizobium, Streptomyces, Thermomonospora and Xanthomonas have been reported in the phyllosphere of different crop plants [9-15].
The endophytic microbes are referred to those microorganisms, which colonizes in the interior of the plant parts, viz: root, stem or seeds without causing any harmful effect on host plant. The word endophyte means 'in the plant' and is derived of the Greek words end on (within) and python (plant). Endophytic microbes enter in host plants mainly through wounds, naturally occurring as a result of plant growth or through root hairs and at epidermal conjunctions. Endophytes may be transmitted either vertically (directly from parent to offspring) or horizontally (among individuals). A given endophytic microbiome can be modified by factors such as the physicochemical structure of the soil, plant growth phase and plant physiological state, as well as by diverse environmental factors [16,17].
The main colonization route used by endophytes seems to be the rhizosphere. Microbes reach the rhizosphere by chemotaxis towards root exudates components followed by attachment. The lipopolysaccharide and exopolysaccharide are bacterial components shown to play roles in attachment of endophytes to plant tissue. The preferred site of attachment and subsequent entry is the apical root zone with a thin-walled surface root layer, such as the cell elongation zone and the root hair zone with small cracks caused by the emergence of lateral roots. Root regions such as the differentiation zone and intercellular spaces in the epidermis have been suggested to be preferential sites for microbial colonization as well. Root cracks, wounds caused, for instance, by arthropods or nematodes, and emergence sites of lateral roots are generally considered as the main 'doors' for microbial penetration. Bacterial traits putatively involved in endophytic colonization of plant roots. For penetration, the bacteria have to produce cellulolytic enzymes required to hydrolyse the exothermal walls, such as endoglucanases and endopolygalacturonidases [18]. These enzymes also seem to be important for spreading through the intercellular space of the root cortex and beyond. Endophytes usually do not enter plant cells. Only a few of them can penetrate the endow dermal barrier and invade the xylem vessels. Endophytic microbes live in plant tissues without causing substantive harm to the host. Endophytic microbes exist within the living tissues of most plant species in form of symbiotic to slightly pathogenic. A large number of endophytic microbial species Achromobacter, Azoarcus, Burkholderia, Collimonas, Curtobacterium, Enterobacter, Flavobacterium, Gluconoacetobacter, Herbaspirillum, Klebsiella, Microbiospora, Micromomospora,Nocardioides, Pantoea, Planomonospora, Pseudomonas, Serratia, Streptomyces and Thermomonospora have been identified from different host plants [6,8,10,15,18-21].
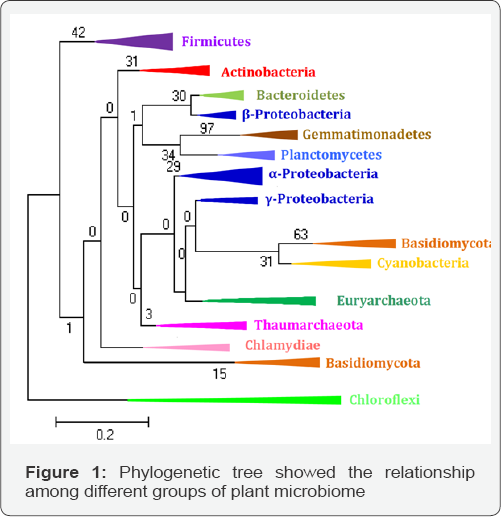
The study on microbial biodiversity of plant associated microbes revealed representative microbes from archaea (Euryarchaeota); bacteria (Acidobacteria, Actinobacteria, Bacteroidetes, Deinococcus-Thermus, Firmicutes and Proteobacteria) and fungi (Ascomycota and Basidiomycota). Literature review suggested that the distribution of microbes although varied in all bacterial phyla, but Proteobacteria were most dominant and ubiquitous followed by Actinobacteria. Among different classes of Proteobacteria i.e. α, β, γ and δ-proteobacteria, the members of γ-proteobacteria were most dominant and have been reported from different crop plants. Least number of microbes was reported from phylum Deinococcus-Thermus and Acid bacteria followed by Bacteroidetes [18,22-26] (Figure 1). There are very few reports of archaea as PGP including rhizospheric as well as endophytic [27-29].
Actinobacteria is a phylum of gram-positive bacteria and divided into five classes' viz. Acidimicrobiia, Actinobacteria, Coriobacteriia, Nitriliruptoria, Rubrobacteria and Thermoleophilia. Members of class Actinobacteria are most dominant and found to associate with plants growing in different habitats as well as extreme environments. It also contains one of the largest of bacterial genera, Streptomyces [30-32]. The rhizospheric Actinobacteria are most dominant in nature and they are of great economic importance to humans because agriculture and forests depend on their contributions to soil systems. Among different groups of microbes, the member Bacillus and Bacillus derived genera are belonged to phylum Firmicutes, which most culturable and colonize with different plants such as wheat, rice, maize, soybean, and chickpea [33-37]. The phylum Firmicutes, have been further distributed into five families, Bacillaceae, Bacillales Incertae Sedis, PaneniBacillaceae, Planococcaceae and Staphylococcaceae and reported from most of crop plants studies [6-8,10,12,15]. Among different phylum the Proteobacteria one of the predominant phylum including many dominant genera including Brevundimonas terrae, Bosea sp. and Methylobacterium sp. from α-proteobacteria; Burkholderia sp, Burkholderia cepacia, Variovoraxginsengisoli,Janthinobacterium lividum and Janthinobacterium sp. from β-proteobacteria and Aeromonas, Pantoea, Providencia, Pseudomonas, Psychrobacter and Yersinia from γ-proteobacteria class [6-8,18,38].
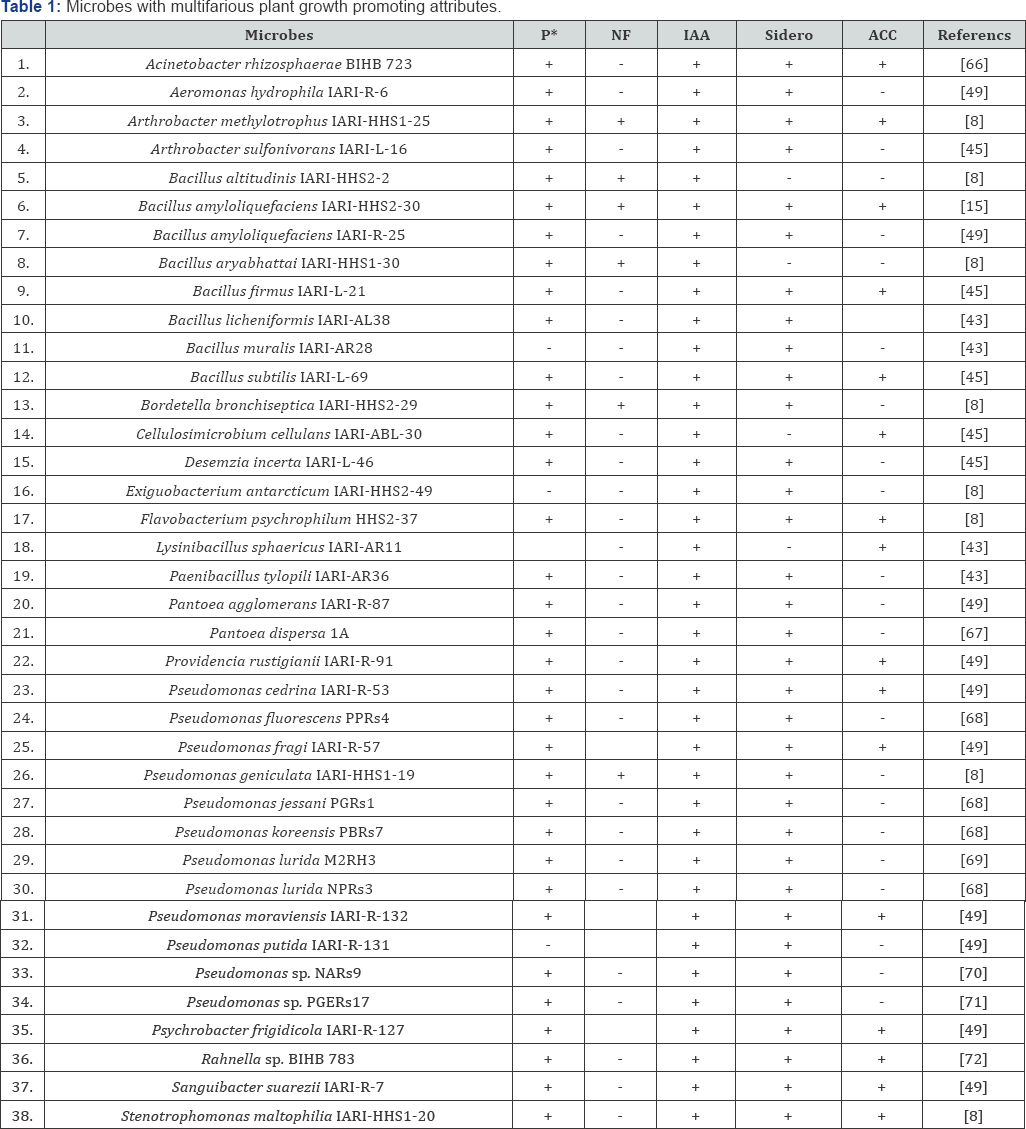
*P-Phosphorus; NF-Nitrogen fixation; IAA- Indole acetic acids; Sidero- Siderophores; ACC-1-aminocyclopropane-1-carboxylate (ACC) deaminase
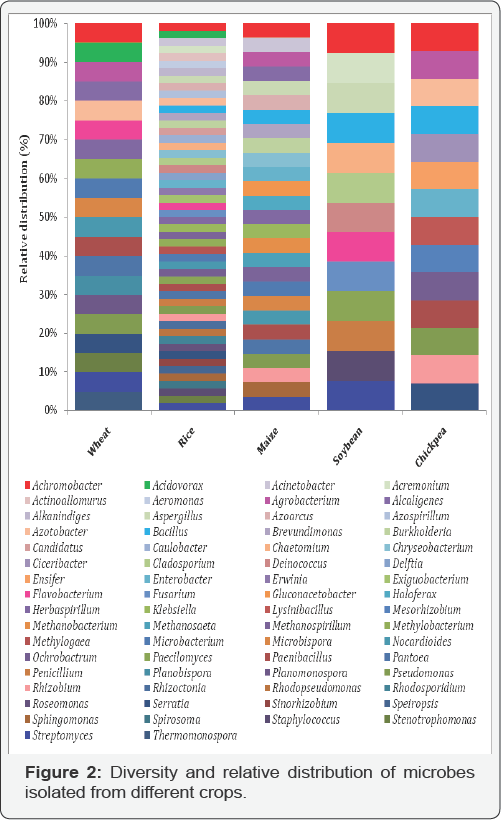
Plant associated microbes have been shown be beneficial by promoting plant growth either directly, e.g. by fixation of atmospheric nitrogen, solubilization of minerals such as phosphorus, potassium and zinc; production of Sidero pores and plant growth hormones such cytokinins, auxins and gibberellins or indirectly, via production of antagonistic substances by inducing resistance against plant pathogens [3,38-41]. Biological nitrogen fixation (BNF) is one of the possible biological alternatives to N-fertilizers and could lead to more productive and sustainable agriculture without harming the environment. Many associative microbes are now known to fix atmospheric nitrogen and supply it to the associated host plants. A variety of nitrogen fixing microbes like Arthrobacter, Azoarcus, Azospirillum, Azotobacter, Bacillus, Enterobacter, Gluconoacetobacter, Herbaspirillum, Klebsiella, Pseudomonas, and Serratia have been isolated from the rhizosphere of various crops, which contribute fixed nitrogen to the associated plants [18,42-44] (Figure 2) (Table 1).
Plant-associated microbes typically produce plant growth hormones such as auxins and gibberellins. The gibberellins production is most typical for the root-associated microbes and auxins production is common to all plant-associated microbes. Auxins can promote the growth of roots and stems quickly (by increasing cell elongation) or slowly (through cell division and differentiation). The production of such growth regulators by microbes provides numerous benefits to the host plant including the facilitation of root system expansion, which enhances the absorption of water and nutrients and improves plant survival. The ability to synthesize these phytohormones is widely distributed among plant-associated microbes [45-47]. Diverse microbial species possess the ability to produce the auxins phytohormone indole acetic acid (IAA). Reviewing the role of bacterial IAA in different microorganism-plant interactions highlights the fact that microbes use this phytohormone to interact with plants as part of their colonization strategy, including phyto-stimulation and circumvention of basal plant defense mechanisms. The IAA application has also been suggested to promote plant growth or suppress weed growth.
Phosphorus (P) is major essential macronutrient for biological growth and development. Microbes offer a biological rescue system capable of solubilizing the insoluble inorganic P of soil and make it available to the plants. The ability of some microbes to convert insoluble P to an accessible form, like orthophosphate, is an important trait in PGP microbes for increasing plant yields. The rhizospheric P-utilizing microbes could be a promising source for plant growth promoting agent in agriculture. P-solubilization is a common trait among microbes associated with different crops. For instance, the majority of microbial populations from wheat, rice, maize, and legumes were able to solubilise mineral phosphates, and a vast number of PGP microbes with P solubilizing property have been reported which include members belonging to Burkholderia, Enterobacter, Halolamina, Pantoea, Pseudomonas, Citrobacter and Azotobacter [48-54] (Table 1). Possible mechanisms for solubilization from organic bound P involve either enzymes namely C-P lyase, nonspecific phosphatases and phytases [55,56]. However, most of the bacterial genera solubilize P through the production of organic acids such as gluconate, ketogluconate, acetate, lactate, oxalate, tartarate, succinate, citrate and glycolate. Type of organic acid produced for P solubilization may depend upon the carbon source utilized as substrate. Highest P solubilization has been observed when glucose, sucrose or galactose has been used as sole carbon source in the medium [27,57].
Ethylene is a stress-induced plant hormone that can inhibit plant growth. Some microbes can lower the level of ethylene in the plant by cleaving the plant-produced ethylene precursor 1-aminocyclopropane-1-carboxylate (ACC). Inoculation of such microbes can mitigate the effect of various stressors by sustaining plant growth in the face of ethylene. ACC-deaminase producing microbes may play a role in regulating ethylene levels after such bursts, ensuring that ethylene levels stay below the point where growth is impaired. Ethylene is a key regulator of the colonization of plant tissue by bacteria which in turn suggests that the ethylene inhibiting effects of ACC- deaminase may be a microbial colonization strategy. Generally, ethylene is an essential metabolite for the normal growth and development of plants [58-61]. This plant growth hormone is produced endogenously by approximately all plants and is also produced by different biotic and abiotic processes in soils and is important in inducing multifarious physiological changes in plants. Apart from being a plant growth regulator, ethylene has also been established as a stress hormone. Under stress conditions like those generated by salinity, drought, water logging, heavy metals and pathogenicity, the endogenous level of ethylene is significantly increased which negatively affects the overall plant growth. PGP microbes which possess the enzyme, 1-aminocyclopropane-1-carboxylate (ACC) deaminase, facilitate plant growth and development by decreasing ethylene levels, inducing salt tolerance and reducing drought stress in plants. Microbial strains exhibiting ACC deaminase activity have been identified in a wide range of genera such as Acinetobacter, Achromobacter, Agrobacterium, Alcaligenes, Azospirillum, Bacillus, Burkholderia, Enterobacter, Pseudomonas, Ralstonia, Serratia and Rhizobium [6,8,61-65] (Figure 2)(Table 1).
The indirect mechanism of plant growth occurs when microbes lessen or prevent the detrimental effects of pathogens on plants by production of inhibitory substances or by increasing the natural resistance of the host [66-72]. Phytopathogenic microbes can control by releasing siderophore, chitinases, antibiotics, fluorescent pigment or by cyanide production [73,74]. Biocontrol systems are eco-friendly, cost-efficient and involved in improving the soil consistency and maintenance of natural soil flora [75-77]. To act efficiently, the Biocontrol agent should remain active under large range of conditions viz., varying pH, temperature and concentrations of different ions. Biocontrol agents limit growth of pathogen as well as few nematodes and insects. Biocontrol microbes can limit pathogens directly by producing antagonistic substances, competition for iron, detoxification or degradation of virulence factors; or indirectly by Inducing Systemic Resistance (ISR) in plants against certain diseases, signal interference, competition for nutrients and niches and interference with activity, survival, germination and speculation of the pathogen. Iron is a necessary cofactor for many enzymatic reactions and is an essential nutrient for virtually all organisms. In aerobic conditions, iron exists predominantly in its ferric state (Fe3+) and reacts to form highly insoluble hydroxides and ox hydroxides that are largely unavailable to plants and microorganisms. To acquire sufficient iron, siderophore produced by bacteria can bind Fe3+ with a high affinity to solubilizing this metal for its efficient uptake.
Bacterial siderophores are low-molecular-weight compounds with high Fe3+ chelating affinities responsible for the solubilization and transport of this element into bacterial cells. Some bacteria produce hydroxamate-type siderophores, and others produce catecholate-types [78,79]. In a state of iron limitation, the siderophore-producing microorganisms are also able to bind and transport the iron-siderophore complex by the expression of specific proteins. The production of siderophores by microorganisms is beneficial to plants because it can inhibit the growth of plant pathogens. siderophores have been implicated for both direct and indirect enhancement of plant growth by plant growth promoting microbes.
Conclusion and Future Prospect
The microbes are capable of colonizing the rhizosphere, phyllosphere as well as living inside the plant tissues as endophytes. Biotechnology has opened up new possibilities concerning the application of these microbes for the beneficial applications in soil for the promotion of plant growth and the biological control of soil-borne pathogens. The nutritional and environmental requirements of these microbes are very diverse. Due to the diverse range of activities as well as the number of microbes in varying habitats around the world, these are important bioresources towards rationalized use of chemicals fertilizers in agriculture. An understanding of plant microbiome for major crops will be of significant importance for exploring efficient use of these microbes.
Acknowledgement
The authors duly acknowledge the Department of Biotechnology, Govt. of India for the financial support provided (Grant No. BT/AGR/BIOFORTI/PHII/NIN/2011), Ministry of Food Processing Industries (MoFPI) Govt. of India grant for infrastructural facility development (F.No. 5-11/2010-HRD) and Vice Chancellor, Eternal University for providing the motivation and research infrastructure.
Competing Interests
The authors declare no conflict of interest.
References
- Barea JM, Pozo MJ, Azcon R, Azcon Aguilar C (2005) Microbial co-operation in the rhizosphere. J Exp Bot 56(417): 1761-1778.
- Lavania M, Chauhan PS, Chauhan S, Singh HB, Nautiyal CS (2006) Induction of plant defense enzymes and phenolics by treatment with plant growth-promoting rhizobacteria Serratia marcescens NBRI1213. Curr Microbiol 52(5): 363-368.
- Tilak K, Ranganayaki N, Pal KK, De R, Saxena AK (2005) Diversity of plant growth and soil health supporting bacteria. Curr Sci 89(1): 136150.
- Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14(1): 1-4.
- Kumar V, Yadav AN, Saxena A, Sangwan P, Dhaliwal HS (2016) Unravelling rhizospheric diversity and potential of phytase producing microbes. SM J Biol 2(1): 1009.
- Verma P, Yadav AN, Khannam KS, Kumar S, Saxena AK, Suman A (2016) Molecular diversity and multifarious plant growth promoting attributes of Bacilli associated with wheat (Triticum aestivum L) rhizosphere from six diverse agro-ecological zones of India. J Basic Microbiol 56(1): 44-58.
- Yadav AN, Verma P, Kumar R, Kumar V, Kumar K (2017) Current applications and future prospects of eco-friendly microbes. EU Voice 3(1): 1-3.
- Verma P, Yadav AN, Khannam KS, Panjiar N, Kumar S, et al. (2015) Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann Microbiol 65(4): 1885-1899.
- Hornschuh M, Grotha R, Kutschera U (2002) Epiphytic bacteria associated with the bryophyte Funaria hygrometrica: effects of Methylobacterium strains on protonema development. Plant Biol 4 (6): 682-687.
- Verma P, Yadav AN, Kazy SK, Saxena AK, Suman A (2014) Evaluating the diversity and phylogeny of plant growth promoting bacteria associated with wheat (Triticum aestivum) growing in central zone of India. Int J Curr Microbiol Appl Sci 3(5): 432-447.
- Holland MA, Davis R, Moffitt S, OLaughlin K, Tayman B (2000) Using "leaf prints” to investigate a common bacterium. Am Biol Teach 62(2): 128-131.
- Verma P, Yadav AN, Kazy SK, Saxena AK, Suman A (2013) Elucidating the diversity and plant growth promoting attributes of wheat (Triticum aestivum) associated acidotolerant bacteria from southern hills zone of India. Natl J Life Sci 10(2): 219-227.
- Tancos K, Cox K (2017) Effects of consecutive streptomycin and kasugamycin applications on epiphytic bacteria in the apple phyllosphere. Plant Dis 101(1): 158-164.
- Dobrovolskaya T, Khusnetdinova K, Manucharova N, Golovchenko A (2017) Structure of epiphytic bacterial communities of weeds. Microbiology 86(2): 257-263.
- Verma P, Yadav AN, Shukla L, Saxena AK, Suman A (2015) Alleviation of cold stress in wheat seedlings by Bacillus amyloliquefaciens IARI-HHS2-30, an endophytic psychrotolerant K-solubilizing bacterium from NW Indian Himalayas. Natl J Life Sci 12(2): 105-110.
- Lian J, Wang Z, Zhou S (2008) Response of endophytic bacterial communities in banana tissue culture plantlets to Fusarium wilt pathogen infection. J Gen Appl Microbiol 54(2): 83-92.
- Mitter B, Pfaffenbichler N, Flavell R, Compant S, Antonielli L (2017) A new approach to modify plant microbiomes and traits by introducing beneficial bacteria at flowering into progeny seeds. Front Microbiol 8:11.
- Suman A, Yadav AN, Verma P (2016) Endophytic Microbes in Crops: Diversity and Beneficial impact for Sustainable Agriculture. Microbial Inoculants in Sustainable Agricultural Productivity, Research Perspectives. Springer-Verlag, pp. 117-143.
- Hallmann J, Quadt-Hallmann A, Mahaffee W, Kloepper J (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43(10): 895-914.
- Quadt Hallmann A, Kloepper J, Benhamou N (1997) Bacterial endophytes in cotton: mechanisms of entering the plant. Can J Microbiol 43(6): 577-582.
- Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 278(1): 1-9.
- Yadav AN (2015) Bacterial diversity of cold deserts and mining of genes for low temperature tolerance. Thesis, IARI, New Delhi/BIT, Ranchi, pp. 234. doi:10.13140/RG.2.1.2948.1283/2.
- Saxena AK, Yadav AN, Rajawat (2016) Microbial diversity of extreme regions: An unseen heritage and wealth. Indian J Plant Genet Resour 29(3): 246-248.
- Shukla L, Suman A, Yadav AN, Verma P, Saxena AK (2016) Syntrophic microbial system for ex-situ degradation of paddy straw at low temperature under controlled and natural environment. J App Biol Biotech 4(2): 30-37.
- Yadav AN, Verma P, Kumar M, Pal KK (2015) Diversity and phylogenetic profiling of niche-specific Bacilli from extreme environments of India. Ann Microbiol 65(2): 611-629.
- Suman A, Verma P, Yadav AN, Saxena AK (2015) Bioprospecting for extracellular hydrolytic enzymes from culturable thermotolerant bacteria isolated from Manikaran thermal springs. Res J Biotechnol 10(4): 33-42.
- Yadav AN, Sharma D, Gulati S, Singh S, Kaushik R (2015) Haloarchaea endowed with phosphorus solubilization attribute implicated in phosphorus cycle. Sci Rep 5: 12293.
- Saxena AK, Kaushik R, Yadav AN, Gulati S, Sharma D (2015) Role of Archaea in sustenance of plants in extreme saline environments. In: Proceeding of 56th Annual Conference of Association of Microbiologists of India and International Symposium on "Emerging Discoveries in Microbiology”. doi:10.13140/RG.2.1.2073.9925.
- Gaba S, Singh RN, Abrol S, Yadav AN, Saxena AK (2017) Draft Genome Sequence of Halolamina pelagica CDK2 Isolated from Natural Salterns from Rann of Kutch, Gujarat, India. Genome Announc 5(6): 1-2.
- Singh RN, Gaba S, Yadav AN, Gaur P, Gulati S, Kaushik R, Saxena AK (2016) First, high quality draft genome sequence of a plant growth promoting and cold active enzymes producing psychrotrophic Arthrobacter agilis strain L77. Stand Genomic Sci 11: 54
- Yadav AN, Verma P, Kumar V, Sachan SG, Saxena AK (2017) Extreme cold environments: a suitable niche for selection of novel psychrotrophic microbes for biotechnological applications. Adv Biotechnol Microbiol 2(2): 1-4.
- Sahay H, Yadav AN, Singh AK, Singh S, Kaushik R, Saxena AK (2017) Hot springs of Indian Himalayas: Potential sources of microbial diversityand thermostable hydrolytic enzymes. 3 Biotech 7(2): 1-411.
- Hung PQ, Annapurna K (2004) Isolation and characterization of endophytic bacteria in soybean Omonrice 12: 92-101.
- Hungria M, Nogueira MA, Araujo RS (2015) Soybean seed coinoculation with "Bradyrhizobium" spp. and Azospirillum brasilense: a new biotechnological tool to improve yield and sustainability. Am J Plant Sci 6(6): 811
- Kämpfer P, Glaeser SP, McInroy JA, Busse H-J (2016) Nocardioides zeicaulis sp nov an endophyte actinobacterium of maize. Int J Syst Evol Microbiol 66(4): 1869-1874.
- Liu Y, Zhai L, Wang R, Zhao R, Zhang X (2015) “Paenibacillus" zeae sp. nov, isolated from maize (Zea mays L) seeds. Int J Syst Evol Microbiol 65(12): 4533-4538.
- Sun L, Qiu F, Zhang X, Dai X, Dong X, Song W (2008) Endophytic bacterial diversity in rice (Oryza sativa L) roots estimated by 16S rDNA sequence analysis. Microb Ecol 55(3): 415-424.
- Verma P, Yadav AN, Khannam KS (2016) Appraisal of diversity and functional attributes of thermotolerant wheat associated bacteria from the peninsular zone of India. "Saudi J Biol Sci. doi:10.1016/j. sjbs.2016.01.042".
- Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71(9): 4951-4959.
- Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41(2): 109-117.
- Kour D, Rana KL, Verma P, Yadav AN, Kumar V, et al. (2017) Biofertilizers: eco-friendly technologies and bioresources for sustainable agriculture. in: Proceeding of International Conference on Innovative Research in Engineering Science and Technology, IREST/PP/014.
- Niste M, Vidican R, Pop R, Rotar I (2013) Stress factors affecting symbiosis activity and nitrogen fixation by Rhizobium Cultured in vitro. ProEnvironment 6(13): 42-45.
- Yadav AN, Sachan SG, Verma P, Saxena AK (2016) Bioprospecting of plant growth promoting psychrotrophic Bacilli from cold desert of north western Indian Himalayas. Indian J Exp Biol 54(2): 142-150.
- Olivares J, Bedmar EJ, Sanjuan J (2013) Biological nitrogen fixation in the context of global change. Mol Plant-Microb IN 26(5): 486-494.
- Yadav AN, Sachan SG, Verma P, Tyagi SP, Kaushik R, et al. (2015) Culturable diversity and functional annotation of psychrotrophic bacteria from cold desert of Leh Ladakh (India). World J Microbiol Biotechnol 31(1): 95-108.
- Chaiharn M, Lumyong S (2011) Screening and optimization of indole-3-acetic acid production and phosphate solubilization from rhizobacteria aimed at improving plant growth. Curr Microbiol 62(1): 173-181.
- Xie H, Pasternak JJ, Glick BR (1996) Isolation and characterization of mutants of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2 that overproduce indoleacetic acid. Curr Microbiol 32(2): 67-71.
- Kumar V, Yadav AN, Verema P, Sangwan P (2017) ß-Propeller phytases: Diversity, catalytic attributes, current developments and potential biotechnological applications. Int J Biol Macromolec 98: 595-609.
- Yadav AN, Sachan SG, Verma P, Saxena AK (2015) Prospecting cold deserts of north western Himalayas for microbial diversity and plant growth promoting attributes. J Biosci Bioeng 119(6): 683-693.
- Illmer P, Schinner F (1992) Solubilization of inorganic phosphates by microorganisms isolated from forest soils. Soil Biol Biochem 24(4): 389-395.
- Kaur R, Saxena A, Sangwan P, Yadav AN (2017) Production and characterization of a neutral phytase of Penicillium oxalicum EUFR-3 isolated from Himalayan region. Nus Biosci 9(1): 68-76.
- Wakelin SA, Warren RA, Harvey PR, Ryder MH (2004) Phosphate solubilization by Penicillium spp. closely associated with wheat roots. Biol Fert Soils 40(1): 36-43.
- Pradhan N, Sukla L (2006) Solubilization of inorganic phosphates by fungi isolated from agriculture soil. Afr J Biotechnol 5(10): 850-854.
- Yadav AN, Rana KL, Kumar V, Dhaliwal HS (2016) Phosphorus Solubilizing Endophytic Microbes: Potential Application for Sustainable Agriculture. EU Voice 2: 21-22.
- Singh P, Kumar V, Agrawal S (2014) Evaluation of phytase producing bacteria for their plant growth promoting activities. Int J Microbiol. doi.org/10.1155/2014/426483
- Kumar V, Singh D, Sangwan P, Gill PK (2015) Management of Environmental Phosphorus Pollution Using Phytases: Current Challenges and Future Prospects. In: Applied Environmental Biotechnology: Present Scenario and Future Trends. Springer, pp. 97114.
- Rodriguez H, Gonzalez T, Goire I, Bashan Y (2004) Gluconic acid production and phosphate solubilization by the plant growth- promoting bacterium Azospirillum spp. Naturwissenschaften 91(11): 552-555.
- Suman A, Verma P, Yadav AN, Srinivasamurthy R, Singh A, Prasanna R (2016) Development of hydrogel based bio-inoculant formulations and their impact on plant biometric parameters of wheat (Triticum aestivum). Int J Curr Microbiol Appl Sci 5(3): 890-901.
- Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118(1): 10-15.
- Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol 119(3): 329-339.
- Siddikee MA, Chauhan P, Anandham R, Han G-H, Sa T (2010) Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J Microbiol Biotechnol 20(11): 1577-1584.
- Khalid A, Akhtar M, Mahmood M, Arshad M (2006) Effect of substrate-dependent microbial ethylene production on plant growth. Microbiology 75(2): 231-236.
- Srivastava AK, Kumar S, Kaushik R, Singh P (2014) Diversity analysis of Bacillus and other predominant genera in extreme environments and its utilization in Agriculture. Technical report, 414402/C30026.
- Sun Y, Cheng Z, Glick BR (2009) The presence of a 1-aminocyclopropane- 1-carboxylate (ACC) deaminase deletion mutation alters the physiology of the endophytic plant growth-promoting bacterium Burkholderia phytofirmans PsJN. FEMS Microbiol Lett 296(1): 131-136.
- Xu M, Sheng J, Chen L, Men Y, Gan L (2014) Bacterial community compositions of tomato seeds and plant growth promoting activity of ACC deaminase producing Bacillus subtilis (HYT-12-1) on tomato seedlings. World J Microbiol Biotechnol 30(3): 835-845.
- Gulati A, Vyas P, Rahi P, Kasana RC (2009) Plant growth-promoting and rhizosphere-competent Acinetobacter rhizosphaeraestrain BIHB 723 from the cold deserts of the Himalayas. Curr Microbiol 58(4): 371-377.
- Selvakumar G, Kundu S, Joshi P (2008) Characterization of a cold- tolerant plant growth-promoting bacterium Pantoea dispersa 1A isolated from a sub-alpine soil in the North Western Indian Himalayas. World J Microbiol Biotechnol 24(7): 955-960.
- Mishra PK, Bisht SC, Ruwari P, Selvakumar G (2011) Alleviation of cold stress in inoculated wheat (Triticum aestivum L) seedlings with psychrotolerant Pseudomonads from NW Himalayas. Arch Microbiol 193(7): 497-513.
- Selvakumar G, Joshi P, Suyal P (2011) Pseudomonas lurida M2RH3 (MTCC 9245), a psychrotolerant bacterium from the Uttarakhand Himalayas, solubilizes phosphate and promotes wheat seedling growth. World J Microbiol Biotechnol 27(5): 1129-1135.
- Mishra PK, Mishra S, Bisht SC (2009) Isolation, molecular characterization and growth-promotion activities of a cold tolerant bacterium Pseudomonas sp. NARs9 (MTCC9002) from the Indian Himalayas. Biol Res 42(3): 305-313.
- Mishra PK, Mishra S, Selvakumar G, Bisht SC (2008) Characterisation of a psychrotolerant plant growth promoting Pseudomonassp. strain PGERs17 (MTCC 9000) isolated from North Western Indian Himalayas. Ann Microbiol 58(4): 561-568.
- Vyas P, Joshi R, Sharma K, Rahi P (2010) Cold-adapted and rhizosphere- competent strain of Rahnella sp. with broad-spectrum plant growth- promotion potential. J Microbiol Biotechnol 20(12): 1724-1734.
- Lottmann J, Heuer H, de Vries J, Mahn A (2000) Establishment of introduced antagonistic bacteria in the rhizosphere of transgenic potatoes and their effect on the bacterial community. FEMS Microbiol Ecol 33(1): 41-49.
- Yadav AN, Sachan SG, Verma P, Kaushik R, Saxena AK (2016) Cold active hydrolytic enzymes production by psychrotrophic Bacilli isolated from three sub-glacial lakes of NW Indian Himalayas. J Basic Microbiol 56(3): 294-307.
- Jha PN, Gupta G, Jha P, Mehrotra R (2013) Association of rhizospheric endophytic bacteria with plants: a potential gateway to sustainable agriculture. Greener J Agric Sci 3(2): 73-84.
- Uppal A, El Hadrami A, Adam L, Tenuta M (2008) Biological control of potato Verticillium wilt under controlled and field conditions using selected bacterial antagonists and plant extracts. Biol Con 44(1): 90100.
- Verma P, Yadav AN, Kumar V, Khan MA, Saxena AK (2017) Microbes in termite management: potential role and strategies. In: Sustainable Termite Management. pp. 1-15. doi.org/10.1016/B978-0-444-63501- 3.00001-6.
- Neilands J (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270(45): 26723-26726.
- Ahmad F, Ahmad I, Khan M (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities . Microbiol Res 163(2): 173-181.






























