An Investigation of Ground Water Aquifer Characteristics of Sarcee Reserve
*Roger Saint-Fort
Department of Environmental Science, Mount Royal University, Canada
Submission: October 06, 2016; Published: December 19, 2016
*Corresponding author: Roger Saint-Fort, Department of Environmental Science, Faculty of Science & Technology, Mount Royal University, 4825 Mount Royal Gate SW, Calgary, AB, T3E 6K6; Email:rsaintfort@mtroyal.ca
How to cite this article: Roger S. An Investigation of Ground Water Aquifer Characteristics of Sarcee Reserve. Int J Environ Sci Nat Res. 2016; 1(1): 555554. DOI: 10.19080/IJESNR.2016.01.555554
Abstract
This study investigates the ground water aquifer characteristics used domestically by the residents of Sarcee Reserve. Local flow systems in the sub-horizontal sandstones and shales probably occur with the well fractured sandstone units acting as collectors for the downward moving ground water of overlying units. Four basic types of ground waters were identified based on the major ions chemistry. The Na, K-HCO3, CO3 type represents 56% of the ground water samples and is most representative of moderate to deep bedrock. A mixed type dominated by SO4-2 or HCO3- anions and either Ca, Mg, or Na, K cations form 30% of this classification. Typically found in ground water discharge areas. A Ca, Mg-HCO3, CO3 type represents 8% of the ground water of this type and occurs in relatively shallow fractured bedrock. The NaSO4 chemical type represents 6% of the ground water samples. It is generally associated with ground waters in surficial deposits. TDS in the ground water samples ranged from 249 to 1600mg/L. Ground water aquifer quality on the reserve is generally good. Reported poor ground water quality in many instances may be directly attributed to poor well materials and construction. Flavor may be affected by geogenic sources of minerals dissolved in its matrix or products of bacteriological growths. However, repeated presence of objectionable taste, odor, or color in the water may cause the community to question its safety for domestic use.
Keywords: Ground water; Aquifer; TDS; Geogenic; Piper diagram; Health
Introduction
Hess [1] estimated that about 6.2 million Canadians rely on ground water as a source of potable drinking water and usual domestic purposes. Ground water is the main source of potable drinking water for the residents of Tssu Tina nation. Due to geogenic activities, ground water in its pristine state, is expected to contain a multitude of naturally occurring minerals dissolved in its matrix. Therefore, in the ultimate sense of drinking water quality, ground water can’t be considered as always sparkling pure water. Generally, the latter contains an array of geogenic sources of minerals dissolved in its matrix. In addition, a wide variety of anthropogenic sources for aquifer contamination exists. These may include septic tanks, landfills, leakage from underground storage tanks and sewage lagoons. While the presence of dissolved contaminants can adversely impact the usefulness. Furthermore, their qualitative as well as quantitative fate and behavior will remain function of complexphysico/biochemical interactions taking place at the interfaces of the vadose and saturated zone systems as contaminants are passing through different hydrologic zones. There is growing concern on the portability of ground water resource in particular dissolved geogenic concentration levels as they may pose a significant health risk to human health [2]. In that regard, there is a large portfolio of research demonstrating the interplay between lack of access to adequate drinking water in communities and good health [3]. The primary objective of this study was to ascertain the degree of ground water aquifer characteristics that is being used by the residents of the reserve.
Study Area
The study area is designated as Sarcee Reserve No. 145. The land area of the reserve covers 283 km2 with an estimated population of 2,000 residents [2]. Sarcee reserve is located in Township 23, Ranges 2, 3 and 4 West of the 5th Meridian on the southwestern edge of the City of Calgary (coordinates 50o 58’N 114o21’W) and extends west to the Bragg Creek Town site. The most important economic activity is the Grey Eagle casino and commercial gravel mining with small scale activities like agriculture and waste management.
Method of Study
The method of study involved field visits, analyzing ground water drilling records, water quality data, aerial photos, topographical and geological maps. Due to the fact that records for wells drilling and water quality characteristics were available for only 80 houses, some interpretation was, to a certain extent, general in nature, however scientifically insightful. Representative ground water samples were collected from various households in clean glass bottles having a foillined, labelled and appropriately handled prior to be analyzed. All analyses were carried out according to standard methods of water analysis.
Climate
The climate of the subject geographical area is largely subcontinental with short and moderately warm summers, brief springs and fall seasons, and rather long cold winters. It is influenced by the interplay between the cold Artic and Pacific air masses. Dry warm westerly s air mass (Chinooks) often flow over the mountains in the winter to assuage the winter [3]. The temperature commonly ranges from between -35 and 25oC. Average annual precipitation is approximately 50 cm.
Geologic Setting
Physiography
Sarcee reserve is physically situated in a transition zone between the Western Alberta Plains to the east and the Foothills of the Rocky Mountains to the west. Elevations vary from approximately 1120m on the eastern edge of the reserve to over 1430 m in the western sector of the reserve. It primarily consists of an upland area bisected into two distinct highlands by a glacial outwash feature known as Six Mile Coulee. To the east, Bullhead Hill is the dominant feature, while to the west, the features known locally as Blueberry Hill and Spy Hill which are an up thrust unit of the Cretaceous Brazeau formation, are the predominant land features [4]. The reserve is drained by the Elbow River which runs along the west and northern boundaries, entering the reserve in its northeast corner to drain into the Glenmore reservoir. The southeast corner of Sarcee reserve is drained by Fish Creek which then travels through South Calgary to empty into the Bow River. Milburn Creek drains the west central highland which empties into the Elbow. The south portion of Six Mile Coulee drains into Fish Creek.
Bedrock Geology
The eastern area of the reserve is underlain by the siltstone and shale sequence of the Tertiary Paskapoo Formation. The occurrence of Late-Cretaceous Tertiary Bears paw Formation stratigraphically crosses the western part of the reserve as well. A tongue of Cretaceous Alberta Group also crosses the reserve [5].
Surficial Geology
The bedrock within the boundary of the reserve is overlain by nearly continuous till cover. The exception is the higher elevations of Blueberry Hill which has exposures of Cretaceous porcupine Hills Formation at the surface [4]. The major unit found on the reserve is the Spy Hill Drift which consists of till and stratified drift deposited by a glacier [5]. This till consists of clay rich silty till. This till is generally structure less but can contain local and isolated deposits of sand and gravel. The drift is generally thin throughout the reserve ranging from 4 to 6 m thick and decreasing to zero at some higher elevations. Within the natural drainage channels such as Six Mile Coulee, Milburn Creek, the Elbow and Fish Creek Valleys, deposits of sand, gravel, lacustrine clays and silts are common in the valley floors. Commercial gravel deposits are being exploited in the area of Two Guns and on the west boundary of the reserve.
Results and Discussions
Hydrogeology
The ground water flow regime for the study area was inferred with a certain degree of interpretation because of data gaps. Vegetation association, aerial photos, and field observations were used to delineate areas of natural ground water recharge and discharge. The lodrge pole brine-brome grass (Bromus sp.), bearberry (Arctostaphylos ava-ursi) are indicative of natural ground water recharge conditions along ridges, where the water table is relatively deep. Meadows, often containing springs and seepages, supporting the growth of willow (Salix sp.), sedge (Carex sp.) and swamp birch (Betulapumoilia var. glandulifera) are indicative of groundwater discharge in areas of more gentle slopes where the water table is relatively shallow. Discharge areas are typically lower elevations and areas near the Elbow River and Fish Creek. Other significant areas of discharge are Six Mile Coulee, Lott Creek, and Milburn Creek. Seepages, springs, swamps, and hummocky ground as ground water discharge features were also observed.
A simple water budget approach to estimate the volume of water entering the recharge areas was used. As surficial deposits thin or absent, it is likely that the recharge areas are quite effective in replenishing the local aquifers. The basic equation is expressed as Eq.(1)
R = [Pr – {(Sc x Pr) + E t} A (1)
Where R is the volume of water recharging the ground waters, Pr is annual precipitation, Sc represents a runoff coefficient, Et is evapotranspiration, and A is site area available for recharge. Values derived for the various variables are Pr = 0.50m/yr; Sc = 0.20; Et = 0.20m/yr; and A = 2800ha. Annual ground water recharge was estimated at 5 x 106m3.
Hydro geochemistry
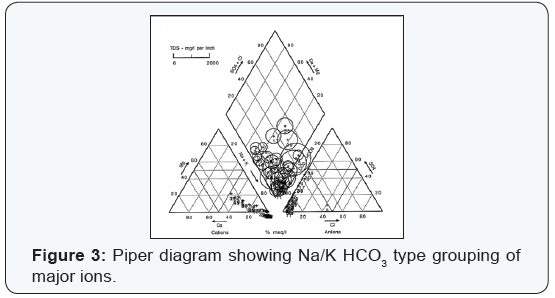
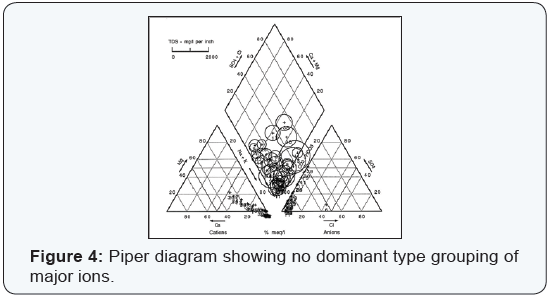
Most natural ground waters can be represented as solutions of three cationic constituents: calcium, magnesium, and the alkali metals; and of the three anionic constituents: sulfate, chloride, and those contributing to alkalinity [6]. The composition of ground water may be represented conveniently by trilinear plotting. These plots may aid prospective ground water users to forecast the type of ground waters at a particular geographical location and depth. Hydro chemical results for single wells were plotted and their respective groupings are shown on hydro chemical facies (Figures 1-4).
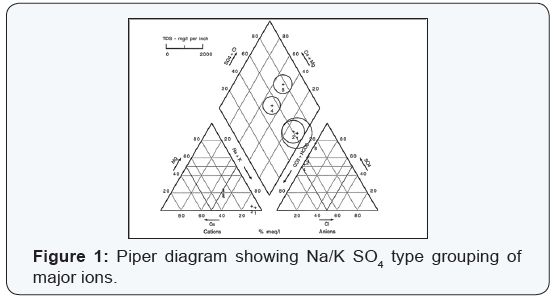
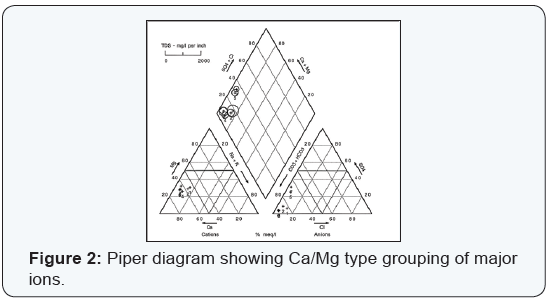
The ground water chemistry appeared highly variable. The major ion chemistry indicates four basic types of ground waters encompassing Sarcee reserve: A Ca, Mg-HCO3, CO3 type represents ground water in relatively shallow fractured bedrock. Such hydrochemistry represents 8% of the ground water samples of this type. Ground waters of the calcium-magnesium bicarbonate type are typical of areas of recharge and of short flow systems. Total dissolved solids (TDS) content, a measure of how much dissolved salts are in the water, range from 249 to 489 mg/L for an average concentration of 337mg/L with a standard deviation of 86. Average well depth below ground surface is approximately 125 ft.
The Na, K-HCO3, CO3 type occurs throughout the area and is most representative of moderate to deep bedrock in area. It was determined that 56% of the ground water samples are of this type. They occur mainly in ground water discharge areas and in the major river valleys. Because of higher sodium content, hardness is generally less than 100 mg/L while TDS contents of up to 1366 mg/L reflect the deeper, more mineralized nature of these ground waters. TDS values ranged from 329 to 1360 mg/L for an average concentration of 610 mg/L with a standard deviation of 225. It is important to note that the 400 mg/L content of TDS generally coincides with a change in dominant cations. Average well depth below ground surface is approximately 160 ft.
A mixed type of the ground waters, dominated by SO4-2 or HCO3- anions and either Ca, Mg, or Na, K cations. In that regard, 30% of the ground water falls under this classification. Wells depth tend to be quite variable ranging spatially from 100 to 200 ft. TDS values ranged from 387 to 1290mg/L for an average concentration of 514 mg/L with a standard deviation of 202. This water type occurs mainly in ground water discharge areas. Low ground water movement may be responsible for locally high TDS and SO4-2 while active ground water flushing has HCO3- as the dominant anion. The data indicate that the maximum TDS content for the mixed type ground water is 1290mg/L, but that about 55% of the ground water has a content less than 500mg/L. The sulfate rich ground water seems to have a much greater effect on the TDS content than does the cation exchange process.
The NaSO4 chemical type represents 6% of the ground water samples analyzed. It is generally associated with ground waters in surficial deposits. Sulfate content is generally highest in relatively shallow less than 50 ft completed in till and glaciofluvial sediments. Sulfate content at this depth is probably related to the gypsum content of these sediments within the annual phreatic range. The highest TDS content of any ground water sample was denoted in the NaSO4 ground water type. TDS value ranged from 830 to 1600mg/L for an average value of 1071mg/L with a standard deviation of 308.All the ground waters have a TDS content greater than 500mg/L. The relatively shallowness and sulfate rich ground waters level do not seem to have a much greater effect on TDS content than does the cation exchange process. Excessive mineralization and ground water discharge configuration are the probable reasons for the quality noted. Sulfates concentration in aquifer formations are generally lower in ground water from bedrock aquifers than in those from aquifers in surficial deposit. This could be ascribed to the reduction process occurring when water comes into contact with methane, a natural gas often from bedrock aquifer in Alberta or other agents such as decomposed organic matter. The two geochemical reduction process [7] are represented by the following reactions:
CaSO4 + CH4 ↔ CaS + CO2 + H2O (1)
CaS + 2 CO2 + 2 H2O ↔ H2S + Ca (HCO3)2 (2)
Waters affected by sulfate reduction gain in bicarbonate as well, and the calcium in the resulting bicarbonate will, by natural softening processes, be exchanged for sodium. The reduction of sulfates then indirectly increases the sodium alkalinity content of bedrock ground waters. A summary of the major contributing minerals to the hydrochemistry and their congruent dissociation are highlighted in the geochemical reactions:
Dolomite: Ca/Mg(CO2) <----->Ca2 + Mg+2 + 2 CO2 [3]
Fluorite: CaF2<----->Ca2 + 2F- [4]
Calcite: CaCO3 <-----> Ca2 + CO3-2 [5]
Epsomite: MgSO4•7 H2O <-----> Mg+2 + SO4 -2 + 7 H2O [6]
Sylvite: KCl<----->K+ + Cl- [7]
Mirabillite: Na2SO4•10 H2O <-----> 2 Na+ + SO4 -2 + 10 H2O [8]
Gypsum: CaSO4•2 H2O <-----> Ca2 + SO4 -2 + 2 H2O [9]
Halite: NaCl<-----> Na+ + Cl- [10]
Many minerals that are affecting the ground waters chemistry in the aquifer formations will be expected to dissolve congruently and incongruently according to the equilibrium concentrations attained in the water.
Ground water aquifer quality on the reserve is generally good. Reported poor ground water quality in many instances may be directly attributed to poor well materials and construction. Flavor may be affected by inorganic salts or metal ions, or products of bacteriological growths. However, repeated presence of objectionable taste, odor, or color in a water supply may cause the community to question its safety for domestic use. Guideline levels for drinking water is typically based on taste quality according to level of TDS and not health risk [8]. Water with extremely low TDS may be insipid and taste flat. The palatability of ground waters on the reserve with a TDS value less than 600 mg/L is regarded as good drinking water. It was found that 50% of the water samples have such designation. The hydrochemistry data indicate that 35% of the ground water samples can be regarded as of fair quality.
They are characterized by TDS concentrations between 600 to 900 mg/L. Poor quality was represented by 10% of the ground water samples. The hydrochemistry is characterized by TDS concentrations between 900 to 1200 mg/L. However, in 5% of ground water samples, TDS concentrations were found to be greater than 1200 mg/L. This makes the palatability of the water as unacceptable. Furthermore, from a health risk standpoint, daily consumption of ground water with high TDS concentrations may have adverse effects on residents experiencing renal, cardiac and circulatory problems [9,10]. Wells with fluorides concentration greater than 2 mg/L in the ground water could lead to dental fluorosis or mottling of the tooth enamel [11].
Geochemical conditions leading to the development of salinity in the ground water formations on the reserve can be conceptualized in terms of the dynamic equilibrium arising between mineral availability and mineral solubility [8]. Furthermore, high soluble chloride minerals including halite (NaCl), will often occur as salt strata originally formed during water evaporation of marine basins. Anhydrite (CaSO4) and gypsum (CaSO4•2 H2O) are considered as the most common of the sulfate bearing sedimentary minerals that may release SO4 - 2 upon dissolution. Geochemical evolution from active use of the ground waters may be described according to the following reactions were exchanged represents cations exchanged on ground water fabric:
CaSO4•2 H2O -----> Ca+2 + SO4 -2 + 2 H2O [11]
Ca+2 + 2 Na (exchanged) <-----> 2 Na+ + Ca (exchanged) [12]
Ca+2 + Mg+2 (exchanged) <-----> Mg+2 + Ca (exchanged) [13]
CaCO3 + H2CO3 -----> Ca+2 + 2 HCO3 - [14]
NaCl -----> Na+ + Cl- [15]
KCl -----> K+ + Cl- [16]
Mg+2 + 2 Na (exchanged) <-----> 2 Na+ + Mg (exchanged) [17]
Ca+2 + 2 K (exchanged) <-----> 2 K+ + Ca (exchanged) [18]
Mg+2 + 2 K (exchanged) <-----> 2 K+ + Mg (exchanged) [19]
Cl- will remain dissolved in the ground water solution matrix, unaffected by various ion exchanges, precipitation, or biochemical degradations.
Well Yield
Local ground water flow systems in the sub-horizontal sandstones and shales formation probably occur with the fractured sandstone units. The latter acting as conduits and collectors for the downward moving of overlying units. Hence, well yields on the reserve are expected to be intrinsically variable and hydraulic conductivities highly anisotropic. Sustainable yield is considered to be the rate at which individual wells can be pumped continuously for a period of 20 years so that at the end of this period water level in the ground water formation will not be drawn below the aquifer’s top [12]. The Paskapoo formation contains sandstone units which are capable of high sustained well yields. This is evidenced by the well yields data in the eastern area of the reserve. Distribution of wells location relative to bedrock geology indicate that the sandstone units within the Paskapoo formation are the major producing horizons. Where drift aquifers are present, they are highly permeable and productive since they consist primarily of glaciofluvial sand and gravel deposits. However, they can also be unpredictable due to variations in porosity and aerial extent. Based on drillers logs, highest yield are recorded in the NE-SE sections with an average yield of 12 gpm ranging from 4 to 60 gpm. Average depth completion is 130ft. Lowest yields are typically denoted the NW-SW sections in ¼ sections 19 and 30 in range 4 and ¼ section 18 in range 3. With an average depth completion of 110ft, the wells yielded on the average 10gpm ranging from 4 to 35gpm.
Conclusion
This study indicates that the ground water quality of the investigated aquifers tend to vary spatially due to natural factors. The ranges of TDS are significantly varying in the water and in some instances exceed the desirable as well as health guidelines permissible limits. Piper diagrams indicate the dominancy of Na, K-HCO3, CO3 water and mixed type dominated by SO4 -2 or HCO3 - anions and either Ca, Mg, or Na, K cations.
Acknowledgement
The author is thankful for the support provided by Health Canada.
References
- Hess PJ (1986) Ground water use in Canada 1981. National Hydrology Research Institute, Inland Waters Directorate, Ottawa. IWD Tech Bull No. 140.
- Statistics Canada (2016) “Population and dwelling counts for Canada, provinces and territories, and census subdivisions (municipalities), 2011 and 2006 censuses (Alberta).
- Longley RW (1967) Climate and weather patterns. In Alberta: A Natural History. (1967)Edited by W.G. Hardy, pp 53-70. Hurting Publishers, Edmonton.
- Rutherford RL (1927) Geology along the Bow River between Cochrane and Kananaskis. Province of Alberta. Report No: 17.
- Wyatt FA, Newton JD, Bowser WE, Odynsky W (1942) Soil survey of Blackfoot and Calgary sheets. Bulletin No. 39, pp 105-118 July.
- Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Am Geoph Union Trans 25(6): 914-928.
- Saint-Fort R (1993) Assessing the ground water quality characteristics of the Hamlet of Carseland. J Environ Sci Health 28(5): 995-1003.
- Australian Drinking Water Guidelines Paper 6 National Water Quality Management Strategy. (2011) National Health and Medical Research Council (NHMRC), National Resource Management Ministerial Council (NRMMC), Commonwealth of Australia, Canberra.
- Sauer HI (1974) Relationship between trace element content of drinking water and chronic disease. Univ. Ill. Bulletin No. 71(108): 39.
- Kavcar P, Sofuoglu A, Sofuoglu SC (2009) A health risk assessment for exposure to trace metals via drinking water ingestion pathway. Int J Hyg Environ Health 212(2): 216-227.
- (2010) Guidelines for Canadian Drinking Water Quality. Guideline Technical Document. Fluoride. Ottawa, Ontario.
- Alberta Environment. Groundwater evaluation guideline. (February 2003).






























